Published online Feb 15, 2025. doi: 10.4251/wjgo.v17.i2.98803
Revised: October 17, 2024
Accepted: October 25, 2024
Published online: February 15, 2025
Processing time: 196 Days and 5.8 Hours
Gastric cancer (GC) poses a significant threat to public health. However, the clinicopathological features and tumor biological behaviors vary among the GC patients, leading to individual variations in lymph node metastasis. Consequ
To investigate the distribution of metastatic lymph nodes in patients with upper and lower GC and to analyze the differences in related pathological elements and prognosis.
Differential analysis between upper and lower GC patients with various cli
Significant differences were observed between the two GC populations regarding tumor diameter, histological grade, pT stage, pN stage, tumor-node-metastasis (pTNM) stage, vascular invasion, and adjuvant chemotherapy usage (all P < 0.05). Lymph node metastasis rates were highest for Siewert type II patients in groups Nos. 1, 3, 2 and 7; for Siewert type III patients in groups Nos. 3, 1, 2 and 7; and for other/unclassified patients in groups Nos. 1, 3, 7, 2. In the lower GC samples, the sequences were Nos. 3, 6, 7, 4. Pathological type, pT stage, pTNM stage, and positive vascular invasion were independent risk factors for development of lymph node metastasis. Age, pa
Upper GC showed a significantly higher malignancy grade and different lymph node metastasis pattern than lower GC.
Core Tip: Upper gastric cancer (GC) has a significantly greater degree of malignancy than lower GC. In upper GC, the rate of lymph node metastasis was greater in groups Nos. 1, 2, 3 and 7 among the different subtypes. In lower GC, the rate of lymph node metastasis was greater in groups Nos. 3–8. Pathological type, histological grade, pT stage, tumor-node-metastasis (pTNM) stage, and vascular invasion independently influenced the occurrence of lymph node metastasis. Age, pathological type, pT stage, pN stage, pTNM stage, vascular invasion, and absence of adjuvant chemotherapy independently influenced prognosis.
- Citation: Yuan XB, Sun G, Niu J, Dong L, Sui Y, Lv YZ. Metastatic lymph node distribution and pathology correlations in upper and lower gastric cancer patients: A multicenter retrospective study. World J Gastrointest Oncol 2025; 17(2): 98803
- URL: https://www.wjgnet.com/1948-5204/full/v17/i2/98803.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i2.98803
Gastric cancer (GC) is a multifactorial disease in which both environmental and genetic factors affect its development. This complexity often results in poor prognosis, as patients are frequently diagnosed at middle to late stages[1]. Despite being the fifth most common cancer globally (approximately 5.6%) and the fourth leading cause of cancer-related deaths (approximately 7.7%), epidemiological evidence indicates a global decline in annual GC cases. This decline is attributed to the proliferation of preventive screening and enhanced hygienic dietary conditions. However, the incidence of upper GCs is increasing annually[2]. Upper and lower GCs are delineated by a line that divides the gastric curvature into three equal parts, joining the upper and lower thirds of the stomach and greater curvature. Given the differences in clinicopathological characteristics and tumor biology across GC sites, there is a trend toward individualized lymph node metastasis patterns that may influence prognosis. Consequently, the study of metastatic lymph node status in upper and lower GCs has emerged as a research focus in recent years[3-5]. For these reasons, this study aimed to delineate the distribution of metastatic lymph nodes in upper and lower GCs by examining differences in related pathological elements and their prognostic implications.
A total of 1806 patients who underwent radical GC surgery at the First Affiliated Hospital of Dalian Medical University, No. 967 The Hospital of the People's Liberation Army Joint Logistics Support Force, the Fifth People's Hospital of Shenyang and the Fifth People's Hospital of Dalian, comprising 817 patients with upper GC and 989 patients with lower GC, were selected from January 2005 to December 2020. The enrolled patients had no prior gastric surgery, harbored no additional tumors beyond the primary cancer, had not received neoadjuvant therapy, showed no distant organ me
Site delineation for upper GC followed the universally recognized Siewert typing system[6]. For GC, site delineation adhered to the 6th edition of the Guidelines for the Treatment of GC by the Japanese Gastric Cancer Association (JGCA)[7]. Pathological typing was guided by the 2019 edition of the World Health Organization Classification of Tumors of the Digestive System[8]. Pathological staging conformed to the 8th edition of the tumor-node-metastasis (TNM) classification by the American Joint Committee on Cancer and the Union for International Cancer Control[9].
Data on sex, age, tumor diameter, pathological type, histological grade, pT stage, pN stage, pTNM stage, surgical method, adjuvant chemotherapy, vascular invasion, and lymph node metastasis were extracted from the hospital medical records. Follow-up commenced upon GC diagnosis via pathological examination, occurring every 3–6 mo for the first 2 years postoperatively and subsequently every 6–12 mo thereafter. The primary endpoint observed was patient survival or death, delineated as overall survival.
The statistical methods of this study were reviewed by Dr. Sui from The No. 967 Hospital of PLA Joint Logistics Support Force. SPSS version 24.0 was used for statistical analysis. Differential analysis between upper and lower GC patients across various clinicopathological factors was performed utilizing the χ² test and rank-sum test. Logistic regression models were used to identify risk factors influencing GC lymph node metastasis, while Cox regression models were used to analyze risk factors affecting patient prognosis. The Kaplan-Meier method was used to construct survival curves associated with prognostic risk factors for GC. To mitigate the influence of confounding variables and retain potentially significant predictors, variables with P < 0.1 in the univariate analysis were included in the multivariate analysis. Sta
Comparative analysis between upper and lower GCs revealed significant differences in tumor diameter, histological grade, pT stage, pN stage, pTNM stage, vascular invasion, and adjuvant chemotherapy usage (all P < 0.05). Among patients with a tumor diameter > 4 cm, low or undifferentiated histology, T4 stage, N3 stage, stage III, and vascular invasion, upper GC patients exhibited significantly greater proportions than lower GC patients, indicating a greater de
| Variables | Upper GC | Lower GC | χ² | P value |
| Gender | 2.582 | 0.108 | ||
| Male | 554 (67.8) | 635 (64.2) | ||
| Female | 263 (32.2) | 354 (35.8) | ||
| Age (year) | 0.485 | 0.486 | ||
| < 60 | 277 (33.9) | 320 (32.4) | ||
| ≥ 60 | 540 (66.1) | 669 (67.6) | ||
| Tumor diameter (cm) | 61.410 | < 0.001a | ||
| < 4 | 453 (55.4) | 723 (73.1) | ||
| ≥ 4 | 364 (44.6) | 266 (26.9) | ||
| Pathological type | 1.941 | 0.379 | ||
| Adenocarcinoma | 654 (80.0) | 813 (82.2) | ||
| Signet-ring cell carcinoma | 139 (17.0) | 155 (15.7) | ||
| Other | 24 (2.9) | 21 (2.1) | ||
| Histological grade | 0.018a | |||
| G1 | 42 (5.1) | 82 (8.3) | ||
| G2 | 234 (28.6) | 295 (29.8) | ||
| G3 | 541 (66.2) | 612 (61.9) | ||
| pT stage | < 0.001a | |||
| T1 | 163 (20.0) | 269 (27.3) | ||
| T2 | 127 (15.5) | 131 (13.2) | ||
| T3 | 267 (32.7) | 473 (47.8) | ||
| T4 | 260 (31.8) | 116 (11.7) | ||
| pN stage | < 0.001a | |||
| N0 | 340 (41.6) | 429 (43.4) | ||
| N1 | 124 (15.2) | 303 (30.6) | ||
| N2 | 135 (16.5) | 152 (15.4) | ||
| N3 | 218 (26.7) | 105 (10.6) | ||
| pTNM stage | < 0.001a | |||
| I | 354 (43.3) | 455 (46.0) | ||
| II | 50 (6.1) | 115 (11.6) | ||
| III | 413 (50.6) | 419 (42.4) | ||
| Vascular invasion | 4.642 | 0.031a | ||
| Positive | 527 (64.5) | 589 (59.6) | ||
| Negative | 290 (35.5) | 400 (40.4) | ||
| Surgery type | 3.370 | 0.066 | ||
| PG | 656 (80.3) | 827 (83.6) | ||
| TG | 161 (19.7) | 162 (16.4) | ||
| Chemotherapy | 58.041 | < 0.001a | ||
| Yes | 448 (19.7) | 713 (72.1) | ||
| No | 369 (45.2) | 276 (27.9) | ||
For patients with Siewert type II upper GC, the percentages of patients with lymph node metastasis in the top four groups, in descending order, were 42.47% in Group No. 1, 37.44% in Group No. 3, 29.68% in Group No. 2 and 27.85% in Group No. 7. Among the Siewert type III upper GC patients, the four groups with the most lymph node metastasis were Group No. 3 (41.11%), Group No. 1 (38.73%), Group No. 2 (30.43%) and Group No. 7 (28.06%). For upper GC patients categorized as other/unclassified, the leading four groups in terms of lymph node metastasis rates were Group No. 1 (42.03%), Group No. 3 (39.13%), Group No. 7 (29.28%) and Group No. 2 (28.41%). In lower GC, the six groups leading to lymph node metastasis rates were Group No. 3 (41.46%), Group No. 6 (39.13%), Group No. 7 (25.58%), Group No. 4 (23.15%), Group No. 5 (21.03%) and Group No. 8 (19.72%). The data are detailed in Table 2.
| Groups | Upper gastric cancer (n = 817) | Lower gastric cancer (n = 989) | ||
| Siewert type II (n = 219) | Siewert type III (n = 253) | Other/unclassified (n = 345) | ||
| Cases/ratio | Cases/ratio | Cases/ratio | Cases/ratio | |
| 1 | 93 (42.47) | 98 (38.73) | 145 (42.03) | 52 (5.26) |
| 2 | 65 (29.68) | 77 (30.43) | 98 (28.41) | 25 (2.53) |
| 3 | 82 (37.44) | 104 (41.11) | 135 (39.13) | 410 (41.46) |
| 4 | 32 (14.61) | 35 (13.83) | 53 (15.36) | 229 (23.15) |
| 5 | 28 (12.78) | 32 (12.65) | 41 (11.88) | 208 (21.03) |
| 6 | 16 (7.31) | 13 (5.14) | 18 (5.22) | 387 (39.13) |
| 7 | 61 (27.85) | 71 (28.06) | 101 (29.28) | 253 (25.58) |
| 8 | 23 (10.50) | 32 (12.65) | 35 (10.14) | 195 (19.72) |
| 9 | 20 (9.13) | 19 (7.51) | 39 (11.30) | 72 (7.28) |
| 10 | 11 (5.02) | 15 (5.93) | 19 (5.51) | 12 (1.21) |
| 11 | 25 (11.42) | 31 (12.25) | 37 (10.72) | 22 (2.22) |
| 12 | 2 (0.91) | 4 (1.58) | 3 (0.87) | 32 (3.24) |
| 13 | 0 | 1 (0.40) | 1 (0.29) | 9 (0.91) |
| 14 | 0 | 0 | 1 (0.29) | 7 (0.71) |
| 15 | 0 | 0 | 0 | 8 (0.81) |
| 16 | 0 | 0 | 0 | 2 (0.20) |
| 17 | 0 | 0 | 0 | 0 |
| 18 | 0 | 0 | 0 | 0 |
| 19 | 0 | 0 | 0 | 0 |
| 20 | 0 | 0 | 0 | 0 |
| 110 | 16 (7.31) | 0 | 8 (2.32) | 0 |
| 111 | 12 (5.48) | 0 | 9 (2.61) | 0 |
| 112 | 0 | 0 | 0 | 0 |
Univariate logistic regression analysis identified the pathological type of signet ring cell carcinoma; moderately differentiated, poorly differentiated, or undifferentiated tumor status; pT stage, T2 to T4; pTNM stage, II or III; and positive vascular invasion as risk factors for lymph node metastasis. Multivariate logistic regression analysis revealed that the pathological type of signet-ring cell carcinoma; moderately differentiated, poorly differentiated, or undifferentiated tumor status; pT stage, T2 to T4; pTNM stage, II or III; and vascular invasion status remained independent risk factors for lymph node metastasis. The data are shown in Table 3.
| Variables | Univariate analysis | Multivariable analysis | |||||||
| OR | 95%CI | P value | OR | 95%CI | P value | ||||
| Gender | |||||||||
| Male | 1.000 | ||||||||
| Female | 1.096 | 0.534–2.250 | 0.102 | ||||||
| Age (year) | |||||||||
| < 60 | 1.000 | ||||||||
| ≥ 60 | 0.432 | 0.121-1.542 | 0.196 | ||||||
| Tumor diameter (cm) | |||||||||
| < 4 | 1.000 | ||||||||
| ≥ 4 | 1.316 | 0.544-3.121 | 0.531 | ||||||
| Tumor location | |||||||||
| Lower | 1.000 | ||||||||
| Upper | 1.075 | 0.891-1.296 | 0.451 | ||||||
| Pathological type | |||||||||
| Other | 1.000 | 1.000 | |||||||
| Adenocarcinoma | 1.754 | 0.962-3.197 | 0.067 | 3.193 | 0.983-10.375 | 0.053 | |||
| Signet-ring cell carcinoma | 2.655 | 1.401-5.029 | 0.003a | 4.846 | 1.431-16.419 | 0.011a | |||
| Histological grade | |||||||||
| G1 | 1.000 | 1.000 | |||||||
| G2 | 2.443 | 1.578-3.782 | < 0.001a | 3.695 | 1.622-8.419 | 0.002a | |||
| G3 | 5.603 | 3.680-8.530 | < 0.001a | 6.369 | 2.834-14.314 | < 0.001a | |||
| pT stage | |||||||||
| T1 | 1.000 | 1.000 | |||||||
| T2 | 3.893 | 2.694-5.471 | < 0.001a | 2.298 | 1.538-3.434 | < 0.001a | |||
| T3 | 14.211 | 10.501-19.230 | < 0.001a | 3.475 | 2.340-5.162 | < 0.001a | |||
| T4 | 23.882 | 16.531-34.502 | < 0.001a | 5.987 | 3.992-8.978 | < 0.001a | |||
| pTNM stage | |||||||||
| I | 1.000 | 1.000 | |||||||
| II | 5.988 | 3.993-8.977 | < 0.001a | 1.465 | 1.087-2.132 | 0.004a | |||
| III | 113.033 | 72.436-176.382 | < 0.001a | 4.833 | 1.596-18.423 | < 0.001a | |||
| Vascular invasion | |||||||||
| Negative | 1.000 | 1.000 | |||||||
| Positive | 9.144 | 7.347-11.380 | < 0.001a | 2.845 | 1.467-5.517 | 0.016a | |||
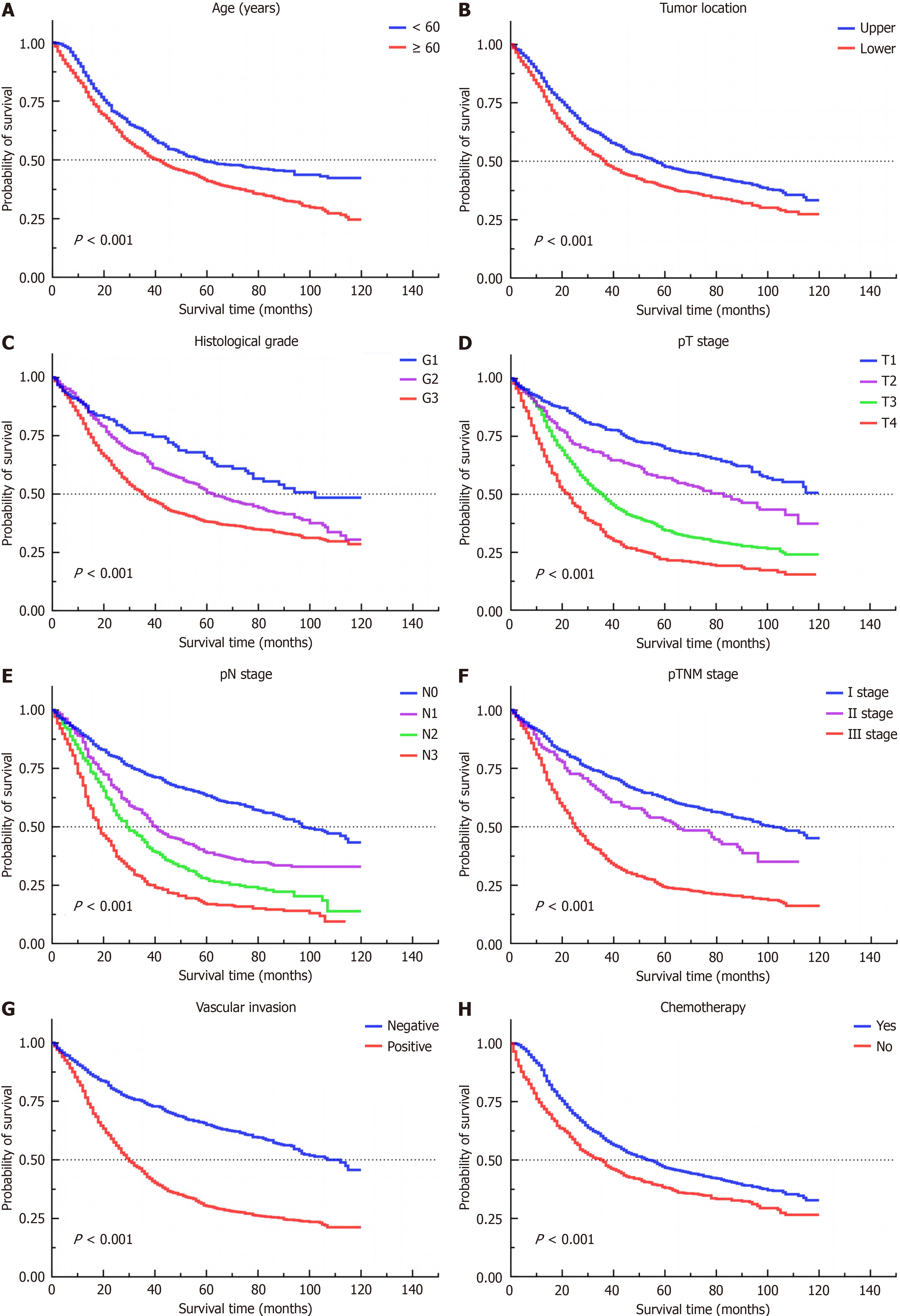
Univariate Cox regression analysis identified factors such as age > 60 years, upper GC status, moderate to undifferentiated tumor grade, pT stage T2 to T4, pN stage N1 to N3, pTNM stage II and III, positive vascular invasion, and the absence of adjuvant chemotherapy as risk factors influencing prognosis. Multivariate Cox regression analysis revealed that age > 60 years, adenocarcinoma status, signet ring cell carcinoma status, pT stages T3 and T4, pN stages N1–N3, pTNM stage III, vascular invasion, and absence of adjuvant chemotherapy were independent risk factors for patient prognosis. The data are shown in Table 4, and survival curves for the risk factors affecting patient prognosis are pre
| Variables | Univariate analysis | Multivariable analysis | ||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Gender | ||||||
| Male | 1.000 | |||||
| Female | 1.106 | 0.976–1.254 | 0.114 | |||
| Age (year) | ||||||
| < 60 | 1.000 | 1.000 | ||||
| ≥ 60 | 1.419 | 1.246-1.617 | < 0.001a | 1.594 | 1.395-1.821 | < 0.001a |
| Tumor diameter (cm) | ||||||
| < 4 | 1.000 | |||||
| ≥ 4 | 1.142 | 0.747-1.746 | 0.534 | |||
| Tumor location | ||||||
| Upper | 1.000 | 1.000 | ||||
| Lower | 1.302 | 1.158-1.464 | < 0.001a | 1.049 | 0.921-1.195 | 0.473 |
| Pathological type | ||||||
| Other | 1.000 | 1.000 | ||||
| Adenocarcinoma | 1.400 | 0.933-2.100 | 0.094 | 1.566 | 1.039-2.362 | 0.032a |
| Signet-ring cell carcinoma | 1.490 | 0.974-2.280 | 0.066 | 1.586 | 1.027-2.450 | 0.037a |
| Histological grade | ||||||
| G1 | 1.000 | 1.000 | ||||
| G2 | 1.457 | 1.096-1.938 | 0.010a | 1.087 | 0.814-1.453 | 0.571 |
| G3 | 1.943 | 1.481-2.549 | < 0.001a | 1.201 | 0.905-1.593 | 0.204 |
| pT stage | ||||||
| T1 | 1.000 | 1.000 | ||||
| T2 | 1.577 | 1.257-1.979 | < 0.001a | 1.205 | 0.946-1.534 | 0.130 |
| T3 | 2.566 | 2.153-3.059 | < 0.001a | 1.411 | 1.128-1.766 | 0.003a |
| T4 | 3.864 | 3.194-4.676 | < 0.001a | 1.801 | 1.395-2.326 | < 0.001a |
| pN stage | ||||||
| N0 | 1.000 | 1.000 | ||||
| N1 | 1.715 | 1.464-2.009 | < 0.001a | 1.328 | 1.083-1.628 | 0.006a |
| N2 | 2.441 | 2.059-2.893 | < 0.001a | 1.645 | 1.282-2.111 | < 0.001a |
| N3 | 3.505 | 2.989-4.110 | < 0.001a | 2.148 | 1.666-2.768 | < 0.001a |
| pTNM stage | ||||||
| I | 1.000 | 1.000 | ||||
| II | 1.383 | 1.102-1.735 | 0.005a | 1.223 | 0.958-1.563 | 0.107 |
| III | 2.651 | 2.331-3.015 | < 0.001a | 1.812 | 1.395-2.354 | < 0.001a |
| Surgery type | ||||||
| PG | 1.000 | |||||
| TG | 1.147 | 0.987-1.333 | 0.124 | |||
| Vascular invasion | ||||||
| Negative | 1.000 | 1.000 | ||||
| Positive | 2.440 | 2.136-2.787 | < 0.001a | 1.813 | 1.512-2.218 | < 0.001a |
| Chemotherapy | ||||||
| Yes | 1.000 | 1.000 | ||||
| NO | 1.433 | 1.271-1.616 | < 0.001a | 1.670 | 1.460-1.910 | < 0.001a |
A total of 817 patients who were diagnosed with upper GC and 989 with lower GC were included in this study, for a total of 1806 patients. Among patients with upper GC, the mean age was 64.91 ± 12.86 years, with a male-to-female ratio of approximately 2.11:1, whereas among patients with lower GC, the mean age was 66.15 ± 12.49 years, with a male-to-female ratio of approximately 1.79:1. Epidemiological evidence indicates that the male-to-female ratio of GC is generally approximately 2:1. Although the overall incidence of GC is declining annually, the incidence of upper GC is increasing annually[2]. Our findings are consistent with these observations, indicating a potential shift toward younger demo
Regarding lymph node metastasis patterns, our study revealed that for upper GC, metastasis rates were significantly greater in lymph node groups Nos. 1, 2, 3 and 7 across different subtypes, whereas for lower GCs, groups Nos. 3–8 exhibited elevated metastasis rates. These findings are consistent with prior research[10,11], highlighting the importance of clinicians diligently evaluating lymph node metastasis in these specified groups during surgical resection and pa
Our study revealed pathological type, histological grade, pT stage, pTNM stage, and vascular invasion as independent risk factors for lymph node me
Our study identified age, pathological type, pT stage, pN stage, pTNM stage, vascular invasion, and the absence of adjuvant chemotherapy as independent risk factors influencing GC prognosis. Caliskan Yildirim et al[17] found through multifactorial analysis that in N3-stage GC, lymph node metastasis rate, histological grade, and adjuvant chemotherapy were independent prognostic factors. Tian et al[18] determined that histological grade, pTNM stage, and adjuvant chemotherapy are independent risk factors for GC prognosis. Muneoka et al[19] identified age at surgery, tumor infiltration depth, tumor location, and lymph node metastasis as independent risk factors for disease-free survival in patients with progressive GC. Multiple studies[20-22] have highlighted lymph node metastasis as a crucial prognostic factor for GC. A Spanish study[23] demonstrated that age is not an independent predictor of disease-free survival in patients with GC. This suggests that the role of age as an independent prognostic factor in GC may lack sufficient clinical evidence, whereas lymph node metastasis, pTNM stage, and histological grade serve as reliable predictors of outcomes.
Given the inherent limitations of retrospective studies, our study is subject to selection bias. In categorizing adjuvant chemotherapy and surgical modalities, simple groupings were utilized owing to the absence of detailed clinical in
Upper GC has been shown to possess a significantly greater degree of malignancy than lower GC. In upper GC, the rate of lymph node metastasis was greater in Groups Nos. 1, 2, 3 and 7 among the different subtypes. In lower GC, the rate of lymph node metastasis was greater in groups Nos. 3–8. Pathological type, histological grade, pT stage, pTNM stage, and vascular invasion independently influenced the occurrence of lymph node metastasis. Age, pathological type, pT stage, pN stage, pTNM stage, vascular invasion, and absence of adjuvant chemotherapy independently influenced patient prognosis.
| 1. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3308] [Article Influence: 551.3] [Reference Citation Analysis (6)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68628] [Article Influence: 13725.6] [Reference Citation Analysis (201)] |
| 3. | Li ZL, Zhao LY, Zhang WH, Liu K, Pang HY, Chen XL, Chen XZ, Yang K, Hu JK. Clinical significance of lower perigastric lymph nodes dissection in Siewert type II/III adenocarcinoma of esophagogastric junction: a retrospective propensity score matched study. Langenbecks Arch Surg. 2022;407:985-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Huang Y, Zheng Z, Xu R, Zhang H, Yin J, Liu X, Zhang J, Chen G, Zhang Z. Assessment of risk factors of lymph node metastasis and prognosis of Siewert II/III adenocarcinoma of esophagogastric junction: A retrospective study. Medicine (Baltimore). 2024;103:e37289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | de Jongh C, Triemstra L, van der Veen A, Brosens LAA, Luyer MDP, Stoot JHMB, Ruurda JP, van Hillegersberg R; LOGICA Study Group. Pattern of lymph node metastases in gastric cancer: a side-study of the multicenter LOGICA-trial. Gastric Cancer. 2022;25:1060-1072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | Siewert JR. Adenocarcinoma of the esophago-gastric junction. Gastric Cancer. 1999;2:87-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer. 2023;26:1-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 841] [Article Influence: 280.3] [Reference Citation Analysis (2)] |
| 8. | Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76:182-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2554] [Cited by in RCA: 2758] [Article Influence: 459.7] [Reference Citation Analysis (3)] |
| 9. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4696] [Article Influence: 521.8] [Reference Citation Analysis (4)] |
| 10. | Brisinda G, Chiarello MM, Fico V, Puccioni C, Crocco A, Bianchi V, Vanella S. Pattern of Distribution of Lymph Node Metastases in Individual Stations in Middle and Lower Gastric Carcinoma. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Arroyo-Martinez Q, Han WH, Eom BW, Yoon HM, Kim YI, Cho SJ, Lee JY, Kim CG, Morales-Conde S, Padillo-Ruiz J, Kook MC, Choi IJ, Kim YW, Ryu KW. The distribution pattern of metastatic lymph nodes after non-curative endoscopic resection in early gastric cancer. J Surg Oncol. 2018;118:1257-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Zhang CD, Yamashita H, Okumura Y, Yagi K, Aikou S, Seto Y. Signature and Prediction of Perigastric Lymph Node Metastasis in Patients with Gastric Cancer and Total Gastrectomy: Is Total Gastrectomy Always Necessary? Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 13. | Yura M, Yoshikawa T, Otsuki S, Yamagata Y, Morita S, Katai H, Nishida T, Yoshiaki T. Oncological safety of proximal gastrectomy for T2/T3 proximal gastric cancer. Gastric Cancer. 2019;22:1029-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 14. | Haruta S, Shinohara H, Hosogi H, Ohkura Y, Kobayashi N, Mizuno A, Okamura R, Ueno M, Sakai Y, Udagawa H. Proximal gastrectomy with exclusion of no. 3b lesser curvature lymph node dissection could be indicated for patients with advanced upper-third gastric cancer. Gastric Cancer. 2017;20:528-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Khalayleh H, Kim YW, Man Yoon H, Ryu KW, Kook MC. Evaluation of Lymph Node Metastasis Among Adults With Gastric Adenocarcinoma Managed With Total Gastrectomy. JAMA Netw Open. 2021;4:e2035810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Chen J, Zhao G, Wang Y. Analysis of lymph node metastasis in early gastric cancer: a single institutional experience from China. World J Surg Oncol. 2020;18:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Caliskan Yildirim E, Acikgoz Y, Ergun Y, Algin E, Bal O. Treatment Outcomes and Prognostic Factors in N3 Stage Gastric Cancer After Curative Resection: A Real World Data. Cancer Manag Res. 2023;15:1085-1096. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Tian H, Liu Z, Zhang Z, Zhang L, Zong Z, Liu J, Ying H, Li H. Clinical Significance of Fibrinogen and Platelet to Pre-Albumin Ratio in Predicting the Prognosis of Advanced Gastric Cancer. J Inflamm Res. 2023;16:4373-4388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Muneoka Y, Akazawa K, Ishikawa T, Ichikawa H, Nashimoto A, Yabusaki H, Tanaka N, Kosugi SI, Wakai T. Nomogram for 5-year relapse-free survival of a patient with advanced gastric cancer after surgery. Int J Surg. 2016;35:153-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 20. | Lee AY, Kim MC, Cho S, Yoo IK, Kim YM, Lee TH, Seo JY, Kim SH, Cho JY. Da Vinci robot-assisted endoscopic full-thickness gastric resection with regional lymph node dissection using a 3D near-infrared video system: a single-center 5-year clinical outcome. Surg Endosc. 2024;38:2124-2133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | Li GZ, Doherty GM, Wang J. Surgical Management of Gastric Cancer: A Review. JAMA Surg. 2022;157:446-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 195] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 22. | Lee J, Cha S, Kim J, Kim JJ, Kim N, Jae Gal SG, Kim JH, Lee JH, Choi YD, Kang SR, Song GY, Yang DH, Lee JH, Lee KH, Ahn S, Moon KM, Noh MG. Ensemble Deep Learning Model to Predict Lymphovascular Invasion in Gastric Cancer. Cancers (Basel). 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 23. | Díaz Del Arco C, Ortega Medina L, Estrada Muñoz L, Molina Roldán E, García Gómez de Las Heras S, Fernández Aceñero MJ. Impact of Age at Diagnosis on Clinicopathological Features, Prognosis, and Management of Gastric Cancer: A Retrospective Single-Center Experience from Spain. Cancers (Basel). 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/