Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.102434
Revised: December 8, 2024
Accepted: January 10, 2025
Published online: March 15, 2025
Processing time: 99 Days and 3.5 Hours
Colorectal cancer (CRC) is one of the most common malignant gastrointestinal tumors worldwide, with high incidence and mortality rates.
To investigate the expression significance of the chromatin-remodeling protein MORC family CW-type zinc finger 4 (MORC4) as a biomarker in CRC patients, and to explore its relationship with pathological features and prognosis.
A total of 143 CRC specimens and 57 adjacent tissue specimens, surgically removed from our hospital between January 2020 and January 2021, were co
Compared with adjacent tissues, the expression rate of MORC4 protein in CRC tissues was significantly higher (P < 0.05). No significant difference was observed in the high expression rate of MORC4 protein in CRC tissues among patients of different gender, age, tumor location, tumor diameter, and primary tumor status
The upregulation of MORC4 expression in CRC patients is closely related to disease severity and prognosis, suggesting its potential as an evaluation biomarker, which warrants further investigation.
Core Tip: To investigate the expression significance of the chromatin-remodeling protein MORC family CW-type zinc finger 4 as a biomarker in colorectal cancer patients, and to explore its relationship with pathological features and prognosis. The upregulation of MORC family CW-type zinc finger 4 expression in colorectal cancer patients is closely related to disease severity and prognosis, suggesting its potential as an evaluation biomarker, which warrants further investigation.
- Citation: Wei L, Wang L, Liu YG, Gao LF. Expression significance of biomarker MORC4 in colorectal cancer patients and its relationship with pathological features and prognosis. World J Gastrointest Oncol 2025; 17(3): 102434
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/102434.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.102434
Colorectal cancer (CRC) is one of the most common malignant gastrointestinal tumors worldwide, with high incidence and mortality rates[1]. According to global cancer statistics[2], CRC ranks third in cancer incidence and second in cancer-related deaths, following lung cancer. Particularly in economically developed regions, the incidence of CRC is steadily rising due to factors such as population aging, lifestyle changes (e.g., high-fat diets, lack of exercise), and increasing rates of obesity and diabetes[3]. Although early screening techniques (such as colonoscopy and computed tomography colonography) and advancements in treatment have improved early diagnosis and treatment of CRC in recent years, and some patients’ prognoses have been improved, the overall survival (OS) rate of patients with advanced CRC remains low, with a 5-year survival rate of less than 15%[4]. The development of CRC involves the combined effects of genetic, environmental, and lifestyle factors, leading to abnormal proliferation and malignant transformation of intestinal cells[5]. In recent years, as tumor molecular biology research has deepened, the molecular mechanisms of CRC have gradually been revealed, and the role of biomarkers in tumor development, diagnosis, and prognosis assessment has garnered increasing attention[6]. Biomarkers not only provide new avenues for early diagnosis and molecular subtyping of CRC but also lay the foundation for personalized treatment and prognosis evaluation[7].
The chromatin-remodeling protein MORC family CW-type zinc finger 4 (MORC4) is a protein involved in DNA repair, gene expression regulation, and cell cycle control[8]. Its abnormal expression in various malignant tumors has attracted widespread attention in recent years. MORC4 may influence tumor occurrence and progression by regulating chromatin remodeling, affecting cell proliferation, differentiation, and apoptosis[9]. Previous studies have shown that MORC4 is highly expressed in various tumors, including breast cancer, liver cancer, and gastric cancer, and is closely related to tumor invasiveness and poor patient prognosis[10]. However, there is a lack of systematic research on the expression of MORC4 in CRC and its relationship with pathological features and prognosis. Therefore, this study aims to explore the expression of MORC4 in CRC and its relationship with patients’ clinicopathological characteristics and prognosis. By analyzing the expression levels of MORC4 in CRC tissues and adjacent normal tissues, we aim to reveal its role in tumor progression and evaluate its potential as a prognostic marker for CRC, providing new theoretical support and potential therapeutic targets for CRC diagnosis and treatment.
A total of 143 CRC specimens and 57 adjacent non-cancerous tissue specimens resected via surgery at our hospital between January 2020 and January 2021 were collected. After embedding in paraffin, immunohistochemistry was performed to examine the expression of the MORC4 protein. Clinical and pathological data of the 143 CRC patients are shown in Table 1. This study was approved by the Ethics Committee of our hospital.
| Pathological characteristics | Cases | Proportion (%) |
| Gender | - | - |
| Male | 81 | 56.64 |
| Female | 62 | 43.36 |
| Age (years) | - | - |
| < 60 | 19 | 13.29 |
| ≥ 60 | 124 | 86.71 |
| Tumor location | - | - |
| Left side | 112 | 78.32 |
| Right side | 31 | 21.68 |
| Tumor diameter (cm) | - | - |
| ≤ 5 | 94 | 65.73 |
| > 5 | 49 | 34.27 |
| Degree of differentiation | - | - |
| Low differentiation | 21 | 14.69 |
| Moderate + high differentiation | 122 | 85.31 |
| T stage | - | - |
| T1 + T2 stage | 13 | 9.09 |
| T3 + T4 stage | 130 | 90.91 |
| N stage | - | - |
| N0 stage | 88 | 61.54 |
| Nx stage | 55 | 38.46 |
| M stage | - | - |
| M0 stage | 127 | 88.81 |
| M1 stage | 16 | 11.19 |
| TNM stage | - | - |
| I + II | 85 | 59.44 |
| III + IV | 58 | 40.56 |
| Serum CEA (μg/L) | - | - |
| < 5 | 67 | 46.85 |
| ≥ 5 | 76 | 53.15 |
The major reagents used in this study were as follows: MORC4 primary antibody (Batch No.: GR256479-6) was purchased from Abcam, MA, United States; secondary antibody (Batch No.: Wp18031101), citrate buffer (Batch No.: 18070601), and hydrogen peroxide (Batch No.: 180105) were provided by Fuzhou Maixin Biotechnology Development Co., Ltd.; DAB (diaminobenzidine) staining solution (Batch No.: Wp18111303), hematoxylin staining reagent (Batch No.: 2018052901), and crystal violet (Batch No.: 20181025) were obtained from Shanghai Beyotime Biotechnology Co., Ltd. All reagents were used in strict accordance with the manufacturer’s instructions during the experiment to ensure the accuracy and reproducibility of the results.
The expression of MORC4 protein in CRC tissues was detected using standard immunohistochemistry techniques. Positive controls were CRC tissue sections with known high MORC4 expression, as confirmed by pre-experiments using western blot and immunohistochemistry. Negative controls included sections incubated without the primary antibody or with an isotype-matched immunoglobulin G antibody at the same concentration and conditions.
Paraffin-embedded sections were prepared from 143 CRC tissues and 57 adjacent normal tissues. During the experiment, the sections were first incubated in 80% methanol for 10 minutes to block endogenous peroxidase activity. The sections were then washed with phosphate buffered saline (PBS), and antigen retrieval was performed using a 0.1 mmol/L citrate buffer solution to ensure full antigen exposure. After another PBS wash, 50 μL of primary anti-MORC4 antibody (dilution ratio 1:100) was added, and the sections were incubated at 37 °C for 1 hour to allow sufficient binding to the target protein. After washing with PBS, horseradish peroxidase-labeled secondary antibody (50 μL) was added, and the sections were incubated again at 37 °C for 1 hour to enhance the antigen-antibody reaction signal. After secondary antibody incubation, the sections were incubated with streptavidin-peroxidase for 1 hour to ensure signal amplification. Finally, the sections were stained using DAB color development and counterstained with hematoxylin. After mounting, the results were observed using a light microscope. Staining results were independently evaluated by two experienced pathologists blinded to the sample identity. MORC4 protein was primarily localized in the nucleus of tumor cells, with positive staining appearing as brown-yellow granules. The percentage of stained cells in each field of view was scored: Less than 5% stained cells = 0 points, 6%-25% = 1 point, 26%-50% = 2 points, 51%-75% = 3 points, and more than 75% = 4 points. The intensity of staining was also scored, with no staining = 0 point, weak staining = 1 point, moderate staining = 2 points, and strong staining = 3 points. The final score was the product of these two scores: 0-1 points were considered negative (-), 2-4 points were weakly positive (+), 6-8 points were moderately positive (++), and 9-12 points were strongly positive (+++). In the analysis, - to + were defined as low expression, while ++ to +++ were defined as high expression.
For the postoperative prognosis follow-up, patient survival data were recorded via telephone or outpatient visits. The follow-up period started from the date of surgery and ended at the patient’s death or the follow-up cutoff date of February 1, 2024. OS was defined as the time from the surgery date to the first occurrence of all-cause death or the follow-up cutoff. Based on the immunohistochemical scoring criteria, patients were divided into the MORC4 high-expression group and the low-expression group, and the survival differences between the two groups were compared.
Experimental data were visualized using GraphPad Prism 8, and data analysis was conducted using SPSS 22.0. Count data were expressed in the form of [n (%)], and group comparisons were made using the χ2 test. For normally distributed measurement data, and independent samples t-tests were conducted for intergroup comparisons. Survival analysis was performed using the Kaplan-Meier method to plot survival curves, and the Log-rank test was used to assess survival differences between patients with different levels of MORC4 expression. Statistical significance was set at P < 0.05.
Immunohistochemical staining showed that compared to adjacent non-cancerous tissues, brownish-yellow nuclear staining was concentrated in CRC tissue samples, and the high expression rate of MORC4 protein in CRC tissue samples (62.24%) was significantly higher than in adjacent non-cancerous tissues (12.28%), with a statistically significant difference (P < 0.05), as shown in Table 2.
| Tissue type | Low expression | High expression | χ² | P value |
| CRC tissue (n = 143) | 54 (37.76) | 89 (62.24) | 40.750 | < 0.001 |
| Adjacent tissue (n = 57) | 50 (87.72) | 7 (12.28) |
There were no statistically significant differences in the comparison of high MORC4 protein expression rates in CRC tissues among patients of different genders, ages, tumor locations, tumor diameters, and T stages (P > 0.05). However, statistically significant differences were observed in patients with different levels of differentiation, N stage, M stage, tumor-nodes-metastasis (TNM) stage, and serum carcinoembryonic antigen (CEA) levels (P < 0.05), as shown in Table 3.
| Pathological characteristics | Cases (n = 143) | High MORC4 expression (n = 89) | χ² | P value |
| Gender | - | - | 0.705 | 0.401 |
| Male | 81 | 48 (59.26) | - | - |
| Female | 62 | 41 (66.13) | - | - |
| Age (years) | - | - | 0.356 | 0.550 |
| < 60 | 19 | 13 (68.42) | - | - |
| ≥ 60 | 124 | 76 (61.29) | - | - |
| Tumor location | - | - | 0.015 | 0.902 |
| Left side | 112 | 70 (62.50) | - | - |
| Right side | 31 | 19 (61.29) | - | - |
| Tumor diameter (cm) | - | - | 0.827 | 0.362 |
| ≤ 5 | 94 | 56 (59.57) | - | - |
| > 5 | 49 | 33 (67.35) | - | - |
| Degree of differentiation | - | - | 5.772 | 0.016 |
| Low differentiation | 21 | 18 (85.71) | - | - |
| Moderate + high differentiation | 122 | 71 (58.20) | - | - |
| T stage | - | - | 0.911 | 0.339 |
| T1 + T2 stage | 13 | 6 (46.15) | - | - |
| T3 + T4 stage | 130 | 83 (63.85) | - | - |
| N stage | - | - | 5.760 | 0.016 |
| N0 stage | 88 | 48 (54.55) | - | - |
| Nx stage | 55 | 41 (74.55) | - | - |
| M stage | - | - | 7.612 | 0.005 |
| M0 stage | 127 | 74 (58.27) | - | - |
| M1 stage | 16 | 15 (93.75) | - | - |
| TNM stage | - | - | 7.706 | 0.005 |
| I + II | 85 | 45 (52.94) | - | - |
| III + IV | 58 | 44 (75.86) | - | - |
| Serum CEA (μg/L) | - | - | 19.270 | < 0.001 |
| < 5 | 67 | 29 (43.28) | - | - |
| ≥ 5 | 76 | 60 (78.95) | - | - |
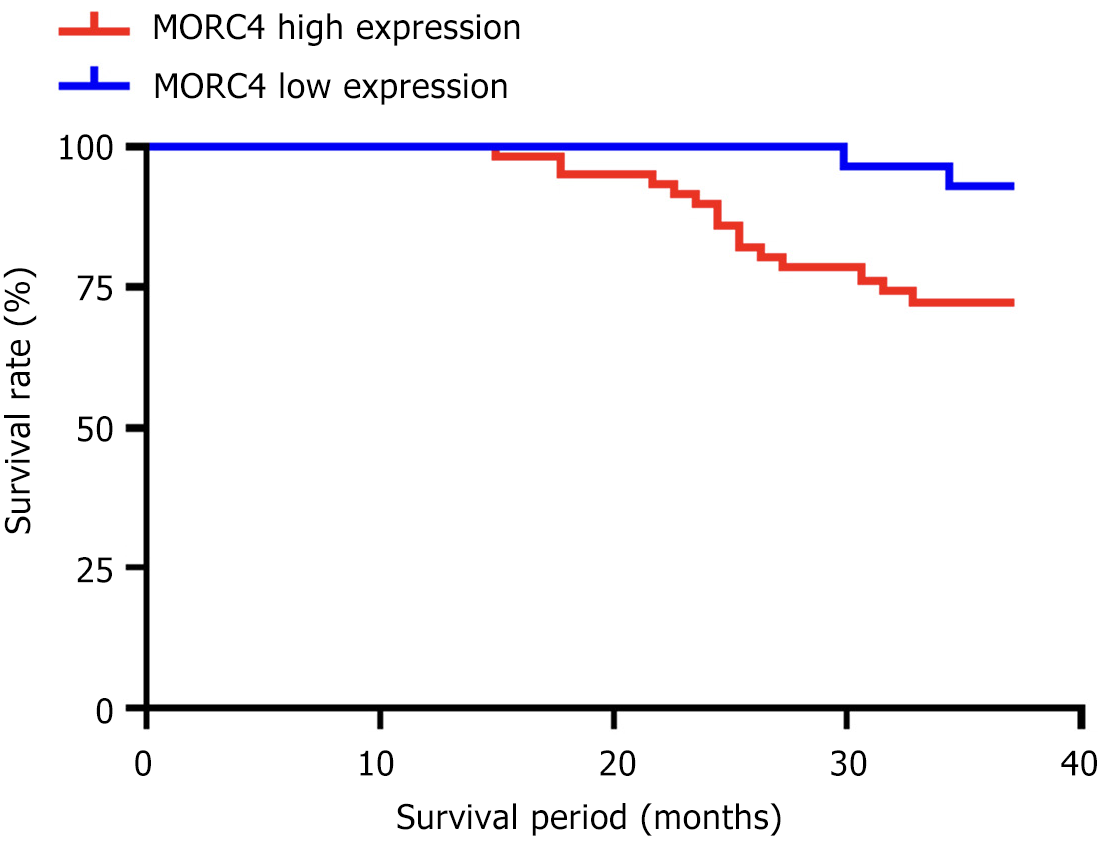
After 3 years of follow-up, of the 143 CRC patients, 112 survived (78.32%), 26 died (18.18%), and 5 were lost to follow-up (3.50%). The postoperative survival time ranged from 15 to 36 months. Survival analysis showed that the 3-year OS rate of patients with high MORC4 expression in CRC tissue was lower than that of patients with low MORC4 expression (P < 0.05), as shown in Figure 1.
In recent years, the incidence and mortality rates of CRC have been rising globally, especially in countries and regions with rapid economic development, where changes in the environment and lifestyle have significantly increased the risk of the disease[11]. The etiological factors of CRC are highly complex and are usually the result of the combined effects of genetic susceptibility and environmental factors[12]. Recent studies have found that behavioral factors such as unhealthy diets, lack of exercise, obesity, and smoking are closely associated with the occurrence of CRC[13]. However, despite advances in medical technology improving the early detection and treatment of CRC, many patients are still diagnosed at an advanced stage, resulting in a poor prognosis[14]. Therefore, actively exploring biomarkers related to CRC prognosis and using them for early diagnosis and personalized treatment is of great clinical value in improving the survival rate of CRC patients.
The MORC protein family is a highly conserved superfamily of nuclear proteins, with its members broadly involved in critical biological processes such as gene expression regulation and DNA damage repair[15]. Members of the MORC protein family typically contain three characteristic domains: First, the N-terminal MutL-type ATPase domain, which plays a key role in DNA repair and gene transcription regulation[16]; second, the CW-type zinc finger domain, which participates in chromatin regulation by recognizing lysine 4 methylation modification of histone 3[17]; and finally, the C-terminal coiled-coil domain, which mainly regulates cell functions through protein-protein interactions and subcellular localization[18]. Additionally, as a member of this family, the MORC4 protein contains four potential small ubiquitin-related modifier binding sites, allowing it to bind to the C-terminal binding protein corepressor, thereby regulating gene expression suppression processes[19]. In recent years, MORC4 has gradually attracted attention in cancer research. Studies have found that this protein plays a crucial role in oncogenesis, participating in various biological behaviors of tumor cells, such as proliferation, migration, invasion, and apoptosis[20]. Other studies have shown that MORC4 exhibits abnormally high expression in several malignancies, particularly in breast cancer, and its high expression is closely associated with tumor malignancy[21]. Knockdown of MORC4 expression can significantly inhibit the growth of breast cancer cells and promote apoptosis, thereby slowing disease progression.
In this study, through the analysis of tissue samples from 143 CRC patients, we found that MORC4 expression levels in CRC tissues were significantly higher than in adjacent normal tissues, indicating that MORC4 may play an important oncogenic role in the development of CRC. Further analysis revealed that MORC4 expression was closely associated with several clinicopathological characteristics. There was no significant difference in the high expression rate of MORC4 between different patients based on gender, age, tumor location, tumor size, or T stage (P > 0.05), suggesting that these factors may not be directly related to MORC4 expression levels. However, high expression of MORC4 was closely associated with tumor differentiation degree, lymph node metastasis, distant metastasis, TNM stage, and serum CEA levels, with statistically significant differences (P < 0.05). These results suggest that high MORC4 expression may reflect a higher aggressiveness and metastatic tendency of CRC, and is associated with poorer prognosis. Additionally, the survival analysis results further support the prognostic value of MORC4. In this study, the OS of CRC patients with high MORC4 expression was significantly lower than that of patients with low expression (P < 0.05), indicating that MORC4 may not only be an oncogenic factor in CRC development but also an important biomarker for assessing patient prognosis. The high expression of MORC4 appears to accelerate the malignant progression of CRC, leading to earlier recurrence and metastasis, thus shortening survival time. Similarities with studies in other types of cancer[22,23] suggest that MORC4 may enhance tumor malignancy by affecting tumor cell survival and apoptotic pathways, promoting cancer cell resistance to treatment and anti-apoptotic properties.
This study, through the analysis of tissue samples from 143 CRC patients, has for the first time clearly identified the significant overexpression of MORC4 in CRC tissues and elucidated its correlation with clinicopathological characteristics and prognosis. It was found that MORC4 expression in CRC tissues is significantly higher than in adjacent normal tissues, suggesting that it may serve as a potential oncogene in the occurrence and development of CRC. Further analysis showed that high MORC4 expression is significantly associated with tumor differentiation, lymph node metastasis, distant metastasis, TNM stage, and serum CEA levels, indicating that MORC4 may play an important role in regulating tumor invasiveness and metastatic capacity. Survival analysis revealed that the OS of patients with high MORC4 expression was significantly lower than that of patients with low expression, suggesting that high MORC4 expression is closely related to poor prognosis and has significant clinical potential. According to the results of this study, MORC4 may not only play an important role in the diagnosis of CRC but also provide reliable evidence for prognosis evaluation. Moreover, the results of this study provide a theoretical basis for future development of therapies targeting MORC4. MORC4 has the potential to become a new therapeutic target, and inhibiting its expression or blocking its function may offer new hope for CRC treatment. Overall, the findings of this study broaden our understanding of the molecular mechanisms of CRC, especially the function and clinical value of MORC4 in CRC, providing important references for future cancer diagnosis, prognosis evaluation, and targeted therapy. It should be noted that despite the progress made in exploring the relationship between MORC4 and the clinical characteristics and prognosis of CRC, there are still some unavoidable limitations. The following points outline the main shortcomings: (1) Small sample size and regional limitations: This study involved only 143 patients, with a relatively limited sample size, and the cases were sourced from a single region, which may limit the generalizability of the findings to a global population or other cohorts. Future studies should expand the sample size and cover more regions and ethnic backgrounds to improve the general applicability of the results; (2) Insufficient in-depth molecular mechanism research: Although this study revealed the high expression of MORC4 in CRC and its correlation with clinical characteristics, it did not delve into the specific molecular mechanisms by which MORC4 affects CRC. Future studies should combine cell experiments and animal models to analyze in detail the specific roles of MORC4 in signal transduction, gene expression regulation, cell cycle, proliferation, and invasion/metastasis to better understand its function as an oncogene; (3) Lack of prospective research and long-term follow-up data: This is a retrospective study that relies on existing medical records, which may introduce selection bias. Additionally, due to the limited follow-up period, the long-term impact of high MORC4 expression on survival rates was not fully observed. Future studies should be designed prospectively and extend follow-up times to comprehensively assess the impact of MORC4 on the long-term prognosis of CRC patients; (4) Insufficient consideration of other confounding factors: This study did not thoroughly evaluate the potential impact of different treatment methods (e.g., surgery, radiotherapy, chemotherapy) on MORC4 expression, which may limit understanding of MORC4’s role in different treatment contexts. Future studies should include different treatment factors in the analysis to explore the interaction between treatment approaches and MORC4 expression; (5) Limitations of using a single biomarker: Although the high expression of MORC4 and its potential diagnostic and prognostic value in CRC were validated, relying on a single biomarker for cancer diagnosis or prognosis assessment remains limited. Future studies should combine multiple molecular markers (e.g., CEA, carbohydrate antigen 19-9) and use multi-factor models to further enhance diagnostic accuracy and prognostic assessment capabilities in CRC; (6) Lack of comparison with other cancer types: This study focused only on MORC4 expression and function in CRC but did not explore its performance and functional differences in other cancers. MORC4 has been reported to be overexpressed in various malignancies, including breast cancer and gastric cancer. Future studies could conduct comparative analyses of MORC4’s role in different types of tumors to determine whether it has a universal oncogenic function or plays a crucial role only in specific cancers; and (7) Further exploration of therapeutic targeting: Although this study proposed that MORC4 could be a potential therapeutic target for CRC, no experimental validation of MORC4 inhibitors has yet been conducted. Future research should focus on the development of MORC4 inhibitors and their preclinical evaluation. In vitro studies could utilize established CRC cell lines to assess effects on cell proliferation, apoptosis, and invasion, while in vivo studies could employ xenograft models to determine therapeutic efficacy and potential toxicity. These studies would not only validate the feasibility of targeting MORC4 but also bridge the gap between basic research and clinical application, offering promising avenues for translational cancer research. In conclusion, while this study reveals the critical role of MORC4 in CRC, its clinical utility in diagnosis, prognosis, and treatment requires further validation through larger-scale and more comprehensive investigations.
| 1. | Rawla P, Sunkara T, Barsouk A. Epidemiology of colorectal cancer: incidence, mortality, survival, and risk factors. Prz Gastroenterol. 2019;14:89-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 444] [Cited by in RCA: 1155] [Article Influence: 165.0] [Reference Citation Analysis (12)] |
| 2. | Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M, Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr Drug Targets. 2021;22:998-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (3)] |
| 3. | Li J, Ma X, Chakravarti D, Shalapour S, DePinho RA. Genetic and biological hallmarks of colorectal cancer. Genes Dev. 2021;35:787-820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 376] [Article Influence: 75.2] [Reference Citation Analysis (6)] |
| 4. | Bresalier RS. Colorectal Cancer Screening in a Changing World. Gastroenterol Clin North Am. 2022;51:577-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 5. | Done JZ, Fang SH. Young-onset colorectal cancer: A review. World J Gastrointest Oncol. 2021;13:856-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (6)] |
| 6. | Helsingen LM, Kalager M. Colorectal Cancer Screening - Approach, Evidence, and Future Directions. NEJM Evid. 2022;1:EVIDra2100035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 49] [Article Influence: 12.3] [Reference Citation Analysis (2)] |
| 7. | Baranov E, Nowak JA. Pathologic Evaluation of Therapeutic Biomarkers in Colorectal Adenocarcinoma. Surg Pathol Clin. 2023;16:635-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Yang Z, Zhuang Q, Hu G, Geng S. MORC4 is a novel breast cancer oncogene regulated by miR-193b-3p. J Cell Biochem. 2019;120:4634-4643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Luo J, Zeng S, Tian C. MORC4 Promotes Chemoresistance of Luminal A/B Breast Cancer via STAT3-Mediated MID2 Upregulation. Onco Targets Ther. 2020;13:6795-6803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Strack E, Rolfe PA, Fink AF, Bankov K, Schmid T, Solbach C, Savai R, Sha W, Pradel L, Hartmann S, Brüne B, Weigert A. Identification of tumor-associated macrophage subsets that are associated with breast cancer prognosis. Clin Transl Med. 2020;10:e239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Yang W, Zheng H, Lv W, Zhu Y. Current status and prospect of immunotherapy for colorectal cancer. Int J Colorectal Dis. 2023;38:266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 12. | Chetroiu D, Pop CS, Filip PV, Beuran M. How and why do we screen for colorectal cancer? J Med Life. 2021;14:462-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 13. | Rajendran S, Barbon S, Pucciarelli S. Spotlight on Circadian Genes and Colorectal Cancer Crosstalk. Endocr Metab Immune Disord Drug Targets. 2021;21:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (3)] |
| 14. | The Lancet Gastroenterology Hepatology. Controversy over colonoscopy for colorectal cancer screening. Lancet Gastroenterol Hepatol. 2022;7:1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 15. | Wang H, Zhang L, Luo Q, Liu J, Wang G. MORC protein family-related signature within human disease and cancer. Cell Death Dis. 2021;12:1112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 16. | Zhong Z, Xue Y, Harris CJ, Wang M, Li Z, Ke Y, Liu M, Zhou J, Jami-Alahmadi Y, Feng S, Wohlschlegel JA, Jacobsen SE. MORC proteins regulate transcription factor binding by mediating chromatin compaction in active chromatin regions. Genome Biol. 2023;24:96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Yang F, Xie HY, Yang LF, Zhang L, Zhang FL, Liu HY, Li DQ, Shao ZM. Stabilization of MORC2 by estrogen and antiestrogens through GPER1- PRKACA-CMA pathway contributes to estrogen-induced proliferation and endocrine resistance of breast cancer cells. Autophagy. 2020;16:1061-1076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Liu YY, Liu HY, Yu TJ, Lu Q, Zhang FL, Liu GY, Shao ZM, Li DQ. O-GlcNAcylation of MORC2 at threonine 556 by OGT couples TGF-β signaling to breast cancer progression. Cell Death Differ. 2022;29:861-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 19. | Tencer AH, Cox KL, Wright GM, Zhang Y, Petell CJ, Klein BJ, Strahl BD, Black JC, Poirier MG, Kutateladze TG. Molecular mechanism of the MORC4 ATPase activation. Nat Commun. 2020;11:5466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Deng Y, Li Z. Effects of PRSS1-PRSS2 rs10273639, CLDN2 rs7057398 and MORC4 rs12688220 polymorphisms on individual susceptibility to pancreatitis: A meta-analysis. Genomics. 2020;112:848-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Duan X, Guo G, Pei X, Wang X, Li L, Xiong Y, Qiu X. Baicalin Inhibits Cell Viability, Migration and Invasion in Breast Cancer by Regulating miR-338-3p and MORC4. Onco Targets Ther. 2019;12:11183-11193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 22. | Liang Y, Wu D, Qu Q, Li Z, Yin H. MORC4 plays a tumor-promoting role in colorectal cancer via regulating PCGF1/CDKN1A axis in vitro and in vivo. Cancer Gene Ther. 2023;30:985-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Wang B, Mao J, Wang L, Zhao Y, Wang B, Yang H. Exosome-mediated transfer of lncRNA RP3-340B19.3 promotes the progression of breast cancer by sponging miR-4510/MORC4 axis. Cancer Cell Int. 2024;24:312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |