Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.112853
Revised: September 5, 2025
Accepted: October 20, 2025
Published online: December 15, 2025
Processing time: 126 Days and 2.6 Hours
Treatment of metastatic gastric cancer (mGC) relies primarily on chemotherapy, which can be effectively combined with immunotherapy. Despite these interven
We report a patient with mGC who received S-1 plus oxaliplatin with sintilimab as first-line therapy, followed by maintenance therapy with S-1 and sintilimab. After 6 months, a partial response was observed, with a 71.7% reduction in the target lesion. After 13 months, the reduction reached 76.3%. Treatment was tem
Sintilimab combined with chemotherapy demonstrated improved progression-free survival and warrants further investigation in mGC.
Core Tip: Chemotherapy combined with immunotherapy is the standard first-line treatment for metastatic gastric cancer. However, the first-line median progression-free survival is only about 7 months. This article reports a case of metastatic gastric cancer in which the patient received S-1 plus oxaliplatin regimen with sintilimab, followed by maintenance therapy with S-1 and sintilimab. The patient achieved a partial response and a progression-free survival of 23 months. Moreover, the treatment was temporarily paused for 6 months due to pneumonia. However, upon re-evaluation 6 months later, the tumor continued to regress. This combination therapy has shown encouraging results and warrants further study.
- Citation: Pan J, Li P, Zhou YH, Pan TT, Chen YT, Chu XY. Long-term survival after treatment of gastric cancer with S-1 plus oxaliplatin regimen and sintilimab: A case report. World J Gastrointest Oncol 2025; 17(12): 112853
- URL: https://www.wjgnet.com/1948-5204/full/v17/i12/112853.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i12.112853
Gastric cancer (GC) is a common malignant tumor of the digestive tract. According to the International Agency for Research on Cancer, in 2022, there were 968350 new cases of GC and 659853 deaths worldwide, ranking fifth among malignant tumors for both incidence and mortality[1]. Currently, chemotherapy remains the mainstay for treating me
A 77-year-old male was admitted to our hospital with abdominal pain for three days in September 2022.
The patient experienced upper abdominal pain 3 days after eating. The pain was dull and intermittent, occurring 8 to 10 times daily, with each episode lasting approximately 5 to 10 minutes. Pain intensity was rated 3 to 4 on the numeric rating scale and resolved spontaneously. The patient reported no acid reflux, belching, nausea, vomiting, abdominal distension, or melena.
This patient was diagnosed with hypertension based on the symptoms of dizziness and abnormal elevation of blood pressure in 2017.
He smoked about 20 cigarettes per day for 15 years and quit smoking 5 years ago. He had no history of alcohol abuse. His family history was notable for his father’s death from GC and his brother’s similar demise, whereas the cause of his mother’s death remained unknown.
All vital signs were stable, and physical examination revealed no notable abnormalities.
Laboratory findings showed slightly decreased hemoglobin (99 g/L) and otherwise normal blood counts.
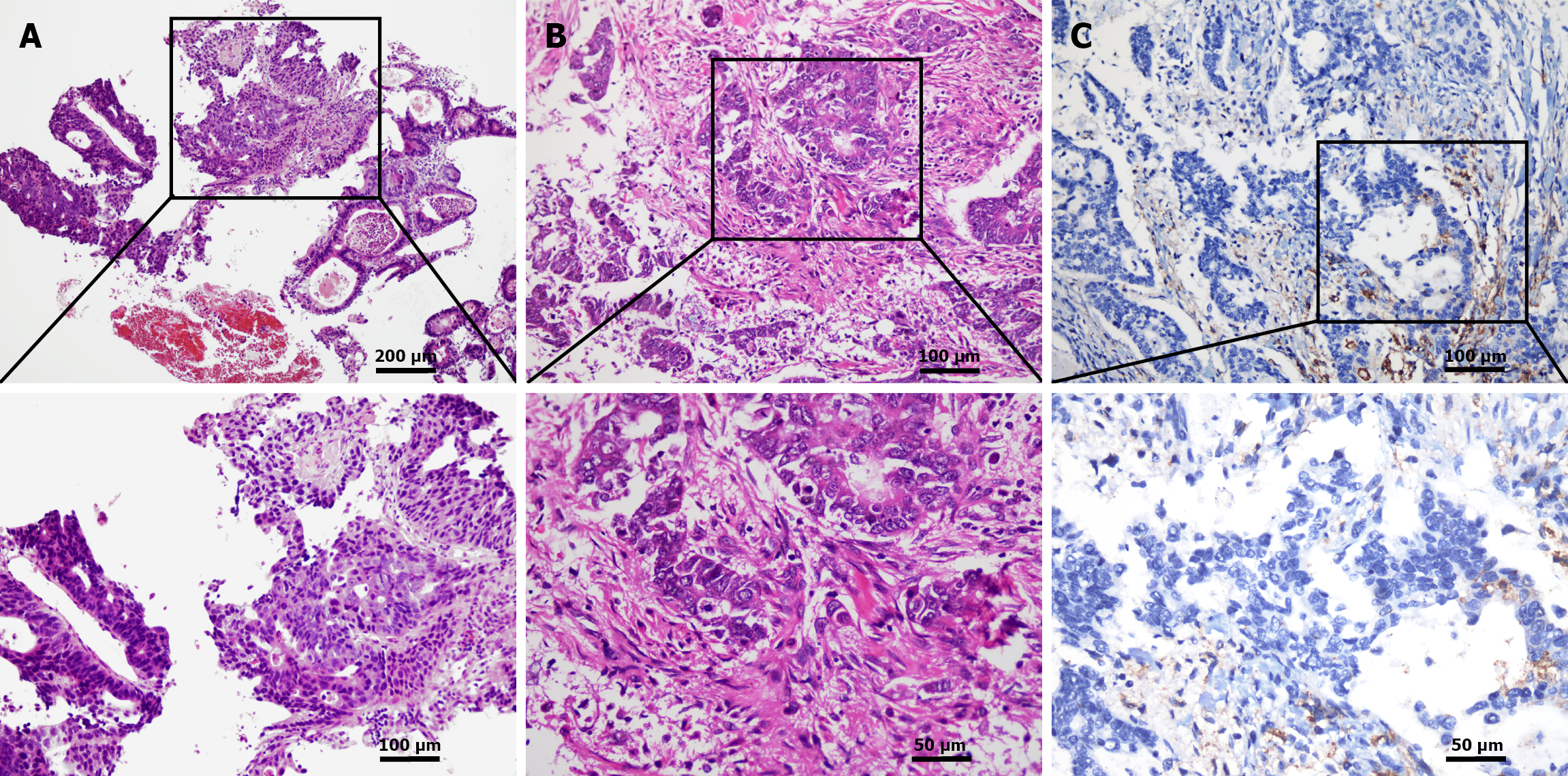
A gastroscopy performed on September 7, 2022, revealed an irregular lesion at the cardia, approximately 4 cm × 5 cm, characterized by significant mucosal infiltration, bleeding, and invasion of the cardia. Pathological examination of the endoscopic biopsy confirmed moderately differentiated adenocarcinoma (Figure 1A). A subsequent contrast-enhanced computed tomography (CT) scan of the abdomen on September 22, 2022, demonstrated localized thickening of the gastric wall extending from the cardia to the lesser curvature, suggestive of GC, along with slightly enlarged lymph nodes in the hepatogastric space.
A discussion at the tumor board meeting was conducted, and radical open gastrectomy with esophagojejunostomy followed by adjuvant chemotherapy was planned.
The patient’s preoperative clinical stage was stage IIA GC.
After providing full informed consent, the patient agreed to undergo surgical treatment. On September 27, 2022, the patient underwent open radical total gastrectomy with esophagojejunostomy. Intraoperative blood loss was approximately 100 mL. During surgery, two units of red blood cells and 140 mL of frozen plasma were transfused. The postoperative hospital stay lasted 12 days. A feeding jejunostomy was not performed. Postoperative pathology revealed moderately to poorly differentiated adenocarcinoma, measuring approximately 3.5 cm × 3.2 cm × 1 cm, with infiltration through the entire gastric wall into the serosal fibrous adipose tissue. Neural invasion and intravascular tumor thrombi were identified. Notably, the upper and lower resection margins, the submitted anastomotic site, and the omental tissue were free of tumor. No metastatic carcinoma was detected in the lymph nodes along the lesser curvature (0/10) or the greater curvature (0/2) (Figure 1B). Immunohistochemistry results indicated HER-2 (+), MutL homolog 1 (+), MutL homolog 12 (+), MutS homolog6 (+), and postmeiotic segregation increased 2 (+). Next-generation sequencing results showed programmed death ligand 1 (combined positive score = 3; tumor proportion score < 1%; Figure 1C) and a tumor mutation burden of 8.2 mutations/Mb. The patient’s postoperative tumor-node-metastasis stage was stage IIA (T3N0M0). The patient underwent two cycles of adjuvant chemotherapy using the SOX regimen at the Department of General Surgery, Jinling Hospital in November and December 2022 (oxaliplatin 200 mg IV on day 1; S-1 60 mg per os twice daily from day 1 to day 14, every 3 weeks). The patient self-reported the discontinuation of chemotherapy due to nausea and vomiting, and no further postoperative adjuvant treatment was administered thereafter.
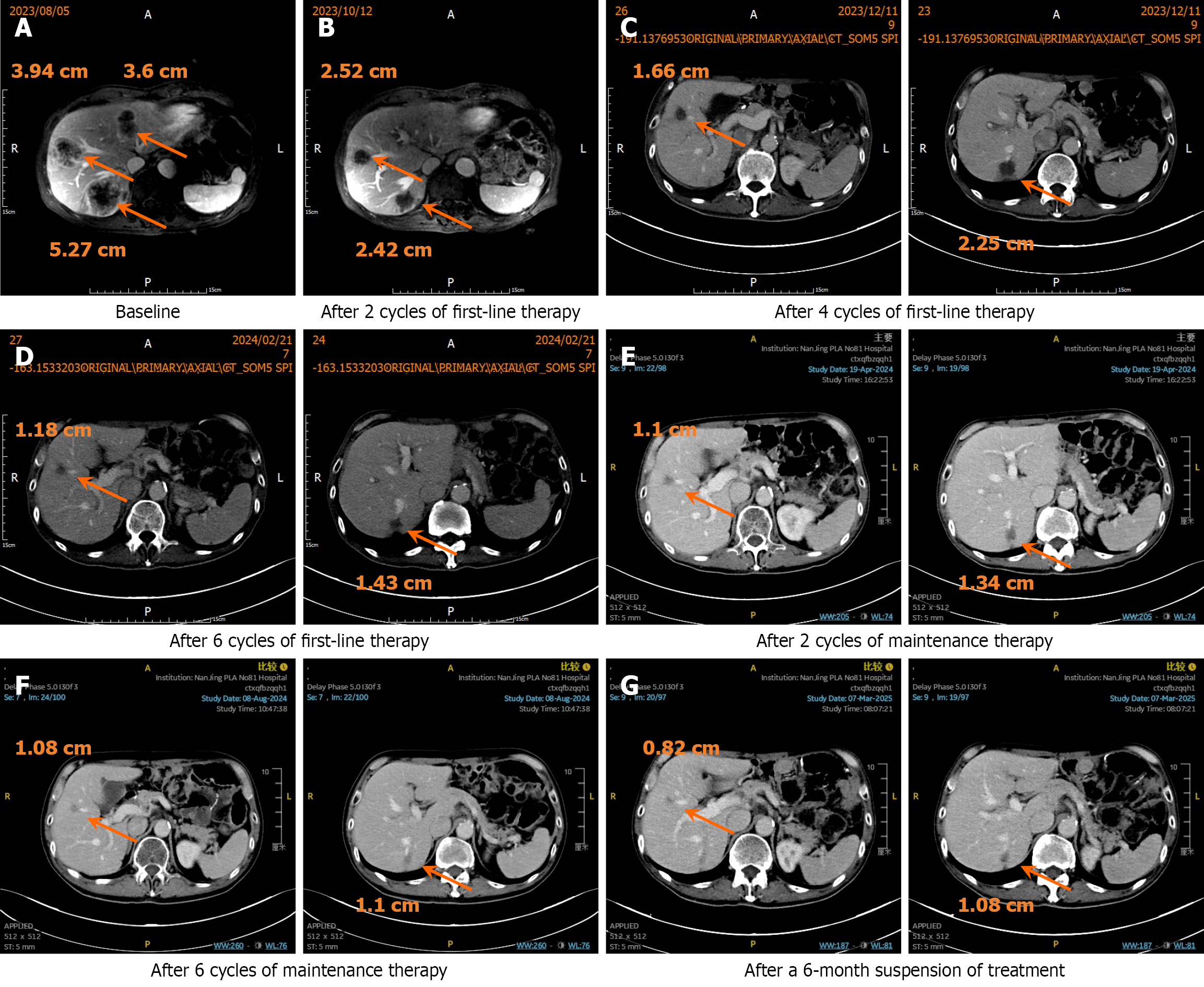
On August 5, 2023, an upper abdominal magnetic resonance imaging revealed multiple liver lesions, the largest of which was approximately 5.2 cm. The patient was diagnosed with stage IV GC with liver metastasis. We did not perform a percutaneous biopsy of the liver lesion. From August 9, 2023, to January 9, 2024, the patient received first-line therapy consisting of the SOX regimen combined with sintilimab for six cycles (sintilimab 200 mg IV on day 1; oxaliplatin 200 mg IV on day 1; S-1 40 mg in the morning and 60 mg in the evening from day 1 to day 14, every 3 weeks). According to the National Comprehensive Cancer Network guidelines, positron emission tomography/CT is not routinely recommended for assessment of chemotherapy. Therefore, we performed contrast-enhanced CT of the chest and abdomen instead. After two cycles of first-line therapy, the evaluation indicated a PR with a target lesion reduction of 46.4%. After six cycles, the evaluation continued to show a PR with target lesion reductions of 71.7%. From February 23, 2024, to September 2, 2024, the patient received maintenance treatment with sintilimab combined with S-1 for eight cycles (sintilimab 200 mg IV on day 1; S-1 40 mg in the morning and 60 mg in the evening from day 1 to day 14, every 3 weeks). The efficacy evaluation indicated a maintained PR with a 76.3% reduction in target lesions (Figure 2).
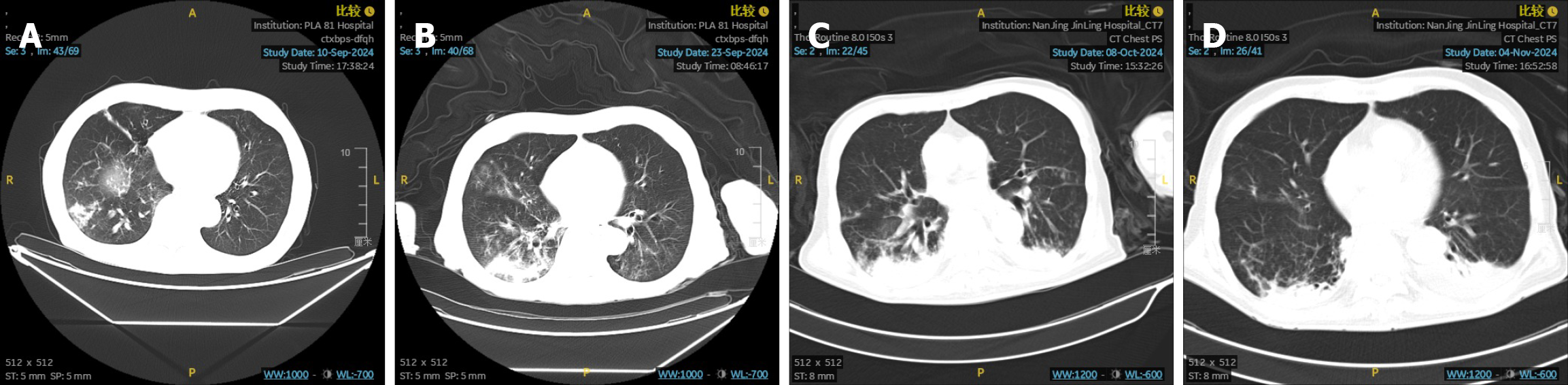
On September 10, 2024, the patient was diagnosed with pneumonia and treated with cefoperazone sulbactam sodium combined with moxifloxacin in the Emergency Department. Subsequently, on September 23, 2024, the patient was admitted to the Respiratory Department and treated with piperacillin and tazobactam due to the worsening infection. On October 8, 2024, the patient required admission to the intensive care unit and was administered imipenem and cilastatin due to further aggravation of the infection. A chest CT scan conducted on November 4, 2024, revealed bronchiectasis with infection in the right lung. Subsequently, there was clinical and radiological improvement (Figure 3). During the anti-infective treatment, the use of sintilimab and S-1 was discontinued. Following discharge from the hospital, due to the patient’s poor physical condition, the patient requested a temporary discontinuation of antitumor treatment. After thorough communication, the patient opted for close observation and follow-up. From September 2024 to February 2025, the patient did not undergo any antitumor therapy. A CT scan on March 7, 2025, indicated that the liver lesions continued to shrink, with target lesions reduced by 79.4% (Figure 2). Subsequently, on March 12, April 9, and May 19, 2025, the patient received three cycles of maintenance therapy with sintilimab combined with S-1 (sintilimab 200 mg IV on day 1; S-1 40 mg in the morning and 60 mg in the evening from day 1 to day 14, every 3 weeks). The patient tolerated the therapy well, with the main adverse reactions being grade 1 anemia and grade 1 fatigue, which were considered primarily related to chemotherapy and not associated with sintilimab.
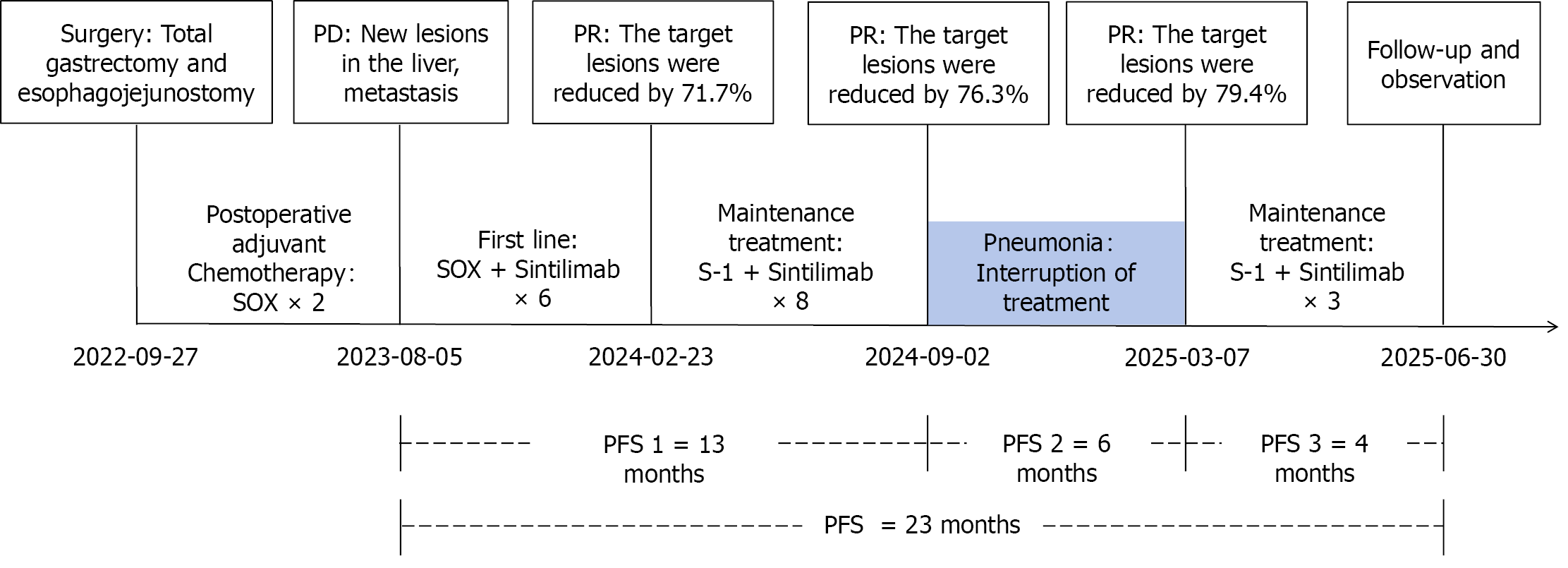
The patient is currently undergoing maintenance therapy, maintaining a good quality of life. The case timeline is presented in Figure 4.
mGC is associated with poor long-term survival due to a lack of effective systemic therapy. The CheckMate649 study was a multicenter trial that enrolled a total of 1581 patients, including 208 Chinese patients[4]. This study confirmed the efficacy of nivolumab combined with chemotherapy as a first-line treatment for mGC. However, GC is highly heterogeneous, exhibiting significant differences between Eastern and Western populations[8]. The ORIENT-16 study is the first randomized, double-blind, phase III trial conducted in China, demonstrating substantial benefits of immunotherapy combined with chemotherapy as a first-line treatment for mGC[7]. The results of this study showed that sintilimab combined with chemotherapy provided significant survival benefits, with a mOS of 15.2 months and a mPFS of 7.1 months, along with a median duration of response of nearly 10 months and a favorable safety profile. Based on these findings, the China State Food and Drug Administration approved sintilimab for first-line treatment of mGC in 2023. Accordingly, we administered sintilimab in combination with the SOX chemotherapy regimen to this patient.
After the first two cycles of first-line treatment, the index patient achieved a 46.4% reduction in target lesions from baseline at the first evaluation. This finding suggests that the SOX regimen combined with sintilimab demonstrates substantial tumor regression in a short timeframe. A phase II single-arm clinical study conducted by Li et al[9] also reported similar findings. This study assessed the efficacy and safety of the FLOT (fluorouracil plus leucovorin, oxali
The index patient not only exhibited significant tumor regression within a short period but also achieved favorable long-term efficacy. From initiation of the SOX regimen combined with sintilimab until treatment was interrupted due to pneumonia, PFS reached 13 months. After resolution of pneumonia and resumption of treatment, PFS extended to 23 months by July 2025. In contrast, large-sample randomized controlled trials have reported mPFS ranging from 6.9 months to 7.7 months, unlike the aforementioned small-sample phase II clinical studies[4-7]. The survival data for this patient exceed the median values reported in the aforementioned studies due to various factors, as discussed below.
First, the patient had an intestinal-type gastric adenocarcinoma, according to the Lauren classification. This classification, based on GC histology and morphology, has been clinically utilized for many years[11]. It categorizes GC into intestinal (50%), diffuse (30%), and mixed types (20%)[12]. The histological characteristics of intestinal type patients include a tubular glandular structure of tumor cells observable under the microscope, whereas diffuse type patients exhibit poorly differentiated tumor cells with weak cell adhesion. Patients with the intestinal type are more prone to liver metastasis and generally have a better prognosis. In contrast, those with the diffuse type are more susceptible to peri
Second, the patient had only hepatic metastases without any evidence of extrahepatic metastasis. Approximately 37% of GC patients develop liver metastasis following surgical resection, which is associated with a relatively favorable prognosis[14]. In contrast, 5% of GC patients present with peritoneal metastasis at the time of surgery, and the incidence of peritoneal metastasis after radical gastrectomy is 29%. This condition is associated with a mOS of only about 4 months, indicating a poor prognosis[15]. Furthermore, the tumor burden associated with liver metastases is also linked to prognosis. Hori et al[16] classified patients with liver metastasis from GC based on the number of liver metastases and the size of the largest diameter. The mOS was 10.2 months for patients with fewer than 4 liver metastases and a maximum diameter of less than 5 cm, compared to 3.1 months for those with 5 or more liver metastases and a maximum diameter of 5 cm or greater. In the present case, the patient had only three hepatic lesions with the maximum diameter of 5.27 cm at the baseline assessment, indicating a relatively low tumor burden and a favorable prognosis.
Moreover, the patient exhibited notable sensitivity to immunotherapy. Due to pneumonia, both immunotherapy and chemotherapy had to be suspended for a period of up to six months. However, follow-up CT scans conducted after this six-month interval revealed that the liver metastases had continued to shrink, with no new lesions detected, resulting in sustained PR. This finding suggests that once the treatment activates the body’s immune surveillance mechanism, it may function autonomously for an extended period, even after treatment cessation, thereby exerting persistent antitumor effects. Gauci et al[17] investigated the survival outcomes of 262 cancer patients following the interruption of immunotherapy. Through careful screening, 76 patients were identified as responders to immunotherapy. Among these, 39 patients had to interrupt their treatment for various reasons. Of these, 15 cases experienced disease recurrence, whereas the remaining 24 patients maintained therapeutic efficacy for at least 10 months to 20 months post-treatment discontinuation. The results indicate that, even after treatment cessation due to immune-related adverse events or in accordance with clinical study protocols, only 38% of the patients experienced disease progression, whereas 62% demonstrated sustained response. The durability of this therapeutic effect may be attributed to the capacity of immunotherapy to elicit a polyclonal and memory-adaptive antitumor immune response. This immune response can control the tumor initiation and progression, as well as kill the tumor cells, which can be maintained over the long term[18,19].
In summary, the combination of the SOX regimen with sintilimab for the treatment of mGC demonstrates favorable short-term efficacy and long-term survival. It may be related to the intestinal type, as classified by Lauren, the presence of liver-only metastasis, and notable sensitivity to immunotherapy. In clinical practice, GC patients with intestinal type and liver-only metastasis are not uncommon. Moreover, the patient demonstrated good tolerance to treatment, with no adverse reactions attributable to immunotherapy. Nevertheless, future studies with larger sample sizes are needed to validate these findings and improve survival in mGC.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12605] [Article Influence: 6302.5] [Reference Citation Analysis (6)] |
| 2. | Onoyama T, Ishikawa S, Isomoto H. Gastric cancer and genomics: review of literature. J Gastroenterol. 2022;57:505-516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 3. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5528] [Article Influence: 345.5] [Reference Citation Analysis (3)] |
| 4. | Janjigian YY, Ajani JA, Moehler M, Shen L, Garrido M, Gallardo C, Wyrwicz L, Yamaguchi K, Cleary JM, Elimova E, Karamouzis M, Bruges R, Skoczylas T, Bragagnoli A, Liu T, Tehfe M, Zander T, Kowalyszyn R, Pazo-Cid R, Schenker M, Feeny K, Wang R, Lei M, Chen C, Nathani R, Shitara K. First-Line Nivolumab Plus Chemotherapy for Advanced Gastric, Gastroesophageal Junction, and Esophageal Adenocarcinoma: 3-Year Follow-Up of the Phase III CheckMate 649 Trial. J Clin Oncol. 2024;42:2012-2020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 151] [Article Influence: 75.5] [Reference Citation Analysis (0)] |
| 5. | Rha SY, Oh DY, Yañez P, Bai Y, Ryu MH, Lee J, Rivera F, Alves GV, Garrido M, Shiu KK, Fernández MG, Li J, Lowery MA, Çil T, Cruz FM, Qin S, Luo S, Pan H, Wainberg ZA, Yin L, Bordia S, Bhagia P, Wyrwicz LS; KEYNOTE-859 investigators. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24:1181-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 416] [Article Influence: 138.7] [Reference Citation Analysis (0)] |
| 6. | Qiu MZ, Oh DY, Kato K, Arkenau T, Tabernero J, Correa MC, Zimina AV, Bai Y, Shi J, Lee KW, Wang J, Poddubskaya E, Pan H, Rha SY, Zhang R, Hirano H, Spigel D, Yamaguchi K, Chao Y, Wyrwicz L, Disel U, Cid RP, Fornaro L, Evesque L, Wang H, Xu Y, Li J, Sheng T, Yang S, Li L, Moehler M, Xu RH; RATIONALE-305 Investigators. Tislelizumab plus chemotherapy versus placebo plus chemotherapy as first line treatment for advanced gastric or gastro-oesophageal junction adenocarcinoma: RATIONALE-305 randomised, double blind, phase 3 trial. BMJ. 2024;385:e078876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 133] [Article Influence: 66.5] [Reference Citation Analysis (0)] |
| 7. | Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L, Shu Y, Li J, Zhao J, Pan H, Luo S, Qin Y, Guo Q, Bai Y, Ling Y, Yang J, Yan Z, Yang L, Tang Y, He Y, Zhang L, Liang X, Niu Z, Zhang J, Mao Y, Guo Y, Peng B, Li Z, Liu Y, Wang Y, Zhou H; ORIENT-16 Investigators. Sintilimab Plus Chemotherapy for Unresectable Gastric or Gastroesophageal Junction Cancer: The ORIENT-16 Randomized Clinical Trial. JAMA. 2023;330:2064-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 263] [Article Influence: 87.7] [Reference Citation Analysis (1)] |
| 8. | Guan WL, He Y, Xu RH. Gastric cancer treatment: recent progress and future perspectives. J Hematol Oncol. 2023;16:57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 590] [Reference Citation Analysis (5)] |
| 9. | Li N, Li Z, Fu Q, Zhang B, Zhang J, Wan XB, Lu CM, Wang JB, Deng WY, Ma YJ, Bie LY, Wang MY, Li J, Xia QX, Wei C, Luo SX. Efficacy and safety of neoadjuvant sintilimab in combination with FLOT chemotherapy in patients with HER2-negative locally advanced gastric or gastroesophageal junction adenocarcinoma: an investigator-initiated, single-arm, open-label, phase II study. Int J Surg. 2024;110:2071-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D, Wang W, Wang H, Wang H, He K, Li Z, Lu Y, Zhang J, Zhao K, Zhang Y, Xu N, Li Z, Liu Y, Wang Y, Wang Y, Teng L. Efficacy and safety of neoadjuvant sintilimab, oxaliplatin and capecitabine in patients with locally advanced, resectable gastric or gastroesophageal junction adenocarcinoma: early results of a phase 2 study. J Immunother Cancer. 2022;10:e003635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 11. | Yang S, Gu X, Tao R, Huo J, Hu Z, Sun F, Ni J, Wang X. Relationship between histological mixed-type early gastric cancer and lymph node metastasis: A systematic review and meta-analysis. PLoS One. 2022;17:e0266952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 12. | Wu LW, Jang SJ, Shapiro C, Fazlollahi L, Wang TC, Ryeom SW, Moy RH. Diffuse Gastric Cancer: A Comprehensive Review of Molecular Features and Emerging Therapeutics. Target Oncol. 2024;19:845-865. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | van der Kaaij RT, Koemans WJ, van Putten M, Snaebjornsson P, Luijten JCHBM, van Dieren JM, Cats A, Lemmens VEPP, Verhoeven RHA, van Sandick JW. A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur J Cancer. 2020;130:23-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 14. | Wang C, Zhang Y, Zhang Y, Li B. A bibliometric analysis of gastric cancer liver metastases: advances in mechanisms of occurrence and treatment options. Int J Surg. 2024;110:2288-2299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Acs M, Piso P, Glockzin G. Peritoneal Metastatic Gastric Cancer: Local Treatment Options and Recommendations. Curr Oncol. 2024;31:1445-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Hori S, Honda M, Kobayashi H, Kawamura H, Takiguchi K, Muto A, Yamazaki S, Teranishi Y, Shiraso S, Kono K, Kamiga T, Iwao T, Yamashita N. A grading system for predicting the prognosis of gastric cancer with liver metastasis. Jpn J Clin Oncol. 2021;51:1601-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 17. | Gauci ML, Lanoy E, Champiat S, Caramella C, Ammari S, Aspeslagh S, Varga A, Baldini C, Bahleda R, Gazzah A, Michot JM, Postel-Vinay S, Angevin E, Ribrag V, Hollebecque A, Soria JC, Robert C, Massard C, Marabelle A. Long-Term Survival in Patients Responding to Anti-PD-1/PD-L1 Therapy and Disease Outcome upon Treatment Discontinuation. Clin Cancer Res. 2019;25:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 109] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, Hruban C, Guthrie VB, Rodgers K, Naidoo J, Kang H, Sharfman W, Georgiades C, Verde F, Illei P, Li QK, Gabrielson E, Brock MV, Zahnow CA, Baylin SB, Scharpf RB, Brahmer JR, Karchin R, Pardoll DM, Velculescu VE. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017;7:264-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 631] [Cited by in RCA: 761] [Article Influence: 84.6] [Reference Citation Analysis (0)] |
| 19. | Alsaed B, Bobik N, Laitinen H, Nandikonda T, Ilonen I, Haikala HM. Shaping the battlefield: EGFR and KRAS tumor mutations' role on the immune microenvironment and immunotherapy responses in lung cancer. Cancer Metastasis Rev. 2025;44:56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/