Published online Dec 15, 2025. doi: 10.4251/wjgo.v17.i12.105800
Revised: May 16, 2025
Accepted: November 7, 2025
Published online: December 15, 2025
Processing time: 302 Days and 15.7 Hours
The incidence of early-onset colorectal cancer (EOCRC, < 45 years of age at onset) is on the rise among adults, including African Americans (AA).
To examine differences between EOCRC and late-onset colorectal cancer (LOCRC) among AA patients and any effect during coronavirus disease (COVID) by comparing data during pre-COVID (2015-2019) and the COVID era (2020-2023).
We conducted a retrospective review of Howard University Hospital records from 2015 to 2023 for colorectal cancer patients that included demographics, clinicals, pathology, and colonoscopy records. A three-year interval analysis was performed to compare post-COVID era (2020-2023) to preceding years to discern temporal trends.
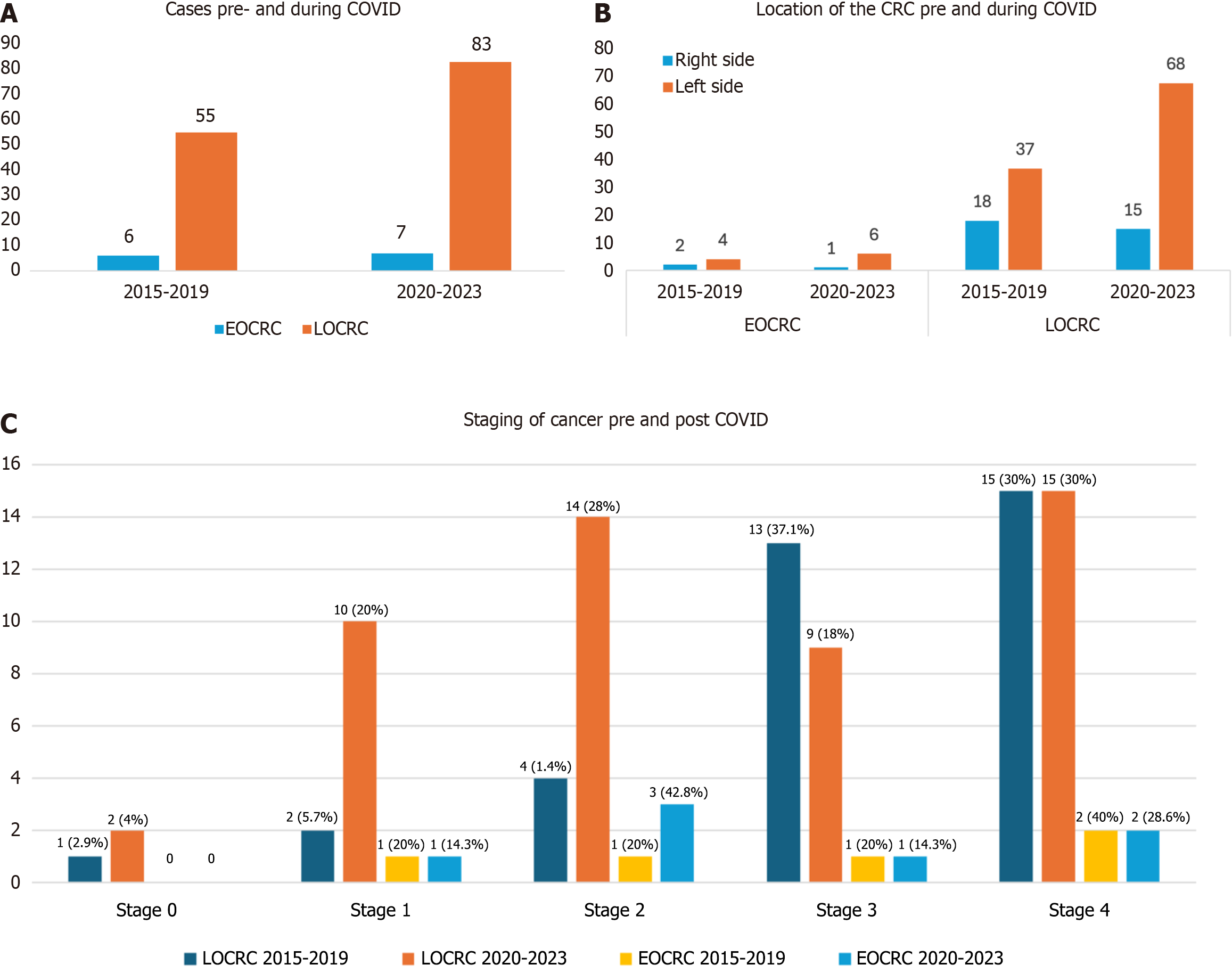
The study included 138 LOCRC and 13 EOCRC cases of which > 80% of patients were AA. Compared to pre-COVID, LOCRC cases increased in number from 55 to 83, and EOCRC cases increased from 6 to 7 during COVID. There was no change in mean age for LOCRC (64.7 years vs 65.3 years) but mean age increased for EOCRC (37.3 years vs 41.5 years). Males predominated in both groups particularly during the pandemic. More than 65% of LOCRC patient colonoscopies were for diagnostic purposes. Gastrointestinal bleeding as a colonoscopy indication and reduced bowel preparation quality were increased during the pandemic. EOCRC patients showed a shift from stage 4 (49.2%) to stage 2 (30%) and LOCRC patients staging trends changed from stage 4 (40%) to stage 3 (28.6%).
We report increase in colorectal cancer cases during the COVID-19 era, especially among young AA males. EOCRC and LOCRC patients showed distal location predominance, most commonly in recto-sigmoid region. The decrease in staging or metastasis, which might be due to growing awareness and earlier detection among patients.
Core Tip: The incidence of early-onset colorectal cancer (CRC) is rising, especially in African Americans, who face higher CRC rates and mortality. The coronavirus disease 2019 pandemic disrupted screenings, leading to increased early-onset colorectal cancer and late-onset CRC cases. This study highlights how the pandemic worsened racial disparities in CRC outcomes, emphasizing the need for targeted public health interventions. Lower metastasis rates may reflect early detection or stage 4 patients avoiding care and dying outside hospitals. Addressing these inequities is crucial to improving CRC outcomes, particularly among African Americans disproportionately affected by delayed screenings and healthcare disruptions during the pandemic.
- Citation: Chirumamilla LG, Brim H, Challa SR, Oskrochi G, Deverapalli M, Rashid R, Rashid M, Aduli F, Kibreab A, Laiyemo A, Sherif ZA, Shayegh N, Shokrani B, Zafar R, Carethers JM, Ashktorab H. Early and late-onset colorectal cancer in African Americans during COVID-19. World J Gastrointest Oncol 2025; 17(12): 105800
- URL: https://www.wjgnet.com/1948-5204/full/v17/i12/105800.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i12.105800
In the late 1990s, colorectal cancer (CRC) ranked fourth among causes of cancer deaths for both men and women. In 2024, it is the primary cause of cancer-related deaths among men and the second leading cause among women[1]. In recent decades, there has been a notable rise in CRC cases among individuals under 50 years old, commonly termed early-onset CRC (EOCRC)[2]. According to the American Cancer Society’s projections for 2024, approximately 152810 new cases of CRC are expected in the United States, with men accounting for 81540 cases and women for 71270 cases. From 2011 to 2019, incidence rates dropped by about 1% annually, mainly among older adults, and has been attributed to screening[1,3,4]. However, since the 1990s, the age-adjusted incidence EOCRC has been rising alarmingly by 2% to 4% per year in many countries, with even sharper increases among those younger than 30[1,5-9]. According to an analysis of Sur
The distribution of CRC is uneven across United States subpopulations, with significant differences in incidence, cancer stage, and mortality by race and ethnicity[10]. Since before the early 1990s until 2023, African American (AA) individuals have experienced higher CRC incidence and mortality rates than any other racial or ethnic group[10]. Based on findings from the Surveillance, Epidemiology, and End Results program, the overall incidence of CRC among Black Americans is 41.9 per 100000, compared to 37.0 per 100000 for White Americans[10-13]. The coronavirus disease 2019 (COVID-19) pandemic had far-reaching effects, including a 90% drop in colonoscopies and biopsies by mid-April 2020 due to delayed non-urgent procedures like CRC screenings[14]. This could lead to an estimated 18800 missed CRC diagnoses and 1.7 million missed colonoscopies, likely resulting in increased advanced CRC cases and deaths in the United States[11,13]. Treatment of CRC depends on the stage and specific tumor features. Common chemotherapy regimens include 5-fluorouracil/Leucovorin with either oxaliplatin or irinotecan, often combined with targeted drugs like bevacizumab or cetuximab for advanced disease[14,15]. Immunotherapy with drugs such as pembrolizumab is effective in tumors with high microsatellite instability[16]. Newer treatments also target specific mutations like BRAF and KRAS[17].
The incidence of EOCRC is rising among AA, who tend to have more advanced and metastatic tumors compared to those with late-onset CRC (LOCRC). AA are disproportionately affected by cancer overall, facing significant challenges in prevention, detection, treatment, and survival due to complex systemic racial disparities[10]. The COVID-19 pandemic has further exacerbated these disparities by causing screening delays[12,13]. In this study, our aim focused on understanding the emerging trends in EOCRC and overall CRC presentation in the COVID era.
In this single-center retrospective cohort study conducted at Howard University Hospital (HUH), a historically Black inner-city hospital located in Washington, DC, we utilized de-identified retrospective 151 unique medical record data for histopathologic-confirmed CRCs cases. The data were collected from HUH’s Electronic Medical Record (EMR) Database for the period from January 1, 2015 to December 31, 2023. Histologically confirmed cases were classified by the HUH Pathology Department using the International Classification of Diseases, Ninth Edition codes. Colonoscopy reports were verified through the hospital’s EMRs system. The use of de-identified EMRs and pathology reports of patients was approved Medical Ethics Committee of Howard University Internal Review Board, approval No. IRB-12-CMED-76.
Patient data were retrospectively collected from EMRs. The patient data included demographics, body mass index (BMI), comorbidities, and risk factors for CRC. Clinical characteristics encompassed the symptoms at presentation, indication and findings for colonoscopy, laboratory values and pathology results of biopsied samples. All patient data were coded in an Excel file for statistical analysis. Data and variables were streamlined and homogenized during data collection and database construction.
Inclusion criteria encompassed patients with histopathologic confirmed colorectal carcinomas diagnosed through colonoscopy, flexible sigmoidoscopy, or surgical biopsies. The included subtypes of colorectal carcinomas were primary adenocarcinoma, squamous cell carcinoma, neuroendocrine tumors and lymphomas, sarcomas were categorized as others. Patients aged 45 years or older were assigned to the LOCRC group, while those aged 44 or younger were assigned to the EOCRC group. Our cohort consisted of 13 EOCRC individuals and 138 LOCRC individuals. We excluded cases with a previous history of adenocarcinoma, those currently admitted for other reasons, and CRC cases diagnosed at other hospitals without available reports.
Statistical analysis of the trend for EOCRC and LOCRC was conducted for 2015-2023. A three-year interval analysis was performed, with a special focus on the COVID-19 period (2020-2023) for a comparison with the preceding years (2015-2019) to discern temporal trends. Significance of differences in trends of CRC by demographic and clinicopathological features were evaluated using two-sample tests of proportions and the χ2 test for variables with more than two categories. P values less than 0.05 were considered significant.
We identified a total of 151 CRC patients, categorized into LOCRC and EOCRC cases. Among these 138 patients were diagnosed with LOCRC, with 55 patients diagnosed pre-COVID (2015-2019) and 83 diagnosed during the COVID-19 pandemic (2020-2023). Additionally, 13 patients were diagnosed with EOCRC with 6 patients diagnosed pre-COVID and 7 patients diagnosed during the COVID-19 pandemic (Figure 1A). The observed increase in number especially for the LOCRC cases during the COVID period suggests a potential impact of the pandemic on the diagnosis or reporting of CRC cases. The mean age of LOCRC patients pre-COVID was 64.76 years while during COVID it was 65.3 years, showing no significant difference. For EOCRC patients, the mean age pre-COVID was 37.33 years and during COVID, it was 41.57 years (Tables 1 and 2). In terms of gender distribution 56.4% of LOCRC patients were male pre-COVID compared to 67.5% during COVID (P = 0.186). Similarly, 66.7% of EOCRC patients were male pre-COVID compared to 100% during COVID (P = 0.097) (Tables 1 and 2).
| Characteristics | LOCRC | P value | |
| 2015-2019 (n = 55) | 2020-2023 (n = 83) | ||
| Mean age | 64.76 | 65.3 | - |
| Male | 31 (56.4) | 56 (67.5) | 0.186 |
| Female | 24 (43.6) | 27 (32.5) | - |
| Race | 0.146 | ||
| African Americans | 50 (90.9) | 68 (82.9) | |
| White | 1 (1.8) | 5 (6.1) | |
| Asian | 1 (1.8) | 4 (4.9) | |
| Hispanic | 2 (3.6) | 0 | |
| Others | 1 (1.8) | 5 (6.1) | |
| BMI (kg/m2) | 0.877 | ||
| Underweight < 18.9 | 5 (9.3) | 4 (4.9) | |
| Normal (19-24.9) | 19 (35.2) | 33 (40.7) | |
| Overweight (25-29.9) | 15 (27.9) | 20 (24.7) | |
| Obese (30-34.9) | 15 (27.9) | 24 (29.6) | |
| Smoking | 22 (40.7) | 37 (44.6) | 0.658 |
| Alcohol | 28(33.7) | 22 (40.7) | 0.778 |
| Unemployed | 31 (57.4) | 53 (69.7) | 0.033 |
| Comorbidities | |||
| Hypertension | 37 (68.5) | 49 (59.8) | 0.3 |
| Diabetes | 18 (33.3) | 18 (22) | 1.44 |
| Heart diseases | 6 (11.1) | 13 (15.7) | 0.45 |
| IBD | 0 | 1 (1.2) | 0.42 |
| CKD | 8 (15.1) | 7 (8.4) | 0.23 |
| Ongoing radiation treatment | 7 (13.5) | 14 (17.1) | 0.57 |
| Cholecystectomy | 3 (5.6) | 5 (6.1) | 0.89 |
| Indication for colonoscopy | 0.53 | ||
| Screening | 4 (7.3) | 10 (12) | |
| Surveillance | 9 (16.4) | 19 (22.9) | |
| Diagnostic | 42 (76.4) | 54 (65) | |
| Diagnostic indication | |||
| GI bleeding | 8 (32) | 13 (39.4) | 0.54 |
| Change in bowel habits | 0 | 1 (6.1) | 0.48 |
| Abnormal imaging | 7 (28) | 8 (24.2) | 0.79 |
| Polyp location | |||
| Cecum | 6 (10.9) | 10 (12) | 0.84 |
| Ascending colon | 9 (16.3) | 13 (15.7) | 0.46 |
| Transverse colon | 10 (17.9) | 14 (17) | 0.21 |
| Descending colon | 7 (12.7) | 12 (14.5) | 0.77 |
| Sigmoid colon | 5 (9.1) | 13 (15.7) | 0.26 |
| Rectum | 11 (20) | 17 (20.5) | 0.71 |
| Size of the mass | 0.52 | ||
| < 10 mm | 18 (66.7) | 31 (73.8) | |
| > 10 mm | 9 (33.3) | 11 (26.2) | |
| Colonoscopy withdrawal time (in minutes) | 0.17 | ||
| 6:00-10:00 | 5 (27.8) | 5 (8.2) | |
| 10.01-15:00 | 3 (16.7) | 7 (11.5) | |
| 15.01-20:00 | 4 (22.2) | 16 (26.2) | |
| 20.01-25:00 | 1 (5.6) | 16 (26.2) | |
| 25.01-30:00 | 1 (5.6) | 6 (9.8) | |
| > 30 | 4 (22.2) | 11 (18) | |
| Quality of bowel preparation | 0.61 | ||
| Excellent/good | 43 (95.6) | 53 (80.3) | |
| Adequate | 2 (4.4) | 10 (15.2) | |
| Poor | 0 | 3 (2.7) | |
| Type of associated polyp | |||
| Hyperplastic | 8 (25) | 12 (26.1) | 0.91 |
| Tubular adenoma | 16 (50%) | 29 (64.4) | 0.21 |
| Tubulo-villous | 2 (6.1) | 7 (15.2) | 0.206 |
| Villous | 1 (3.1) | 0 | 0.233 |
| Sessile serrated | 5 (15.2) | 0 | 0.07 |
| Type of carcinoma | 0.08 | ||
| Adenocarcinoma | 38 (80.9) | 51 (76.1) | |
| Small cell carcinoma | 1 (2.1) | 7 (10.4) | |
| Neuroendocrine tumor | 7 (14.9) | 4 (6) | |
| Previous colonoscopy | 10 (18.2) | 23 (27.7) | |
| Metastasis | 24 (55.8) | 26 (38.2) | 0.07 |
| Characteristics | EOCRC | P value | |
| 2015-2019 (n = 6) | 2020-2023 (n = 7) | ||
| Mean age | 37.33 | 41.57 | - |
| Male | 4 (66.7) | 7 (100) | 0.097 |
| Female | 2 (33.3) | 0 | - |
| Race | |||
| African Americans | 6 (100) | 7 (100) | - |
| White | 0 | 0 | - |
| Asian | 0 | 0 | - |
| Hispanic | 0 | 0 | - |
| Others | 0 | 0 | - |
| BMI (kg/m2) | 0.37 | ||
| Underweight < 18.9 | 1 (25) | 0 | |
| Normal (19-24.9) | 1 (25) | 1 (14.3) | |
| Overweight (25-29.9) | 0 | 1 (14.3) | |
| Obese (30-34.9) | 2 (50) | 5 (71.4) | |
| Smoking | 2 (50) | 5 (83.3) | 0.26 |
| Alcohol | 2 (50) | 4 (66.7) | 0.58 |
| Unemployed | 2 (33.3) | 1 (14.3) | 0.42 |
| Comorbidities | |||
| Hypertension | 0 | 1 (14.3) | 0.38 |
| Indication for colonoscopy | 0.91 | ||
| Surveillance | 1 (16.7) | 1 (14.3) | |
| Diagnostic | 5 (83.3) | 6 (85.7) | |
| Diagnostic indication | |||
| GI bleeding | 2(33.3) | 4(57.4) | 0.69 |
| Change in bowel habits | 1 (16.7) | 3 (42.8) | 0.047 |
| Abnormal imaging | 2 (33.3) | 1 (14.3) | 0.86 |
| Polyp location | |||
| Cecum | 1 (16.7) | 0 | 0.26 |
| Ascending colon | 1 (16.7) | 1 (14.3) | 0.91 |
| Transverse colon | 1 (16.7) | 1 (14.3) | 0.91 |
| Descending colon | 1 (16.7) | 0 | 0.26 |
| Sigmoid colon | 0 | 0 | - |
| Rectum | 1 (16.7) | 1 (14.3) | 0.91 |
| Size of the mass | 0.03 | ||
| < 10 mm | 4 (66.7) | 6 (85.7) | |
| > 10 mm | 2 (33.3) | 1 (14.3) | |
| Colonoscopy withdrawal time (in minutes) | 0.601 | ||
| 6:00-10:00 | 1 (33.3) | 0 | |
| 10.01-15:00 | 0 | 1 (14.3) | |
| 15.01-20:00 | 1 (33.3) | 2 (28.6) | |
| 20.01-25:00 | 1 (33.3) | 2 (28.6) | |
| 25.1-30:00 | 0 | 1 (14.3) | |
| > 30 | 0 | 1 (14.3) | |
| Quality of bowel preparation | 0.33 | ||
| Excellent/good | 6 (100) | 6 (85.7) | |
| Adequate | 0 | 0 | |
| Poor | 0 | 1 (14.3) | |
| Type of associated polyp | |||
| Hyperplastic | 1(25) | 1(100) | 0.17 |
| Tubular adenoma | 3 (75) | 1 (100) | 0.58 |
| Tubulo-villous | 0 | 0 | |
| Villous | 0 | 0 | |
| Sessile serrated | 0 | 0 | |
| Type of carcinoma | |||
| Adenocarcinoma | 4 (66.7) | 7 (100) | 0.19 |
| Small cell carcinoma | 1 (16.7) | 0 | |
| Neuroendocrine tumor | 1 (16.7) | 0 | |
| Others | 0 | 0 | |
| Previous colonoscopy | 1 (16.7) | 0 | |
| Metastasis | 2 (33.3) | 1 (14.3) | 0.414 |
Racial distribution for LOCRC showed high percentages of AA in both groups, with 90.9% pre-COVID and 82.9% during COVID. The percentage of White patients increased from 1.8% pre-COVID to 6.1% during COVID with other races including Asian and Hispanic showing variations (P = 0.146). All EOCRC patients in both groups were AA with no representation from other racial groups (Tables 1 and 2). This racial distribution could be due to our hospital predominantly serving an AA population. A significant increase in unemployment was observed during COVID (69.7%) compared to pre-COVID (57.4%; P = 0.033) for LOCRC patients. However, unemployment was lower for EOCRC patients during COVID (14.3%) compared to pre-COVID (33.3%, P = 0.42) (Tables 1 and 2).
The distribution of BMI categories did not show significant differences between the groups (P = 0.877) in LOCRC. However, among EOCRC patients, there was an increase in the percentage of overweight and obesity during COVID compared to pre-COVID (50% vs 83%; P = 0.37). During COVID, smoking prevalence increased in both LOCRC (44.6% vs 40.7% pre-COVID) and EOCRC patients (83.3% vs 50% pre-COVID), while alcohol consumption remained similar between the groups for both cancer types. In LOCRC, hypertension was more common pre-COVID (68.5%) than during COVID (59.8%; P = 0.3). The prevalence of diabetes, heart disease, and chronic kidney disease showed non-significant variations, while there was a slight increase in ongoing radiation treatment during COVID (17.1% vs 13.5% pre-COVID). In the EOCRC group, hypertension was present in 14.3% of patients during COVID, with no such cases pre-COVID.
In both LOCRC and EOCRC, colonoscopy was primarily for diagnostic and not screening purposes, with LOCRC showing 76.4% diagnostic procedures pre-COVID and 65% during COVID, and EOCRC showing 83.3% diagnostic procedures pre-COVID and 85.7% during COVID. Screening and surveillance colonoscopies showed minor variations, with no screening procedures for EOCRC patients as would be expected. Gastrointestinal (GI) bleeding was the most common indication for diagnostic colonoscopy in both groups with increased presentation frequency during COVID compared to pre-COVID (LOCRC: 39.4% vs 32%; EOCRC: 57.4% vs 33.3%). In EOCRC patients, altered bowel habits were significantly increased from pre-COVID to COVID (16.7% vs 42.8, P = 0.047). The quality of bowel preparation for colonoscopy decreased during COVID compared to pre-COVID in both LOCRC and EOCRC patients. In LOCRC, 95.6% had excellent preparation pre-COVID, which dropped to 80% during COVID. Similarly, in EOCRC, 100% had excellent preparation pre-COVID, decreasing to 85.7% during COVID. The colonoscopy withdrawal times did not show significant differences between the groups in pre and during COVID time (LOCRC: P = 0.17, EOCRC: P = 0.67).
Polyp location did not show significant differences between EOCRC and LOCRC groups. However, in EOCRC patients, a larger proportion of cancer masses were < 10 mm during COVID (85.7%) compared to pre-COVID (66.7%) (P = 0.03). In contrast, no significant difference in mass size was noted between the two periods for LOCRC patients. In LOCRC patients there were variations in the types of associated polyps and carcinoma with a notable but not statistically significant increase in tubular adenomas and small cell carcinomas during COVID. Metastasis was slightly higher pre-COVID (55.8%) compared to during COVID (38.2%), approaching statistical significance (P = 0.07). In EOCRC patients, all patients during COVID had adenocarcinoma, compared to 66.7% pre-COVID. Metastasis was slightly higher pre-COVID (33.3%) compared to during COVID (14.3%; P = 0.414). For both EOCRC (66.6% vs 87.5%) and LOCRC (67.2% vs 81.9%) there was an increase in the number of left-sided CRC cases during the COVID period compared to the pre-COVID period (Figure 1B). For LOCRC, the proportion of stage 4 cases decreased modestly from 42.9% before COVID to 30% during the pandemic. Similarly, EOCRC Stage 4 cases dropped from 40% pre-COVID to 28.6% during the COVID period (Figure 1C).
In this study involving a single tertiary hospital with a high percentage AA population, we identified several effects that the COVID-19 pandemic had on CRC diagnosis. We noted increase in numbers of both LOCRC and EOCRC cases during the pandemic compared to the years before. This rise could be due to several factors, such as disruptions in routine healthcare services and delay in cancer screenings[12,13]. The significant drop-in colonoscopy screening rates during the pandemic, as reported in previous studies, likely contributed to delayed diagnoses and potentially more advanced disease at presentation. This is reflected in the increased incidence of LOCRC and EOCRC observed in our study during the pandemic period[18,19], and the high level of diagnostic colonoscopies vs screening procedures. Our study highlights age-related differences between LOCRC and EOCRC patients. The mean age for LOCRC remained stable pre-COVID and during the pandemic, while EOCRC patients saw an increase from 37.33 years to 41.57 years. This rise may reflect diagnostic delays or demographic shifts during the pandemic coupled with the lack of screening for EOCRC-aged patients. These findings underscore the need for tailored screening programs and adaptable healthcare strategies to ensure timely detection and treatment for both age groups, even during public health crises.
Our study highlights significant racial disparities in CRC incidence, with AA disproportionately affected. This mirrors national data showing higher CRC incidence and mortality rates among Black individuals compared to other racial groups[10,11,20]. By focusing on a predominantly Black inner-city population, our study underscores the urgent need for targeted public health strategies to address these disparities. Recent studies suggest that tumors in AA may have different molecular profiles compared to those in Whites, indicating that the pathways leading to cancer development and progression might vary by race[21,22]. Additionally, consequentially, variations in the gut microbiome have been increasingly linked to the rising incidence of EOCRC and may also contribute to the higher CRC rates observed in AA[7,10]. We observed that all EOCRC patients were AA, indicating a perceived higher prevalence among this group. Gender differences were also evident, with more males affected by both EOCRC and LOCRC similar to previous studies[11]. During the COVID-19 pandemic, the proportion of male EOCRC patients increased from 66.7% pre-COVID to 100%, suggesting potential differences in risk factors that might be attributed to lifestyle changes due to people staying more at home, such as increased alcohol consumption, smoking, and obesity, all known contributors to higher CRC risk[23], however, small samples size should be considered. Secondly, research says estrogen may offer a protective effect against CRC, which could partly explain lower incidence rates in premenopausal women[24,25].
In pre-pandemic and during COVID, the colonoscopies in both EOCRC and LOCRC patients were largely diagnostic, with GI bleeding being a common reason. During the pandemic, there was a significant increase in GI bleeding as an indication, likely because patients delayed seeking care. Among AA, screening colonoscopies decreased notably during the pandemic. This reduction is linked to social factors such as healthcare disparities and lack of health insurance as well as the shutdown of preventive care that may have not fully recovered to date compared to pre-pandemic levels. AA are disproportionately affected by these issues, which restrict access to preventive services like CRC screening[10,13,26,27]. The pandemic’s economic impact exacerbated these challenges, with many losing jobs and health insurance. Additional barriers like limited access to healthcare facilities, transportation issues, and distrust in the medical system further lowered screening rates. Addressing these disparities requires targeted public health interventions and policies to improve healthcare access and equity for underserved communities particularly to not only recover screening rates that dropped during the pandemic, but to increase screening rates beyond those numbers. Many alternatives exist to potentially increase screening rates in the AA population[3,4].
The quality of bowel preparation for colonoscopy decreased significantly during the pandemic for both EOCRC and LOCRC patients. This decrease in bowel preparation quality is consistent with other studies that have noted similar trends due to disruptions in routine medical care and patient reluctance to seek timely medical attention during the pandemic[28]. Efforts to improve patient preparation for colonoscopy and ensure high-quality diagnostic procedures are essential, particularly amidst ongoing public health challenges.
Our study provides valuable insights into the histopathological findings and staging of CRC in EOCRC and LOCRC cases before and during the COVID-19 pandemic. Our study found that left-sided CRC was more common than right-sided CRC in both EOCRC and LOCRC cases, both pre- and during the COVID-19 pandemic, with a higher number of left-sided cases during the pandemic. Left-sided CRC is generally more prevalent than right-sided CRC across various populations and studies[29,30]. For EOCRCs their location is predominantly left-sided, particularly rectosigmoid[7]. These cancers often present symptoms earlier due to the left-sided location, prompting timely medical evaluation and intervention. We observed a metastasis rate in both EOCRC and LOCRC that was slightly higher pre-COVID compared to during COVID (P > 0.05)[29-31]. EOCRC and LOCRC patients showed a shift from stage 4 to stage 2 and stage 3 respectively. The lower metastasis rates during the pandemic could be attributed to increased awareness and earlier detection among patients, or alternatively, stage 4 patients did not present and may have died outside of the hospital due to pandemic conditions.
Our study is the first to analyze CRC cases before and during the COVID-19 pandemic, providing unique insights into its impact on CRC diagnosis and management. It focuses on a predominantly AA population. Using histopathologically confirmed cases ensures data accuracy and reliability. Covering a significant period, the study offers a comprehensive examination of temporal trends. Detailed demographic, clinical, and pathological data collection enhances our understanding of CRC in different age groups, making our findings highly relevant for future research and public health interventions.
This study has some limitations. First, the relatively small sample size may limit the generalizability of our findings, as larger cohorts might provide more robust data. Additionally, this was a single-center study conducted in a historically Black inner-city tertiary hospital, which may not fully represent the broader population. Furthermore, the impact of the COVID-19 pandemic on healthcare access and diagnostic practices may have influenced our results, and these factors should be considered when interpreting the findings. Future research with larger, multi-center cohorts and prospective designs will be essential to validate and expand upon these results.
Future efforts should focus on developing effective CRC screening programs for younger populations and high-risk groups, especially AA males. Public health campaigns need to raise awareness about CRC risk factors, early detection, and lifestyle changes. Improving healthcare access and reducing disparities in CRC outcomes, particularly in underserved and minority communities, is crucial. Additionally, ongoing research into the biological mechanisms of early-onset CRC is essential to inform personalized treatments and improve patient outcomes.
In conclusion, our findings underscore the urgent need for targeted interventions and research to address the rising incidence of EOCRC. Notably, incidence rates among individuals under 50 have increased by approximately 2.4% annually from 2012 to 2021, while mortality rates have risen by about 1% per year in this age group[31]. These trends may have been further exacerbated by delays in screening and care during the COVID-19 pandemic. By prioritizing equitable healthcare access, implementing evidence-based screening programs starting at age 45, and strengthening public health initiatives, we can work toward reducing the burden of CRC and improving outcomes for all patients especially those disproportionately affected.
| 1. | Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2279] [Cited by in RCA: 6057] [Article Influence: 3028.5] [Reference Citation Analysis (4)] |
| 2. | Ullah F, Pillai AB, Omar N, Dima D, Harichand S. Early-Onset Colorectal Cancer: Current Insights. Cancers (Basel). 2023;15:3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 3. | Carethers JM. Commencing colorectal cancer screening at age 45 years in U.S. racial groups. Front Oncol. 2022;12:966998. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 4. | Carethers JM. Improving Noninvasive Colorectal Cancer Screening. N Engl J Med. 2024;390:1045-1046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Spaander MCW, Zauber AG, Syngal S, Blaser MJ, Sung JJ, You YN, Kuipers EJ. Young-onset colorectal cancer. Nat Rev Dis Primers. 2023;9:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 172] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 6. | Potter JD. Rising rates of colorectal cancer in younger adults. BMJ. 2019;365:l4280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Venugopal A, Carethers JM. Epidemiology and biology of early onset colorectal cancer. EXCLI J. 2022;21:162-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 8. | Ashktorab H, Vilmenay K, Brim H, Laiyemo AO, Kibreab A, Nouraie M. Colorectal Cancer in Young African Americans: Is It Time to Revisit Guidelines and Prevention? Dig Dis Sci. 2016;61:3026-3030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Carethers JM. The Increasing Incidence of Colorectal Cancers Diagnosed in Subjects Under Age 50 Among Races: CRaCking the Conundrum. Dig Dis Sci. 2016;61:2767-2769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Carethers JM. Racial and ethnic disparities in colorectal cancer incidence and mortality. Adv Cancer Res. 2021;151:197-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 11. | Ashktorab H, Kupfer SS, Brim H, Carethers JM. Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology. 2017;153:910-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 209] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 12. | National Institutes of Health. Cancer Stat Facts: Colorectal Cancer. [cited 11 February 2025]. Available from: https://seer.cancer.gov/statfacts/html/colorect.html. |
| 13. | van den Puttelaar R, Lansdorp-Vogelaar I, Hahn AI, Rutter CM, Levin TR, Zauber AG, Meester RGS. Impact and Recovery from COVID-19-Related Disruptions in Colorectal Cancer Screening and Care in the US: A Scenario Analysis. Cancer Epidemiol Biomarkers Prev. 2023;32:22-29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 14. | Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, Aranda Aguilar E, Bardelli A, Benson A, Bodoky G, Ciardiello F, D'Hoore A, Diaz-Rubio E, Douillard JY, Ducreux M, Falcone A, Grothey A, Gruenberger T, Haustermans K, Heinemann V, Hoff P, Köhne CH, Labianca R, Laurent-Puig P, Ma B, Maughan T, Muro K, Normanno N, Österlund P, Oyen WJ, Papamichael D, Pentheroudakis G, Pfeiffer P, Price TJ, Punt C, Ricke J, Roth A, Salazar R, Scheithauer W, Schmoll HJ, Tabernero J, Taïeb J, Tejpar S, Wasan H, Yoshino T, Zaanan A, Arnold D. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27:1386-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2286] [Cited by in RCA: 2539] [Article Influence: 253.9] [Reference Citation Analysis (41)] |
| 15. | Neugut AI, Lin A, Raab GT, Hillyer GC, Keller D, O'Neil DS, Accordino MK, Kiran RP, Wright J, Hershman DL. FOLFOX and FOLFIRI Use in Stage IV Colon Cancer: Analysis of SEER-Medicare Data. Clin Colorectal Cancer. 2019;18:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 16. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7494] [Article Influence: 681.3] [Reference Citation Analysis (2)] |
| 17. | Kopetz S, Grothey A, Yaeger R, Van Cutsem E, Desai J, Yoshino T, Wasan H, Ciardiello F, Loupakis F, Hong YS, Steeghs N, Guren TK, Arkenau HT, Garcia-Alfonso P, Pfeiffer P, Orlov S, Lonardi S, Elez E, Kim TW, Schellens JHM, Guo C, Krishnan A, Dekervel J, Morris V, Calvo Ferrandiz A, Tarpgaard LS, Braun M, Gollerkeri A, Keir C, Maharry K, Pickard M, Christy-Bittel J, Anderson L, Sandor V, Tabernero J. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N Engl J Med. 2019;381:1632-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 1060] [Article Influence: 151.4] [Reference Citation Analysis (5)] |
| 18. | Carethers JM, Sengupta R, Blakey R, Ribas A, D'Souza G. Disparities in Cancer Prevention in the COVID-19 Era. Cancer Prev Res (Phila). 2020;13:893-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Mazidimoradi A, Hadavandsiri F, Momenimovahed Z, Salehiniya H. Impact of the COVID-19 Pandemic on Colorectal Cancer Diagnosis and Treatment: a Systematic Review. J Gastrointest Cancer. 2023;54:171-187. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 20. | Aguiar S, Riechelmann RP, de Mello CAL, da Silva JCF, Diogenes IDC, Andrade MS, de Miranda Marques TMD, Stevanato PR, Bezerra TS, Silva MLG, Lopes A, Curado MP. Impact of COVID-19 on colorectal cancer presentation. Br J Surg. 2021;108:e81-e82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 21. | Augustus GJ, Ellis NA. Colorectal Cancer Disparity in African Americans: Risk Factors and Carcinogenic Mechanisms. Am J Pathol. 2018;188:291-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 134] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 22. | Staudacher JJ, Yazici C, Bul V, Zeidan J, Khalid A, Xia Y, Krett N, Jung B. Increased Frequency of KRAS Mutations in African Americans Compared with Caucasians in Sporadic Colorectal Cancer. Clin Transl Gastroenterol. 2017;8:e124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 23. | Yazici C, Wolf PG, Kim H, Cross TL, Vermillion K, Carroll T, Augustus GJ, Mutlu E, Tussing-Humphreys L, Braunschweig C, Xicola RM, Jung B, Llor X, Ellis NA, Gaskins HR. Race-dependent association of sulfidogenic bacteria with colorectal cancer. Gut. 2017;66:1983-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 24. | Das PK, Saha J, Pillai S, Lam AK, Gopalan V, Islam F. Implications of estrogen and its receptors in colorectal carcinoma. Cancer Med. 2023;12:4367-4379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 25. | Barzi A, Lenz AM, Labonte MJ, Lenz HJ. Molecular pathways: Estrogen pathway in colorectal cancer. Clin Cancer Res. 2013;19:5842-5848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 26. | Bromley EG, May FP, Federer L, Spiegel BM, van Oijen MG. Explaining persistent under-use of colonoscopic cancer screening in African Americans: a systematic review. Prev Med. 2015;71:40-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 27. | Shi L, Lebrun LA, Zhu J, Tsai J. Cancer screening among racial/ethnic and insurance groups in the United States: a comparison of disparities in 2000 and 2008. J Health Care Poor Underserved. 2011;22:945-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 65] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Ren B, Yang Y, Lv Y, Liu K. Survival outcome and prognostic factors for early-onset and late-onset metastatic colorectal cancer: a population based study from SEER database. Sci Rep. 2024;14:4377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 29. | Chang SH, Patel N, Du M, Liang PS. Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database. Clin Gastroenterol Hepatol. 2022;20:e1365-e1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Ra H, Jeong S, Lee H, Chung JW, Kim KO, Lee WS, Kim J, Kwon KA, Kim JH. Clinicopathological Differences between Right and Left Colorectal Cancer by Sex. J Clin Med. 2024;13:2810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 31. | American Cancer Society. Colorectal Cancer Facts & Figures 2023-2025. 2023. [cited 11 February 2025]. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2023.pdf. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/