Published online Mar 15, 2025. doi: 10.4251/wjgo.v17.i3.100521
Revised: December 18, 2024
Accepted: January 2, 2025
Published online: March 15, 2025
Processing time: 179 Days and 15 Hours
The problem of gastric cancer (GC) prevention remains relevant for a long time. Various methods of population serological screening of atrophic gastritis and precancerous changes in the gastric mucosa have been created at present. Modern endoscopic and morphological methods of verification of the diagnosis of precancerous diseases and changes in the gastric mucosa have been introduced into the practice of gastroenterologists and oncologists. GC risk stratification systems allow the formation of risk groups that require population screening. Practical hints for population serological screening of atrophic gastritis, endoscopic and morphological verification of precancerous changes and diseases of the stomach recommend using it: When developing state programs for the prevention of stomach cancer; when implementing preventive measures for stomach cancer by doctors of all specialties; the authors also offer the possibility of use by anyone over the age of 40, provided that they seek methodological help from their doctor; in the work of health schools in any medical and preventive institutions. The use of an assessment system of certain risk factor signatures with prognostic value would add significant assistance to preventive measures against GC.
Core Tip: The authors present a consistent system of population-based serological screening for precancerous diseases and changes in the gastric mucosa as a first-line screening strategy. The use of modern endoscopic and morphological diagnostic methods to verify pre-cancerous pathology of the stomach is recommended as a second line of screening for precancerous condition of the stomach. A detailed description of the markers of these pathological changes is given in the manuscript. Recent advances in research to identify the risk factors for stomach cancer have made it possible to use specific science to predict this deadly disease.
- Citation: Kotelevets SM, Chukov SZ. Gastric cancer diagnosis and prevention: Detecting precancerous at community level. World J Gastrointest Oncol 2025; 17(3): 100521
- URL: https://www.wjgnet.com/1948-5204/full/v17/i3/100521.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i3.100521
Stomach cancer retains its status as the most common type of cancer worldwide. In 2020, more than a million new cases of stomach cancer were detected. According to expert estimates, 1 out of 13 deaths worldwide is due to stomach cancer. Stomach cancer ranks fifth in morbidity and fourth in mortality worldwide. Stomach cancer in men is twice as common as in women, and is the most commonly diagnosed type of cancer and the leading cause of cancer death in specific countries of South and Central Asia. The incidence of stomach cancer is high in East Asia and Eastern Europe, compared with rates in North America and Northern Europe, where they are usually low and close to those in African regions[1]. Data are presented in Table 1.
| Rating number | Region | Age-standardized (W) incidence rate per 100000 | |
| Male | Female | ||
| 1 | Eastern Asia | 32.5 | 13.2 |
| 2 | Eastern Europe | 17.4 | 7.1 |
| 3 | South America | 12.1 | 6.0 |
| 4 | Western Asia | 11.4 | 6.1 |
| 5 | Southern Europe | 10.2 | 5.0 |
| 6 | Melanesia | 9.9 | 6.2 |
| 7 | Micronesia/Polynesia | 9.7 | 5.5 |
| 8 | Caribbean | 9.0 | 5.0 |
| 9 | Central America | 8.7 | 6.1 |
| 10 | Western Europe | 8.2 | 3.8 |
| 11 | South Central Asia | 7.4 | 3.7 |
| 12 | South-Eastern Asia | 7.3 | 4.0 |
| 13 | Australia/New Zealand | 6.4 | 2.8 |
| 14 | Northern Europe | 6.2 | 3.1 |
| 15 | Northern America | 5.4 | 3.1 |
| 16 | Northern Africa | 5.4 | 3.5 |
| 17 | Eastern Africa | 4.9 | 4.2 |
| 18 | Western Africa | 4.8 | 3.5 |
| 19 | Southern Africa | 4.7 | 2.4 |
| 20 | Middle Africa | 4.6 | 3.8 |
In 2000, stomach cancer was diagnosed in 880000 people worldwide and became the cause of death in 650000[2]. In the following years, the incidence and mortality from stomach cancer continued to grow steadily; the disease still occupies a leading position[3,4]. The prognosis for the next few years is negative, and a further increase in the incidence of stomach cancer is expected[5]. This state of the problem may indicate a low implementation of modern technologies for screening and diagnosis of gastric cancer (GC), which inevitably affects the effectiveness of preventive and therapeutic measures in this disease.
Targeted prevention of GC should include infection screening and screening of precancerous diseases. Screening strategies of the first line should preferably be non-invasive, inexpensive, allowing large groups of people to be examined, both from risk groups for stomach cancer and also symptomatic patients without established hereditary or acquired risk factors, for example, living in regions with a high incidence of stomach cancer. Then, in a smaller popula
To verify H. pylori infection, a respiratory urease test with carbon C13 or the determination of the H. pylori antigen in feces is recommended. The serological method for the detection of H. pylori (anti-HP IgG) is recommended when is it necessary to screen for infection in a large population. In populations at high risk of developing GC, screening for H. pylori infection is advisable, starting in childhood, by determining antibodies to H. pylori IgG in blood serum using enzyme immunoa
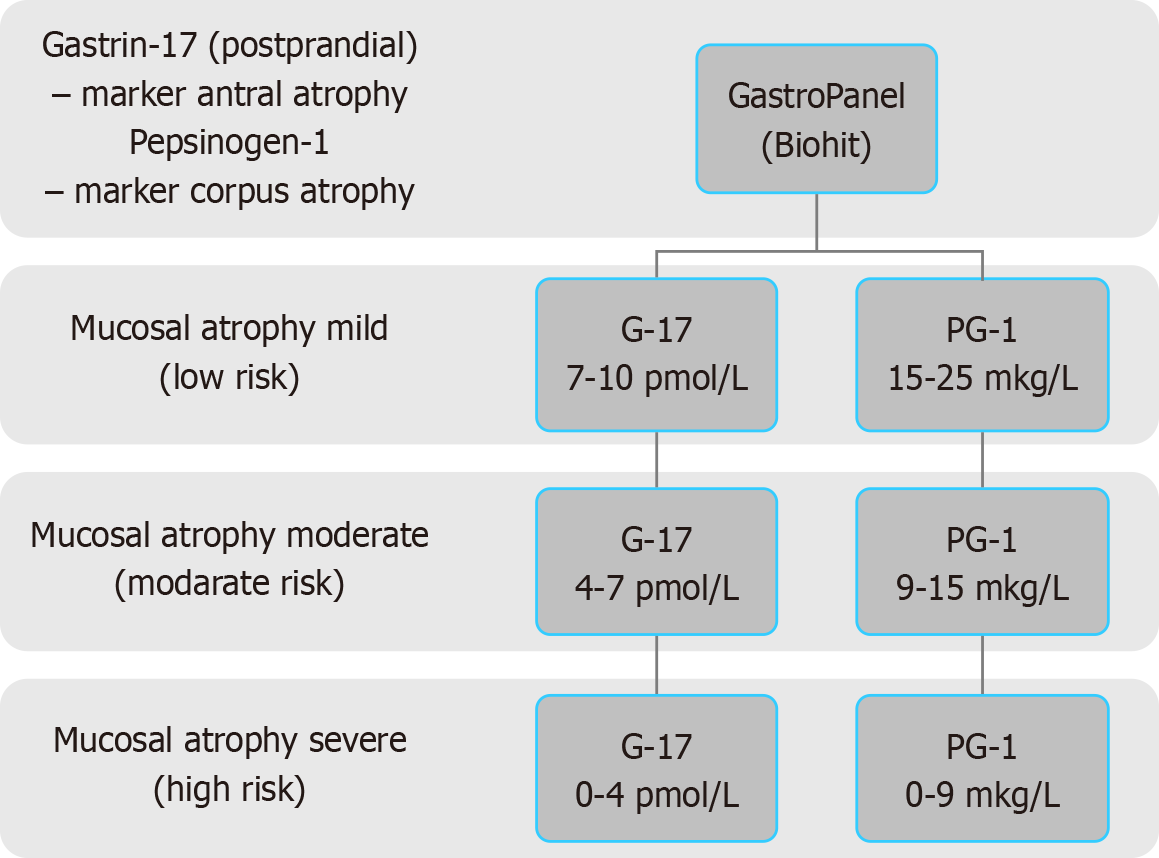
According to current concepts, serological screening for atrophic gastritis should start at the age of 40[7-9]. To date, the most well-known and evidence-based serological markers of the atrophic status of the gastric mucosa are such indicators as: Gastrin-17, pepsinogen-I, pepsinogen-II, pepsinogen-I/pepsinogen-II ratio. It’s crucial to detect only postprandial levels of gastrin-17 because fasting gastrin-17 distorts the results due to functional decrease in the secretory activity of the G cells of the gastric glands. This functional decrease in G-cell secretion does not mean the presence of atrophy of the stomach antral mucosa. Thus, the detection of atrophic gastritis by screening strategies of the first line is carried out by determination of the levels of postprandial gastrin-17, and fasting pepsinogen-I and pepsinogen-II in blood serum. In addition, it is necessary to calculate the ratio of pepsinogen-I/pepsinogen-II. The conclusion about the presence or ab
| No | Indicator | Normal | Signs of atrophic gastritis |
| 1 | Gastrin-17 (postprandial) | > 10 pmol/L | < 10 pmol/l-a sign of atrophic gastritis of the antrum |
| 2 | Pepsinogen-I | > 30 μg/L | < 30 μg/l-a sign of atrophic gastritis of the corpus |
| 3 | Anti-HP IgG | < 30 EIU | > 30-a sign of Helicobacter pylori positive gastritis |
| No | Indicator | Normal | Signs of atrophic gastritis |
| 1 | Ratio pepsinogen-I/pepsinogen-II | > 3 | < 3-an integral sign of atrophic gastritis |
| 2 | Anti-HP IgG | < 30 EIU | > 30-a sign of Helicobacter pylori positive gastritis |
According to serological criteria of varying severity of atrophy for the antrum and for the body of the stomach, the severity of atrophy of the gastric mucosa can be determined. In turn, the severity of the atrophy allows to estimate the risk of developing stomach cancer. The Kyoto Consensus has divided chronic atrophic gastritis, depending on the risk of stomach cancer, into two levels. The detection of the mild and moderate gastric atrophy corresponds to the low risk of stomach cancer, while severe gastric mucosal atrophy is an indicator of high risk of stomach cancer development[10]. The risk of stomach cancer should be determined based on Tables 4 and 5, which are presented below. The flowchart is shown in Figure 1.
| No. | The risk level of gastric cancer | The degree of stomach mucosal atrophy | Antrum (gastrin-17 pmol/L) | Corpus (pepsinogen-1 μg/L) |
| 1 | Low | No atrophy | > 10 | > 25 |
| 2 | Low | Mild | 7-10 | 15-25 |
| 3 | Low | Moderate | 4-7 | 9-15 |
| 4 | High | Severe | 0-4 | 0-9 |
| Localization | Gastric corpus-PG-1 (μg/L) | |||||
| Gastric antrum-G -17 (pmol/L) | Serology | 0 (> 25) | 1 (> 25) | 2 (15-25) | 3 (9-15) | 4 (0-9) |
| 0 (> 10) | 1 | 1 | 1 | 2 | 5 | |
| 1 (> 10) | 1 | 2 | 2 | 2 | 5 | |
| 2 (7-10) | 2 | 2 | 2 | 3 | 5 | |
| 3 (4-7) | 2 | 2 | 4 | 5 | 10 | |
| 4 (0-4) | 18 | 18 | 36 | 36 | 90 | |
Patients with serologically determined severe gastric mucosal atrophy should be referred to the screening strategies of the second line.
In summary, the advantages of serological screening for the detection of atrophic gastritis are as follows: (1) It allows for the primary non-invasive detection of gastric mucosal atrophy by assessing the levels of serological markers of atrophic gastritis in the antrum and the body of the stomach; (2) It substantially shortens the number of patients exposed to the screening strategies of the second line (endoscopic and histological stages of diagnosis), as only patients with serologically detected severe atrophy with a high risk of developing stomach cancer are referred; and (3) Noninvasive screening of severe gastric mucosal atrophy reduces the number of patients exposed to screening strategies of the second line, which significantly reduces the financial costs for performing the high-tech diagnostic methods.
Morphological markers of varying degrees of atrophy of the stomach mucosa of the antrum and the body were paired with serological markers. As a result, it allowed to determine the risk level of GC based on the serological criteria of mild, moderate and severe atrophy[11]. Rugge et al[12] proposed a system Operative Link on Gastritis Assessment (OLGA) for the stratification the risk of GC development in atrophic gastritis. We have undertaken testing of this system based on the combination of serological and histological examination data. Our results show the acceptability of using serological screening to monitor patients at high risk of developing cancer; that allowed us to propose the serological criteria for determining the stages of atrophic gastritis according to the classification OLGA. The OLGIM system-determines the stage, form and extent of intestinal metaplasia. Both systems together assess the risk of developing GC. Intestinal metaplasia should be diagnosed at the second stage using endoscopic and histological methods[12]. Kotelevets SM, Chekh SA proposed to evaluate histological and serological markers of gastric mucosa atrophy according to the OLGA classification[13]. The scheme of OLGA system and the results of our study are presented in Tables 6 and 7.
| Atrophy antrum | Corpus | |||
| No atrophy | Mild atrophy | Moderate atrophy | Severe atrophy | |
| No atrophy | Stage 0 | Stage I | Stage II | Stage III |
| Mild atrophy | Stage I | Stage I | Stage II | Stage III |
| Moderate atrophy | Stage II | Stage II | Stage III | Stage IV |
| Severe atrophy | Stage III | Stage III | Stage IV | Stage IV |
| Localization | Gastric corpus-PG-1 (μg/L) | ||||
| Gastric antrum –G-17 (pmol/L) | Serology | > 25 | 15-25 | 9-15 | 0-9 |
| > 10 | Stage 0 | Stage I | Stage II | Stage III | |
| 7-10 | Stage I | Stage I | Stage II | Stage III | |
| 4-7 | Stage II | Stage II | Stage III | Stage IV | |
| 0-4 | Stage III | Stage III | Stage IV | Stage IV | |
Thus, patients with severe atrophic gastritis detected by serological screening have a high risk of stomach cancer. These patients should be referred to the screening strategies of the second line-invasive, including endoscopy with biopsy and subsequent histology.
Patients with severe atrophy detected in at least one of the screening strategies of the second line: Diagnostic endo
At the stage of invasive screening, the patient undergoes an endoscopic examination followed by a biopsy for histology. Endoscopic technologies currently make it possible to visualize changes in the mucosa in detail, to carry out their qualitative and morphometric assessment, and to identify the areas suspicious for precancerous changes with a high degree of accuracy[14,15]. Despite the endoscopic diagnosis of precancerous changes has achieved significant success, the diagnostic potentials of histological recognition of precancerous changes in the gastric mucosa have not lost their sig
| The degree of atrophy in the biopsy | The percentage of loss of gastric glands |
| Mild atrophy | Less than 30% of the glands are lost |
| Moderate atrophy | Loss of glands is 30%-60% |
| Severe atrophy | Loss of more than 60% of the glands |
According to our data, the most accurate and effective histological assessment of the severity of atrophic gastritis is revealed in biopsies performed in accordance with the updated classification of Kimura-Takemoto atrophic gastritis. Сomparative study of the method of obtaining biopsies and their histological assessment showed the advantage of detecting atrophic gastritis of varying severity in accordance with the updated classification of atrophic gastritis of Kimura-Takemoto compared with the updated Sydney system[18]. six biopsies obtained according to the updated Kimura-Takemoto classification of atrophic gastritis should be subjected to endoscopic monitoring of the stomach at least once a year. In summary, the strategies for the diagnosis of the precancerous conditions of the stomach should be organized in the manner that provides the possibility of assessment of large groups of population, especially in known areas with high risk of GC incidence. At the first stage, these strategies should be non-invasive and inexpensive, but still capable of providing reliable, highly informative results. Based on the results of large-scale screening, a group of patients with a high risk of developing stomach cancer should be identified based on the diagnosis of atrophic gastritis with severe atrophy. In this group of patients, an invasive diagnosis of atrophic gastritis is used with an assessment of the risk of developing stomach cancer.
Markers of atrophic gastritis are used for its detection in population screening. This is a frequent use of serological markers. The relevance of using the GastroPanel® test panel continues today[19]. The use of such a marker as H. pylori for the detection of gastric precancer will also be effective[20]. A pan-specific marker is S100a8 for CD11b+Ly6G+ cells that occur in chronic inflammation of the stomach, which worsens the metaplastic pathology of the mucosa[21]. The role of other genetic markers of precancerous changes in the gastric mucosa is very important. It has been shown that a single nucleotide polymorphism (SNP) in the autophagy gene ATG16 L1 (rs2241880, G-allele) causes the progression of precancerous changes in the gastric mucosa[22]. Confirmation of the carcinogenic effect of deoxycholic acid (DCA), which is the main component of bile acid of duodenogastric reflux, was obtained in experimental models. Therefore, DCA and duodenogastric reflux can be considered as a marker of gastric carcinogenesis[23]. MicroRNA gastrokine 3 is a specific marker of gastric corpus metaplasia in mice and may be considered as a promising marker of carcinogenesis and gastric precancer in humans[24]. Specific markers for the gastric corpus and antrum exist in addition to the traditional serological markers gastrin-17 and pepsinogens. Ninety-one protein is reduced in the tissues of the gastric corpus in atrophic gastritis and interleukin-6, human epididymal protein 4 are reduced in antral atrophic gastritis[25,26]. Positive antibodies to parietal cells, as well as antibodies associated with systemic lupus erythematosus, autoimmune thyroiditis, antinuclear antibodies can serve as markers of precancerous lesions in the stomach[27]. More and more studies are devoted to the association between long non-coding RNA and precancerous lesions of the gastric mucosa. The expression level of LINC00659 gradually increases with the progression of atrophic gastritis, intestinal metaplasia and dysplasia. LINC00659 may have the potential to become a new marker for precancerous changes and diseases of the stomach[28]. GC may be associated with Epstein-Barr virus (EBVaGCs) and therefore EBVaGCs may be a biomarker for gastric precancer[29]. The process of gastric carcinogenesis is closely related to the transformation and differentiation of 4 subgroups of fibroblasts in stromal cells. The proportions of fibroblasts differ at different stages of gastric carcinogenesis. The proportions of each subgroup of fibroblasts have a characteristic level of expression of the corresponding markers: PDGFRA, FBLN2, ACTA2 and PDGFRB. Based on such markers, it is possible to make a prognostic model of gastric carcinogenesis from atrophy of the gastric mucosa and intestinal metaplasia to dysplasia[30]. Some substances are markers of anti-carcinogenesis and reflect the regression of precancerous changes. For example, quercetin inhibits cell proliferation and promotes apoptosis. Such markers can be used to effectively monitor the treatment of precancerous changes[31]. In addition to markers of precancerous changes in the gastric mucosa and precancerous disease (atrophic gastritis), markers of GC are being intensively studied. It has been established that the expression of DSCC1 and GINS1 increases in GC and genes associated with SPAG5 and ASPM, are overexpressed in GC tissues[32,33]. 5-hydroxytryptamine and the LGR5 are markers of precancerous mucosal changes and GC simultaneously[34].
The main risk factors for GC have long been identified. These include age, H. pylori infection, male gender, smoking, farmer's profession, low annual family income, illiteracy, alcohol consumption, hot food consumption, fast food intake, history of gastritis, peptic ulcer disease and gallbladder disease[35]. In developed countries, GC is largely associated with H. pylori[36]. Smoking and alcohol consumption are considered proven risk factors for the occurrence and development of GC[37,38]. Nicotine is the main risk factor in tobacco smoking. It stimulates mutagenesis, as well as tumor angiogenesis and metastasis[39]. People with low levels of education and victims of socioeconomic inequality were more likely to develop neoplastic lesions[40]. Additional risk factors for GC are recent (Table 9).
| Risk factors for gastric cancer | |
| Main risk factors | Additional risk factors |
| Age | Absence healthy lifestyle |
| Helicobacter pylori infection | Underweight or overweight significantly |
| Male gender | Periodontosis |
| Smoking | |
| Farmer's profession | |
| Low annual family income | |
| Illiteracy | |
| Alcohol consumption | |
| Hot food consumption | |
| Fast food intake salt consumption | |
| History of gastritis | |
| Peptic ulcer disease | |
| Gallbladder disease, bile reflux | |
| Genetic factors | |
These include lack of healthy lifestyle associated with geographic differentiation. For example, the Asia-Pacific region[41]. An important additional risk factor for GC is body mass index. Being underweight or overweight significantly increases the risk of GC[42]. Periodontosis is also a very important risk factor for GC. Mechanisms of gastric carcinogenesis in periodontal diseases have been identified. Lipopolysaccharide Porphyromonas gingivalis has a carcinogenic effect on the gastric mucosa[43]. Genetic studies aimed at identifying SNPs associated with the risk of GC have good prospects. Based on them, it is possible to make polygenic risk score[44]. A promising area of research is the identification of prognostic genes on the basis of which it is possible to construct signatures for predicting the long-term risk of GC[45].
Unfortunately, patients with advanced GC continue to seek medical care only after the onset of clinical symptoms (92.1% in Tanzania)[46]. In recent years, experience has been accumulated in the modern promising methods for the diagnosis of precancerous diseases and changes in the gastric mucosa, as well as the possibilities of their treatment. It is very impor
Practical hints for population-based serological screening of atrophic gastritis, endoscopic and morphological verification of precancerous changes and diseases of the stomach, as well as their treatment are recommended for use: When developing state programs for the prevention of stomach cancer; when implementing preventive measures for stomach cancer by doctors of all specialties; the authors also offer the possibility of use by anyone over the age of 40, provided that they seek methodological help from their doctor; in the work of health schools in any medical and preventive institutions. To increase the effectiveness of the implementation of practical recommendations for the prevention of GC, it is necessary to comprehensively use modern markers of atrophic gastritis and precancerous changes in the gastric mucosa. The use of an assessment system of certain risk factor signatures with prognostic value would add significant assistance to preventive measures against GC.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68587] [Article Influence: 13717.4] [Reference Citation Analysis (201)] |
| 2. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1193] [Cited by in RCA: 1262] [Article Influence: 63.1] [Reference Citation Analysis (20)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56666] [Article Influence: 7083.3] [Reference Citation Analysis (135)] |
| 4. | Kang B, Liu XY, Cheng YX, Tao W, Peng D. Factors associated with hypertension remission after gastrectomy for gastric cancer patients. World J Gastrointest Surg. 2022;14:743-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 5. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3303] [Article Influence: 550.5] [Reference Citation Analysis (6)] |
| 6. | Kotelevets SM, Chekh SA, Chukov SZ. Cancer risk stratification system and classification of gastritis: Perspectives. World J Meta-Anal. 2023;11:18-28. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Benberin V, Bektayeva R, Karabayeva R, Lebedev A, Akemeyeva K, Paloheimo L, Syrjänen K. Prevalence of H. pylori infection and atrophic gastritis among symptomatic and dyspeptic adults in Kazakhstan. A hospital-based screening study using a panel of serum biomarkers. Anticancer Res. 2013;33:4595-4602. [PubMed] |
| 8. | Sipponen P, Kekki M, Haapakoski J, Ihamäki T, Siurala M. Gastric cancer risk in chronic atrophic gastritis: statistical calculations of cross-sectional data. Int J Cancer. 1985;35:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 237] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 9. | Iijima K, Abe Y, Kikuchi R, Koike T, Ohara S, Sipponen P, Shimosegawa T. Serum biomarker tests are useful in delineating between patients with gastric atrophy and normal, healthy stomach. World J Gastroenterol. 2009;15:853-859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, Haruma K, Asaka M, Uemura N, Malfertheiner P; faculty members of Kyoto Global Consensus Conference. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1475] [Cited by in RCA: 1263] [Article Influence: 114.8] [Reference Citation Analysis (0)] |
| 11. | Mikhailovich S. Serological Criteria for Mild, Moderate and Severe Atrophy in Atrophic Gastritis. Biol Med (Aligarh). 2015;7. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 12. | Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, Graham DY. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 362] [Article Influence: 19.1] [Reference Citation Analysis (2)] |
| 13. | Kotelevets SM, Chekh SA. Screening, Monitoring, and Treatment of Precancerous Atrophic Gastritis in the Prospective Study for Seven Years. Asian Pac J Cancer Prev. 2020;21:331-336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Bayguinov PO, Oakley DM, Shih CC, Geanon DJ, Joens MS, Fitzpatrick JAJ. Modern Laser Scanning Confocal Microscopy. Curr Protoc Cytom. 2018;85:e39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Pimentel-Nunes P, Libânio D, Marcos-Pinto R, Areia M, Leja M, Esposito G, Garrido M, Kikuste I, Megraud F, Matysiak-Budnik T, Annibale B, Dumonceau JM, Barros R, Fléjou JF, Carneiro F, van Hooft JE, Kuipers EJ, Dinis-Ribeiro M. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy. 2019;51:365-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 712] [Cited by in RCA: 716] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 16. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3221] [Cited by in RCA: 3622] [Article Influence: 120.7] [Reference Citation Analysis (6)] |
| 17. | Correa P. Clinical implications of recent developments in gastric cancer pathology and epidemiology. Semin Oncol. 1985;12:2-10. [PubMed] |
| 18. | Kotelevets SM, Chekh SA, Chukov SZ. Updated Kimura-Takemoto classification of atrophic gastritis. World J Clin Cases. 2021;9:3014-3023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (1)] |
| 19. | Chapelle N, Petryszyn P, Blin J, Leroy M, Le Berre-Scoul C, Jirka I, Neunlist M, Moussata D, Lamarque D, Olivier R, Tougeron D, Mosnier JF, Matysiak-Budnik T. A panel of stomach-specific biomarkers (GastroPanel®) for the diagnosis of atrophic gastritis: A prospective, multicenter study in a low gastric cancer incidence area. Helicobacter. 2020;25:e12727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Aziz S, Rasheed F, Zahra R, König S. Gastric Cancer Pre-Stage Detection and Early Diagnosis of Gastritis Using Serum Protein Signatures. Molecules. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Kao KD, Grasberger H, El-Zaatari M. The Cxcr2(+) subset of the S100a8(+) gastric granylocytic myeloid-derived suppressor cell population (G-MDSC) regulates gastric pathology. Front Immunol. 2023;14:1147695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Mommersteeg MC, Simovic I, Yu B, van Nieuwenburg SAV, Bruno IMJ, Doukas M, Kuipers EJ, Spaander MCW, Peppelenbosch MP, Castaño-Rodríguez N, Fuhler GM. Autophagy mediates ER stress and inflammation in Helicobacter pylori-related gastric cancer. Gut Microbes. 2022;14:2015238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 23. | Jin D, Huang K, Xu M, Hua H, Ye F, Yan J, Zhang G, Wang Y. Deoxycholic acid induces gastric intestinal metaplasia by activating STAT3 signaling and disturbing gastric bile acids metabolism and microbiota. Gut Microbes. 2022;14:2120744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 74] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 24. | Bockerstett KA, Lewis SA, Noto CN, Ford EL, Saenz JB, Jackson NM, Ahn TH, Mills JC, DiPaolo RJ. Single-Cell Transcriptional Analyses Identify Lineage-Specific Epithelial Responses to Inflammation and Metaplastic Development in the Gastric Corpus. Gastroenterology. 2020;159:2116-2129.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 25. | Chapelle N, Osmola M, Martin J, Blin J, Leroy M, Jirka I, Moussata D, Lamarque D, Olivier R, Tougeron D, Hay-Lombardie A, Bigot-Corbel E, Masson D, Mosnier JF, Matysiak-Budnik T. Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study. Diagnostics (Basel). 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Shuman JHB, Lin AS, Westland MD, Bryant KN, Piazuelo MB, Reyzer ML, Judd AM, McDonald WH, McClain MS, Schey KL, Algood HMS, Cover TL. Remodeling of the gastric environment in Helicobacter pylori-induced atrophic gastritis. mSystems. 2024;9:e0109823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 27. | Zádori N, Németh D, Szakó L, Váncsa S, Vörhendi N, Szakács Z, Frim L, Hegyi P, Czimmer J. Prevalence of Autoimmune-phenomena behind Chronic Gastritis of Unknown Origin, and their Role in the Poor Histological Outcome of the Stomach: A Single-centre, Retrospective Cross-sectional Study. J Gastrointestin Liver Dis. 2022;31:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Zhang J, Zhang Z, Shen D. Upregulated LncRNA-LINC00659 expression by H. pylori infection promoted the progression of gastritis to cancer by regulating PTBP1 expression. Indian J Pathol Microbiol. 2024;67:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Sun Q, Fu Y, Chen X, Li L, Wu H, Liu Y, Xu H, Zhou G, Fan X, Xia H. Prognostic Perspectives of STING and PD-L1 Expression and Correlation with the Prognosis of Epstein-Barr Virus-Associated Gastric Cancers. Gut Liver. 2022;16:875-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 30. | Lee SH, Contreras Panta EW, Gibbs D, Won Y, Min J, Zhang C, Roland JT, Hong SH, Sohn Y, Krystofiak E, Jang B, Ferri L, Sangwan V, Ragoussis J, Camilleri-Broët S, Caruso J, Chen-Tanyolac C, Strasser M, Gascard P, Tlsty TD, Huang S, Choi E, Goldenring JR. Apposition of Fibroblasts With Metaplastic Gastric Cells Promotes Dysplastic Transition. Gastroenterology. 2023;165:374-390. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 40] [Reference Citation Analysis (0)] |
| 31. | Nie K, Zheng Z, Li X, Chang Y, Liu F, Wang X. Explore the active ingredients and potential mechanisms of JianPi QingRe HuaYu Methods in the treatment of gastric inflammation-cancer transformation by network pharmacology and experimental validation. BMC Complement Med Ther. 2023;23:411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 32. | Zhu Y, Hou S, Kang C. Complementary biomarkers of computed tomography for diagnostic grading of gastric cancer: DSCC1 and GINS1. Aging (Albany NY). 2024;16:4149-4168. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Xue M, Ma C, Shan H, Hou S, Kang C. SPAG5 and ASPM play important roles in gastric cancer: An observational study. Medicine (Baltimore). 2024;103:e38499. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 34. | Niu Q, Li L, Zhang C, Qi C, He Q, Zhu Y. Expression of 5-HT Relates to Stem Cell Marker LGR5 in Patients with Gastritis and Gastric Cancer. Dig Dis Sci. 2023;68:1864-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 35. | Chen Z, Zheng Y, Fan P, Li M, Liu W, Yuan H, Liu X, Zhang Z, Wu Z, Wang Y, Ji R, Guo Q, Ye Y, Zhang J, Li X, An F, Lu L, Li Y, Wang X, Zhang J, Guan Q, Li Q, Liu M, Ren Q, Hu X, Lu H, Zhang H, Zhao Y, Gou X, Shu X, Wang J, Hu Z, Xue S, Liu J, Zhou Y. Risk factors in the development of gastric adenocarcinoma in the general population: A cross-sectional study of the Wuwei Cohort. Front Microbiol. 2022;13:1024155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 36. | Shirani M, Pakzad R, Haddadi MH, Akrami S, Asadi A, Kazemian H, Moradi M, Kaviar VH, Zomorodi AR, Khoshnood S, Shafieian M, Tavasolian R, Heidary M, Saki M. The global prevalence of gastric cancer in Helicobacter pylori-infected individuals: a systematic review and meta-analysis. BMC Infect Dis. 2023;23:543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 37. | Rota M, Possenti I, Valsassina V, Santucci C, Bagnardi V, Corrao G, Bosetti C, Specchia C, Gallus S, Lugo A. Dose-response association between cigarette smoking and gastric cancer risk: a systematic review and meta-analysis. Gastric Cancer. 2024;27:197-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 43] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 38. | Dong J, Thrift AP. Alcohol, smoking and risk of oesophago-gastric cancer. Best Pract Res Clin Gastroenterol. 2017;31:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 83] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 39. | Lian S, Li S, Zhu J, Xia Y, Do Jung Y. Nicotine stimulates IL-8 expression via ROS/NF-κB and ROS/MAPK/AP-1 axis in human gastric cancer cells. Toxicology. 2022;466:153062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Sun D, Lei L, Xia C, Li H, Cao M, He S, Zhang Z, Guo G, Song G, Peng J, Chen W. Sociodemographic disparities in gastric cancer and the gastric precancerous cascade: A population-based study. Lancet Reg Health West Pac. 2022;23:100437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Akbari A, Ashtari S, Tabaiean SP, Mehrdad-Majd H, Farsi F, Shojaee S, Agah S. Overview of epidemiological characteristics, clinical features, and risk factors of gastric cancer in Asia-Pacific region. Asia Pac J Clin Oncol. 2022;18:493-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Azizi N, Zangiabadian M, Seifi G, Davari A, Yekekhani E, Safavi-Naini SAA, Berger NA, Nasiri MJ, Sohrabi MR. Gastric Cancer Risk in Association with Underweight, Overweight, and Obesity: A Systematic Review and Meta-Analysis. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 43. | Oriuchi M, Lee S, Uno K, Sudo K, Kusano K, Asano N, Hamada S, Hatta W, Koike T, Imatani A, Masamune A. Porphyromonas gingivalis Lipopolysaccharide Damages Mucosal Barrier to Promote Gastritis-Associated Carcinogenesis. Dig Dis Sci. 2024;69:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 44. | Liu YQ, Wang TP, Yan CW, Zhu M, Yang M, Wang MY, Hu ZB, Shen HB, Jin GF. [Association between polygenic risk score and age at onset of gastric cancer]. Zhonghua Liu Xing Bing Xue Za Zhi. 2021;42:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 45. | Ji D, Yang Y, Zhou F, Li C. A nine-consensus-prognostic -gene-based prognostic signature, recognizing the dichotomized subgroups of gastric cancer patients with different clinical outcomes and therapeutic strategies. Front Genet. 2022;13:909175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 46. | Mabula JB, McHembe MD, Koy M, Chalya PL, Massaga F, Rambau PF, Masalu N, Jaka H. Gastric cancer at a university teaching hospital in northwestern Tanzania: a retrospective review of 232 cases. World J Surg Oncol. 2012;10:257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Chapelle N, Péron M, Mosnier JF, Quénéhervé L, Coron E, Bourget A, Cauchin E, Touchefeu Y, Matysiak-Budnik T. Prevalence, Characteristics and Endoscopic Management of Gastric Premalignant Lesions in France. Dig Dis. 2020;38:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 48. | Dilaghi E, Lahner E, Annibale B, Esposito G. Systematic review and meta-analysis: Artificial intelligence for the diagnosis of gastric precancerous lesions and Helicobacter pylori infection. Dig Liver Dis. 2022;54:1630-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 49. | Guimarães P, Keller A, Fehlmann T, Lammert F, Casper M. Deep-learning based detection of gastric precancerous conditions. Gut. 2020;69:4-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 89] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 50. | Chapelle N, Péron M, Quénéhervé L, Bourget A, Leroy M, Touchefeu Y, Cauchin E, Coron E, Mosnier JF, Matysiak-Budnik T. Long-Term Follow-up of Gastric Precancerous Lesions in a Low GC Incidence Area. Clin Transl Gastroenterol. 2020;11:e00237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 51. | Zhu F, Zhang X, Li P, Zhu Y. Effect of Helicobacter pylori eradication on gastric precancerous lesions: A systematic review and meta-analysis. Helicobacter. 2023;28:e13013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 52. | Cao Y, Wang D, Mo G, Peng Y, Li Z. Gastric precancerous lesions:occurrence, development factors, and treatment. Front Oncol. 2023;13:1226652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 53. | Zhang S, Shen Y, Liu H, Zhu D, Fang J, Pan H, Liu W. Inflammatory microenvironment in gastric premalignant lesions: implication and application. Front Immunol. 2023;14:1297101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/