Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.114390
Revised: October 23, 2025
Accepted: November 13, 2025
Published online: December 15, 2025
Processing time: 84 Days and 0.9 Hours
The clinical and pathological characteristics of primary gastric small cell carci
A 67-year-old male experienced upper abdominal pain without any obvious cause for 1 week. Gastroscopy examination revealed a mass in the gastric body. Patho
GSCC is a highly malignant tumor with a poor prognosis. Whether immune-related drugs are optimal for metastatic GSCC requires further exploration.
Core Tip: The clinical and pathological characteristics of primary gastric small cell carcinoma (GSCC) resemble those of small cell lung cancer, but it is less sensitive to chemotherapy and has a poor prognosis. Here we report a case of a 67-year-old man who was found to have a primary stage IV GSCC with metastasis to the liver. The patient was treated with chemotherapy combined with a programmed cell death ligand 1 (PD-L1) inhibitor. Whether PD-L1 inhibitors are optimal for metastatic GSCC requires further exploration.
- Citation: Zhang XL, Zhang JY, Xie L, Li H, Wang L. Advanced gastric small cell carcinoma with immunotherapy-based treatment: A case report. World J Gastrointest Oncol 2025; 17(12): 114390
- URL: https://www.wjgnet.com/1948-5204/full/v17/i12/114390.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i12.114390
The incidence of gastric small cell carcinoma (GSCC) is low, and most reports in the literature are case reports. Due to the small number of cases, the understanding of GSCC is limited. National Comprehensive Cancer Network and European Society for Medical Oncology guidelines recommend combining programmed cell death ligand 1 (PD-L1) inhibitors (durvalumab or atezolizumab) with platinum-etoposide chemotherapy as the first-line standard treatment for extensive-stage small-cell lung cancer (ES-SCLC)[1,2]. However, whether PD-L1 inhibitors are optimal for metastatic GSCC requires more clinical data.
We here report a case of GSCC, and discuss its clinical characteristics and treatment with chemotherapy and dur
A 67-year-old man was admitted to our hospital due to upper abdominal pain for one week. Self-administration of omeprazole did not achieve significant relief.
The patient’s symptoms had lasted 2 weeks.
The patient had a history of stroke.
The patient was a hepatitis B carrier whose older brother died of hepatocellular carcinoma.
Physical examination of the patient showed no abnormalities.
The neuron-specific enolase blood level was 16.80 ng/mL.
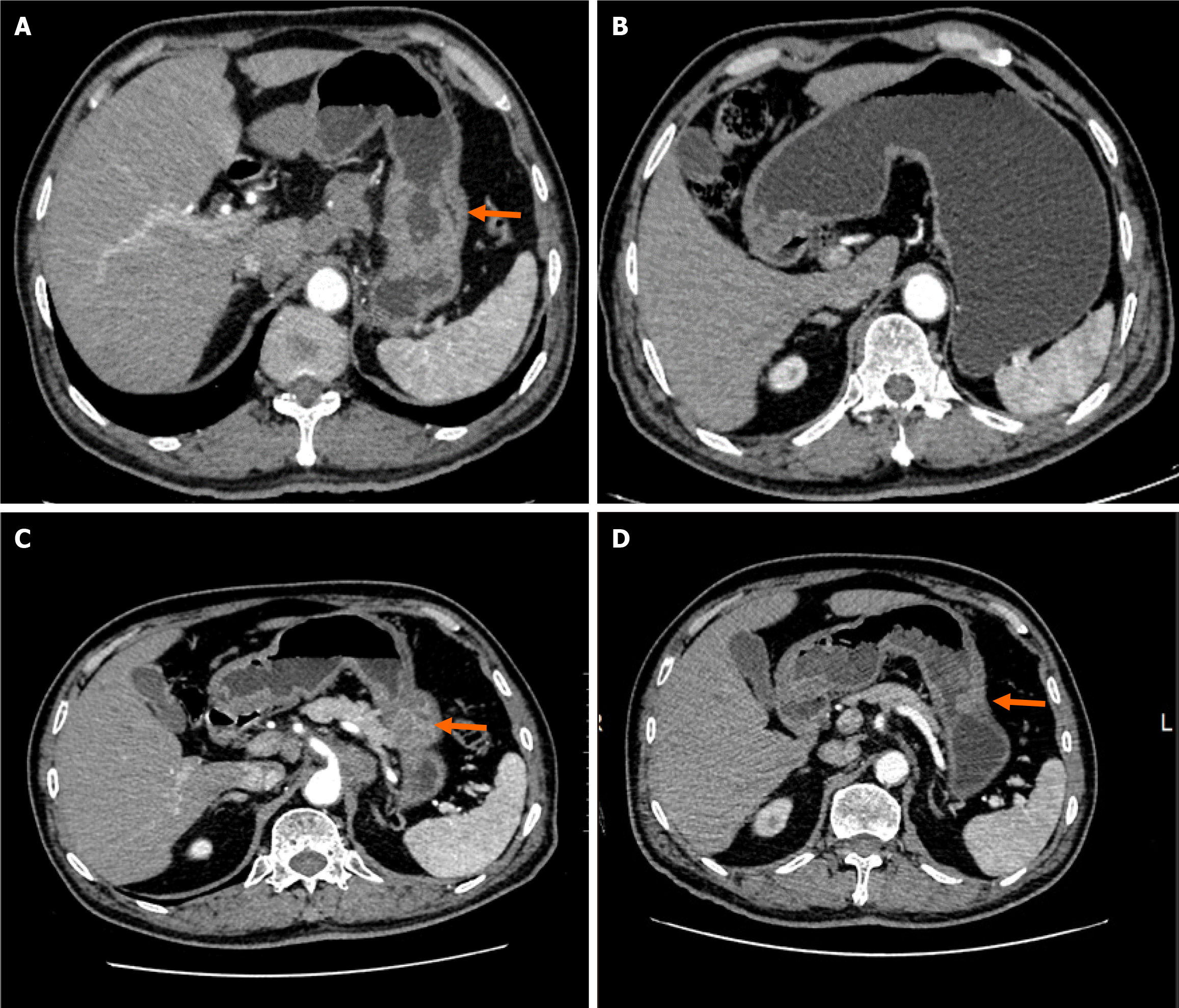
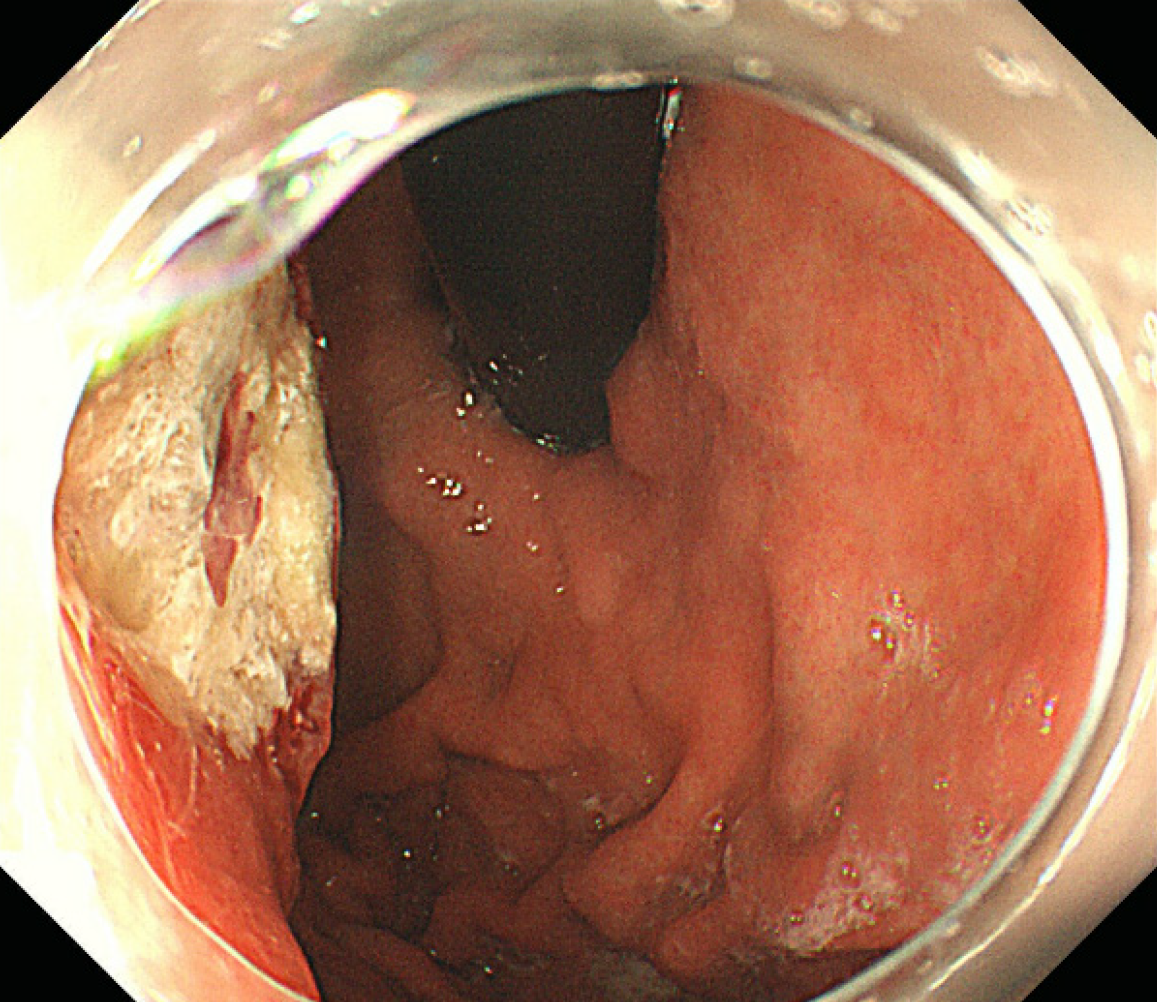
Computed tomography (neck + chest + abdomen; plain scan + enhancement) showed enlarged lymph nodes in the anterior upper mediastinum, occupying space in the greater curvature of the stomach. In addition, multiple intrahepatic metastases with multiple lymph node metastases were observed in the hepatogastric space and around the abdominal aorta (Figure 1). Gastroscopy examination revealed a mass in the gastric body (Figure 2).
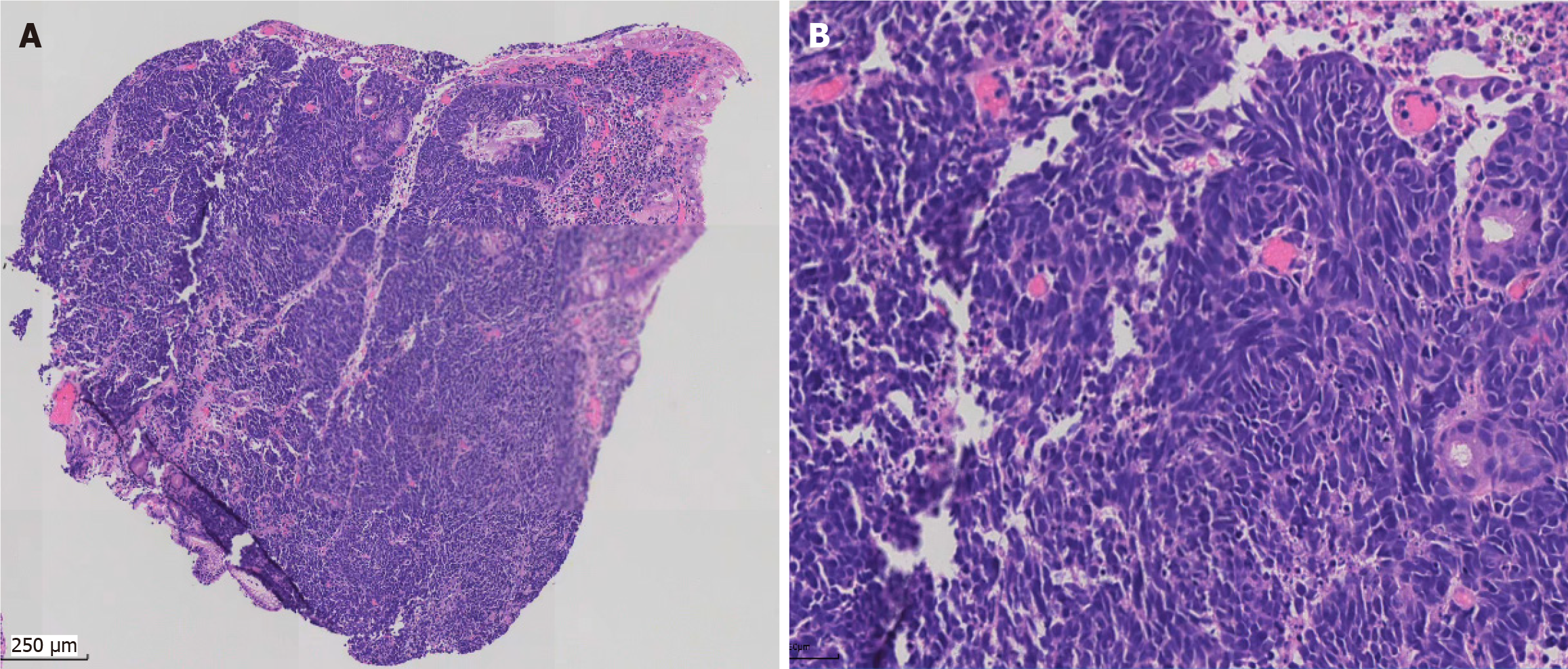
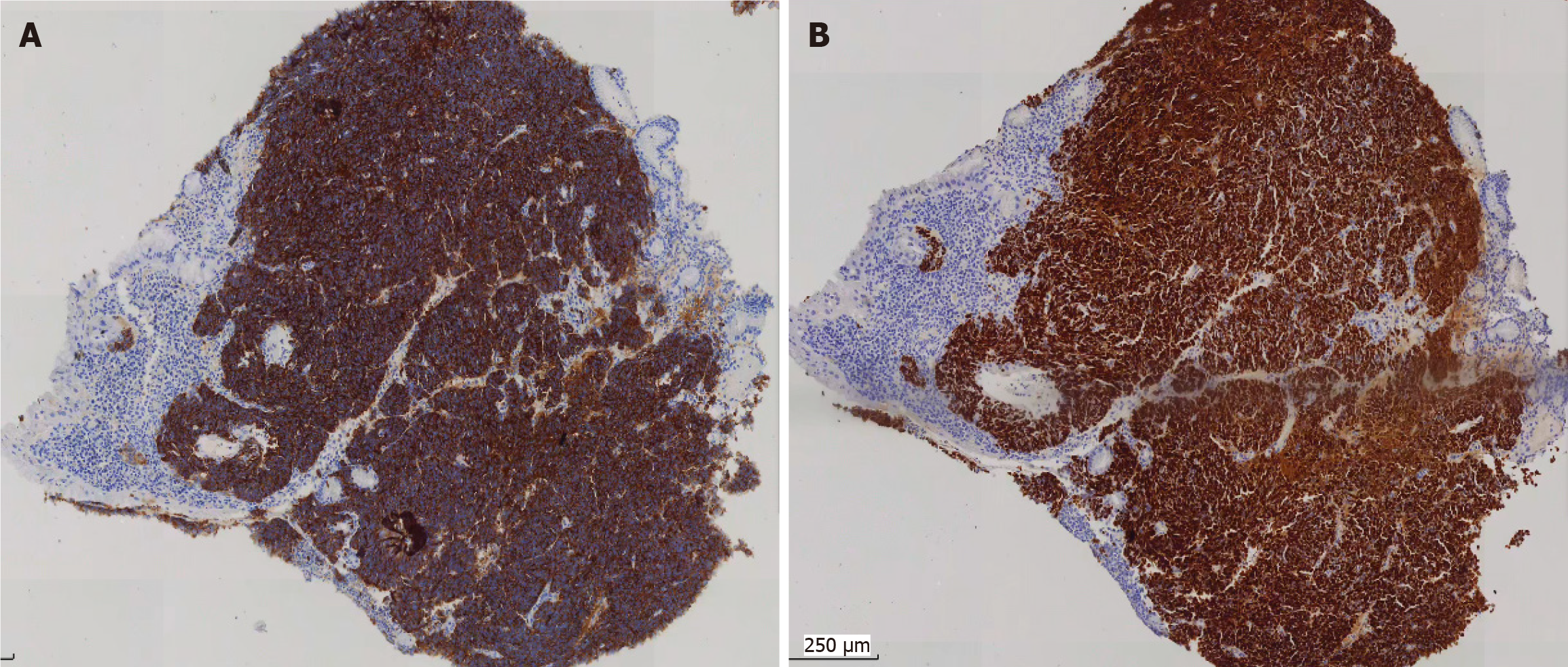
Small blue round cells infiltrated all layers of the gastric wall, and nuclear mitosis was visible (Figure 3). Immunohistochemically, the tumor cells diffusely expressed cytokeratin (CK), synaptophysin, cluster of differentiation 56, and INSM1 indicating that the tumor was a gastric neuroendocrine carcinoma (NEC) (small cell carcinoma) (Figure 4). CK20, p40, CK5/6, chromogranin A (CgA), and somatostatin receptor 2 were all negative. The Ki67 index was approximately 95%. p53 was mutant (nonsense mutation). RB1 showed a loss of expression while INI-1 and BRG1 showed preserved expression. The combined positive score of PD-L1 was 1.
A regimen consisting of cisplatin (day 1, 30 mg/m2 per day) and etoposide (day 1 to day 3, 100 mg/m2 per day) combined with durvalumab (day 1, 1000 mg/day) was given every three weeks as palliative chemotherapy for seven cycles. Durvalumab was then maintained every three weeks. However, the tumor recurred two months after the completion of chemotherapy. A regimen consisting of carboplatin (day 1, 400 mg/m2 per day) and irinotecan (day 1, 100 mg/m2 per day) combined with durvalumab (day 1, 1000 mg/day) was then given every three weeks. Genetic tests showed TP53, RB1, CREBBP, ABCB1, DNMT3A, and HGF gene mutations. The tumor mutation burden (TMB) was low (5.1 mutations/megabase), and microsatellite instability remained stable.
During the 16-month follow-up, the tumor in the gastric body and liver showed marked shrinkage, and the size of the lymph nodes also decreased (Figure 1). The patient did not report any specific discomfort, and there was a significant improvement in mental and physical condition.
GSCC is a specific histological subtype of gastric cancer and a highly malignant NEC[3]. There is a lack of standardized treatment for GSCC and the literature on immunotherapy for GSCC is limited. The treatment of primary small cell carcinoma refers to that of small cell carcinoma of the lung. This article analyzes and summarizes the pathology, clinical manifestations, treatment, and prognosis of GSCC, and adds experience in its clinical diagnosis and treatment.
GSCC has a male predominance, with a median patient age of 62 years[4]. The clinical symptoms and physical changes are similar to those of general gastric cancer, making it difficult to differentiate. Symptoms include pain and discomfort in the upper abdomen, as well as loss of appetite and progressive weight loss. Some patients also experience varying degre
Histologically, the tumor exhibited a nest-like and solid sheet arrangement, with no evidence of glandular differentiation. The cell morphology of GSCC can be round or oval, with scant cytoplasm. Nucleoli are not prominent, and necrosis is commonly observed. Mitotic figures are frequently seen. As a tumor with small blue round cells, it needs to be differentiated from the following tumors: (1) Ewing’s sarcoma or peripheral primitive neuroectodermal tumor: EWS-FLI-1 gene fusion can be detected by reverse transcription-polymerase chain reaction; (2) Alveolar rhabdomyosarcoma: This tumor shows diffuse positivity for MyoD1, myogenin, and desmin. Characteristic chromosomal translocations t (2; 13) and t (1; 13) are observed in this tumor; (3) Lymphoma: Tumor cells are mainly round and express lymphoma markers such as leukocyte common antigen. Immunohistochemically, synaptophysin, CgA, and insulinoma-associated protein 1 are particularly significant and can help diagnosis; and (4) Metastatic small cell carcinoma from the lung: Tumors from the lung express thyroid transcription factor-1 more frequently, and chest computed tomography can identify the primary lesion.
At present, there are some hypotheses regarding the mechanism of GSCC, but its genetic characteristics are still unclear. The pathogenesis and molecular genetic alterations of GSCC are considered to have a high mutation rate and a mutation profile different from that of conventional gastric adenocarcinoma. Both TP53 and RB1 mutation can help distinguish GSCC from neuroendocrine tumor G3. However, with the construction of the human gastric NEC genome atlas, MYC has been identified as a key driver of the disease[5], which could provide new ideas regarding its biological characteristics and treatment. In our case, genetic tests showed TP53 and RB1 mutation, as well as new mutations including ABCB1, DNMT3A, and HGF gene mutations. Deciphering the molecular pathways of GSCC carcinogenesis will not only aid in guiding targeted therapies but also provide references crucial for prognosis.
The treatment for GSCC depends on the clinical stage. If the tumor is localized and there is no distant metastasis, radical resection is recommended, supplemented by postoperative radiotherapy or chemotherapy[6]. For advanced GSCC, neoadjuvant chemotherapy before surgery is recommended, followed by evaluation once every two months. This can improve the resection rate and prognosis of patients[7]. Surgery combined with chemotherapy remains the first-line treatment for GSCC patients. Chemotherapy is usually a platinum-based regimen with etoposide[8]. For stage IV GSCC, the National Comprehensive Cancer Network in the United States recommends chemotherapy as first-line treatment, without mentioning surgical treatment. However, there is still controversy over whether palliative resection of the primary tumor can bring survival benefits to patients with stage IV GSCC[9,10]. Most studies have shown that chemotherapy can improve the prognosis regardless of the stage[11,12]. Studies also showed that patients who experience recurrent metastasis and receive adjuvant radiochemotherapy have a significantly longer overall survival compared to the best supportive care[12]. However, other studies found that chemotherapy did not improve the prognosis of GSCC patients[13].
Immune checkpoint inhibitors (ICIs) have significantly transformed cancer treatment strategies over the past decade[14]. The most extensively studied immune checkpoints are programmed death 1 and its ligand PD-L1. High TMB and high microsatellite instability can enhance the efficacy of immunotherapy and have been approved by the Food and Drug Administration as companion diagnostics for ICIs. A meta-analysis showed that chemo-immunotherapy provided better progression-free survival and overall survival compared with chemotherapy alone as first-line treatment in ES-SCLC, including durvalumab, atezolizumab and nivolumab[15]. However, data on chemo-immunotherapy in GSCC are very rare, and its clinical value is unknown. In our case, surgery was not feasible due to liver metastases and multiple lymph node metastases. Thus, chemotherapy was administered. Whether chemotherapy should be combined with durvalumab is debatable as different doctors have different opinions, and clinical data are limited. Our patient received seven cycles of chemotherapy combined with durvalumab. After chemotherapy, the size of the tumor in the gastric body and lymph node significantly decreased, but the patient refused subsequent surgery due to fear. Durvalumab was then maintained every three weeks. The patient is in good overall condition, which indicates that even in low TMB and microsatellite stable cases, durvalumab is a viable option for patients with GSCC. This is consistent with key insight from pivotal SCLC trials: The clinical benefit of adding ICIs to chemotherapy was independent of these biomarkers. Although high TMB and microsatellite instability-high have been confirmed as predictive biomarkers for ICI efficacy in many solid tumors, this does not apply to chemotherapy immunotherapy combination treatment for SCLC. This case may demonstrate the applicability of principles derived from SCLC to GSCC. The efficacy of this combination in the low-TMB setting may stem from the immunomodulatory effects of chemotherapy. Platinum drugs and etoposide can induce immunogenic cell death, release tumor antigens, and create a proinflammatory microenvironment. This chemotherapy-induced priming may render tumors more sensitive to checkpoint inhibition by a mechanism that is less dependent on the baseline neoantigen load of the tumor. This may challenge the widespread use of TMB as a predictive biomarker and emphasizes that, for certain histologies, synergy between chemotherapy and immunotherapy may be the primary mechanism of action. However, the tumor recurred two months after the completion of chemotherapy. A regimen consisting of carboplatin and irinotecan combined with durvalumab was then given every three weeks. The tumor shrank, and the patient’s condition is stable.
Durvalumab combined with chemotherapy provides significant survival benefits in ES-SCLC. Given the highly aggressive nature and poor prognosis of GSCC, along with the lack of standard treatment options, it is reasonable to adopt one of the most effective therapeutic strategies for SCLC.
| 1. | Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M, Florsheim C, Gold KA, Goldman JW, Grecula JC, Hann C, Iams W, Iyengar P, Kelly K, Khalil M, Koczywas M, Merritt RE, Mohindra N, Molina J, Moran C, Pokharel S, Puri S, Qin A, Rusthoven C, Sands J, Santana-Davila R, Shafique M, Waqar SN, Gregory KM, Hughes M. Small Cell Lung Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:1441-1464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 320] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 2. | Dingemans AC, Früh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, Lantuejoul S, Peters S, Reguart N, Rudin CM, De Ruysscher D, Van Schil PE, Vansteenkiste J, Reck M; ESMO Guidelines Committee. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(☆). Ann Oncol. 2021;32:839-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 416] [Article Influence: 83.2] [Reference Citation Analysis (0)] |
| 3. | Wu QQ, Qiang WG, Wang F, Dai KJ, Xu EC, Luo JD, Li Q, Tang H, Zhou XF, Lu XJ. Management of primary gastric small cell carcinoma in China. Int J Clin Exp Med. 2015;8:1589-1597. [PubMed] |
| 4. | Liu DJ, Fu XL, Liu W, Zheng LY, Zhang JF, Huo YM, Li J, Hua R, Liu Q, Sun YW. Clinicopathological, treatment, and prognosis study of 43 gastric neuroendocrine carcinomas. World J Gastroenterol. 2017;23:516-524. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Griger J, Widholz SA, Jesinghaus M, de Andrade Krätzig N, Lange S, Engleitner T, Montero JJ, Zhigalova E, Öllinger R, Suresh V, Winkler W, Lier S, Baranov O, Trozzo R, Ben Khaled N, Chakraborty S, Yu J, Konukiewitz B, Steiger K, Pfarr N, Rajput A, Sailer D, Keller G, Schirmacher P, Röcken C, Fagerstedt KW, Mayerle J, Schmidt-Supprian M, Schneider G, Weichert W, Calado DP, Sommermann T, Klöppel G, Rajewsky K, Saur D, Rad R. An integrated cellular and molecular model of gastric neuroendocrine cancer evolution highlights therapeutic targets. Cancer Cell. 2023;41:1327-1344.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 6. | Iwamoto M, Gotoda T, Noda Y, Esaki M, Moriyama M, Yoshida N, Takayama T, Kobayashi H, Masuda S. Gastric Neuroendocrine Carcinoma with Rapid Progression. Intern Med. 2020;59:1271-1276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Ma F, Wang B, Xue L, Kang W, Li Y, Li W, Liu H, Ma S, Tian Y. Neoadjuvant chemotherapy improves the survival of patients with neuroendocrine carcinoma and mixed adenoneuroendocrine carcinoma of the stomach. J Cancer Res Clin Oncol. 2020;146:2135-2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 8. | Garcia-Carbonero R, Sorbye H, Baudin E, Raymond E, Wiedenmann B, Niederle B, Sedlackova E, Toumpanakis C, Anlauf M, Cwikla JB, Caplin M, O'Toole D, Perren A; Vienna Consensus Conference participants. ENETS Consensus Guidelines for High-Grade Gastroenteropancreatic Neuroendocrine Tumors and Neuroendocrine Carcinomas. Neuroendocrinology. 2016;103:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 431] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 9. | Toyokawa T, Tanaka H, Muguruma K, Tamura T, Sakurai K, Kubo N, Ohira M, Hirakawa K. Primary gastric small cell carcinoma: a series of seven cases. Anticancer Res. 2015;35:563-567. [PubMed] |
| 10. | Watanabe M, Miyake T, Mikane Y, Syoji R, Kajiwara Y, Aoyama K, Nogami T, Nishikawa H, Shinoura S, Shigemitsu K, Nonaka Y, Hayashi D. [A Case of Gastric Small Cell Carcinoma with Rapid Regrowth after Surgery]. Gan To Kagaku Ryoho. 2019;46:145-147. [PubMed] |
| 11. | Iwasaki K, Barroga E, Enomoto M, Tsurui K, Shimoda Y, Matsumoto M, Miyoshi K, Ota Y, Matsubayashi J, Nagakawa Y. Long-term surgical outcomes of gastric neuroendocrine carcinoma and mixed neuroendocrine-non-neuroendocrine neoplasms. World J Surg Oncol. 2022;20:165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 12. | Han D, Li YL, Zhou ZW, Yin F, Chen J, Liu F, Shi YF, Wang W, Zhang Y, Yu XJ, Xu JM, Yang RX, Tian C, Luo J, Tan HY. Clinicopathological characteristics and prognosis of 232 patients with poorly differentiated gastric neuroendocrine neoplasms. World J Gastroenterol. 2021;27:2895-2909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Lin JP, Zhao YJ, He QL, Hao HK, Tian YT, Zou BB, Jiang LX, Lin W, Zhou YB, Li Z, Xu YC, Zhao G, Xue FQ, Li SL, Fu WH, Li YX, Zhou XJ, Li Y, Zhu ZG, Chen JP, Xu ZK, Cai LH, Li E, Li HL, Xie JW, Huang CM, Li P, Lin JX, Zheng CH. Adjuvant chemotherapy for patients with gastric neuroendocrine carcinomas or mixed adenoneuroendocrine carcinomas. Br J Surg. 2020;107:1163-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Weber MM, Fottner C. Immune Checkpoint Inhibitors in the Treatment of Patients with Neuroendocrine Neoplasia. Oncol Res Treat. 2018;41:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Chen CY, Chen WC, Hung CM, Wei YF. Chemotherapy or chemo-immunotherapy as first-line treatment for extensive-stage small-cell lung cancer: a meta-analysis. Immunotherapy. 2021;13:1165-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/