Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.113431
Revised: September 14, 2025
Accepted: October 21, 2025
Published online: November 15, 2025
Processing time: 80 Days and 20.9 Hours
Early metastasis and recurrence are risk factors that negatively affect the prog
To detect folate receptor (FR)-positive circulating tumor cells (CTCs) and explore their role in the diagnosis and staging of HCC.
This work is a retrospective study that included 128 consecutive patients with benign or malignant disease of the liver from 2020 to 2021. FR + CTCs were col
The FR + CTCs counts showed excellent diagnostic efficacy in patients with HCC, with high sensitivity (0.905) and specificity (0.773) compared with patients with benign disease. Compared with that of the AFP level, the area under the receiver operating characteristic curve of the FR + CTC count is significantly greater (0.900 compared with 0.730, P < 0.05). FR + CTC levels were significantly correlated with macrovascular invasion, tumor size, tumor number, and extrahepatic tumor stage in HCC patients. FR + CTC counts were correlated with DFS in HCC patients after R0 resection. Univariate analysis of DFS revealed that the FR + CTC count, tumor number, Barcelona Clinic Liver Cancer stage and extrahepatic metastasis status were correlated with DFS. Multivariate analysis of DFS revealed that the FR + CTC count and tumor number were correlated with DFS.
Ligand-target polymerase chain reaction is a sensitive tool for quantifying the number of FR + CTCs in HCC patients. These findings could provide new insight for stratifying HCC patients and predicting the recurrence of HCC.
Core Tip: Ligand-target polymerase chain reaction is a sensitive tool for quantifying folate receptor (FR)-positive circulating tumor cells (CTCs) in hepatocellular carcinoma (HCC) patients. FR + CTCs counts were correlated with macrovascular invasion, tumor size, tumor number, extrahepatic tumor stage and disease-free survival time after surgery in HCC patients. These findings could provide new insights for stratifying HCC patients and predicting the recurrence of HCC by quantifying FR + CTCs.
- Citation: Zhang ZY, Zhou M, Liu JJ, Zhang W. Folate receptor-positive circulating tumor cells might function as potential biomarkers for hepatocellular carcinoma. World J Gastrointest Oncol 2025; 17(11): 113431
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/113431.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.113431
Hepatocellular carcinoma (HCC) is among the most common types of liver cancer and has a high mortality rate worldwide. The percentage of HCC patients with vascular invasion at the time of initial HCC diagnosis ranges from 10% to 40%[1]. According to the American Association for the Study of Liver Disease/Barcelona Clinic Liver Cancer (BCLC) staging system and treatment guidelines, HCC associated with vascular invasion is considered an advanced stage of disease. The treatment strategy for intermediate-stage or advanced-stage HCC differs between Eastern and Western populations[2]. On the basis of different guidelines, curative resection or conservative treatment, including transcatheter arterial chemoembolization (TACE) and systemic therapy, is suggested[3]. However, the prognosis of HCC remains unsatisfactory. Two main factors influence the prognosis of HCC: Early metastasis and recurrence after resection and the detection of advanced-stage HCC[4,5]. Early metastasis and recurrence are the main risk factors for poor outcome of HCC patients after resection[6]. Identifying HCC patients with a low possibility of recurrence or early metastasis remains a clinical problem[7]. Another risk factor that can negatively affect prognosis is the poor ability to detect early-stage HCC. Most patients with HCC are initially diagnosed with advanced-stage disease. The stage of HCC is closely correlated with overall survival, indicating that screening for liver cancer is important. Alpha fetoprotein (AFP) is currently the most common serum biomarker for screening for HCC and predicting tumor recurrence. However, its sensitivity and specificity are not sufficient, especially in patients who are AFP negative[8]. Thus, reliable biomarkers that can be used to detect early-stage HCC and diagnose recurrence are urgently needed.
Circulating tumor cells (CTCs), which are known as “liquid biopsies”, are used to monitor the progression, response to therapy and recurrence of different malignant diseases[9]. Folate receptors (FRs) are cysteine-rich cell surface glyco
Ligand-target (LT) polymerase chain reaction (PCR), developed by Shanghai Geno Biotech Co., Ltd. (China), has been used to detect CTCs in patients with lung cancer and pancreatic cancer through enrichment of CTCs using FRs expressed on the cell surface. By labeling with a conjugate of tumor-specific folic acid that can selectively bind to FRs of cancer cells and a synthesized oligonucleotide, CTCs can be converted into detector molecules that can be amplified for quantitative analysis[10]. The diagnostic ability of this technique has been confirmed in lung cancer and pancreatic cancer. This study evaluated the clinical value of FR-positive CTCs in patients with liver cancer.
This work was a retrospective, single-center clinical trial conducted at Hepatic Surgery Center, Tongji Hospital (China). From August 2020 to April 2021, 128 consecutive patients with benign and malignant liver disease were enrolled in this retrospective study. Among these patients, 107 were diagnosed with liver cancer, including HCC. Twenty-one patients were diagnosed with benign liver disease. The diagnosis was based on the histopathological diagnosis after resection, radiation examination via computed tomography/magnetic resonance imaging and laboratory examination. Liver tumor stages were based on the BCLC staging system. All clinical data, including age, sex, preoperative tumor marker (AFP) level, tumor differentiation, tumor size, vascular invasion and distant metastases, and satellite number, were collected. This study was approved by the Ethics Committee of Tongji Hospital (Approval No. TJ-IRB202502081). All procedures were performed in accordance with the guidelines of the Declaration of Helsinki.
All patients included in this study were evaluated by a multidisciplinary team consisting of experienced surgeons, radiologists and oncologists at our hospital. If the tumor was respectable according to the China liver cancer staging system or BCLC staging system, resection treatment was proposed. If the tumor thrombus was combined, it was either resected en bloc or extracted by thrombectomy depending on the location and extent of the tumor thrombus and the lesion in the liver. If the patient did not qualify for resection treatment, TACE or systemic treatments, including lenvatinib or sorafenib, were suggested.
Peripheral blood samples (3 mL) were obtained from all enrolled patients in vacuum tubes containing the anticoagulant ethylenediaminetetraacetic acid for analysis before or one week after treatment. All blood samples were processed within 24 hours. CTCs were enriched and analyzed via a CytoploRare Kit (Shanghai Geno Biotech Co., Ltd., China), a commercially available CTC detection kit approved by China’s Food and Drug Administration. The methods were described previously[11]. The LT-PCR is described in the following article[12]. The sensitivity and specificity have also been re
Follow-up after R0 resection of HCC was conducted via hospital-based follow-up visits or by telephone. All patients were followed up until death or June 1, 2022. After R0 resection in HCC patients, periodic routine follow-up at 3-month intervals was performed for the first 2 years. At the routine follow-up visit, AFP testing, liver function testing, enhanced computed tomography and ultrasonography were performed to determine whether the tumor had recurred. Once recurrence was suspected in the routine examination, enhanced magnetic resonance imaging or digital subtraction angiography was performed for confirmation. Disease-free survival (DFS) was defined from the date of surgery to the date of recurrence detection at the last follow-up.
Continuous data are summarized as the mean ± SD or median (interquartile ranges). To compare CTC counts between the two groups, we used unpaired nonparametric tests. Receiver operating characteristic (ROC) curves were used to determine the threshold of sensitivity and specificity. The Youden index was used to identify the optimal cutoff point and diagnostic efficiency. P < 0.05 was considered to indicate statistical significance. The most efficient cutoff values for discriminating patients with HCC from non-HCC patients were identified via ROC curve analysis, and the area under the ROC curve was calculated. The data were prepared and analyzed via R software. Graphs were constructed with GraphPad PRISM version 8 or R software. Univariate Cox proportional hazards regression analysis was performed to evaluate the associations of biomarkers or clinical parameters with DFS. The P-values were calculated via the Wald test. Variables with P < 0.05 in the univariate analysis were included in the multivariate analysis. Multivariate Cox proportional hazards regression analysis was performed to evaluate the independent prognostic value of the clinical parameters. Kaplan-Meier analysis was used to calculate the DFS. The log-rank test was used to calculate the P-value of the Kaplan-Meier analysis.
All patient characteristics are summarized in Table 1. Among the 128 patients included in this study, 107 patients were confirmed to have HCC. Eighty-four HCC patients were tested for FR + CTC counts before any treatment. Fourteen HCC patients were checked for FR + CTC counts 1 week after radical hepatectomy, and 9 unresectable patients were checked for FR + CTC counts 1 week after receiving TACE and/or systemic treatment. The patients with HCC included in this study had a mean age of 56.2 years, with a range of 27-79 years. A total of 87.6% of the patients were male. The tumor diameter ranged from 5.5-7.0 cm. Twenty-one patients with benign liver disease were also included in this study. Among the patients with benign liver disease, 10 had hemangiomas, 7 had liver cirrhosis, and 4 had hepatolithiasis (Table 1).
| Characteristics | Number of patients |
| HCC | |
| Age, years | 56.2 ± 12.3 |
| Sex | |
| Male | 92 |
| Female | 15 |
| Tumor size (mm) | 6.26 ± 4.1 |
| Tumor stage | |
| BCLC-A | 37 |
| BCLC-B | 41 |
| BCLC-C | 39 |
| Vascular invasion | |
| Macro invasion | 20 |
| Micro invasion | 15 |
| No invasion | 72 |
| Before treatment | 84 |
| After R0 resection | 14 |
| After conservative treatment | 9 |
| Benign disease | |
| Age | 54.3 (25-70) |
| Histological type | |
| Liver cirrhosis | 7 |
| Hemangioma | 10 |
| Hepatolithiasis | 4 |
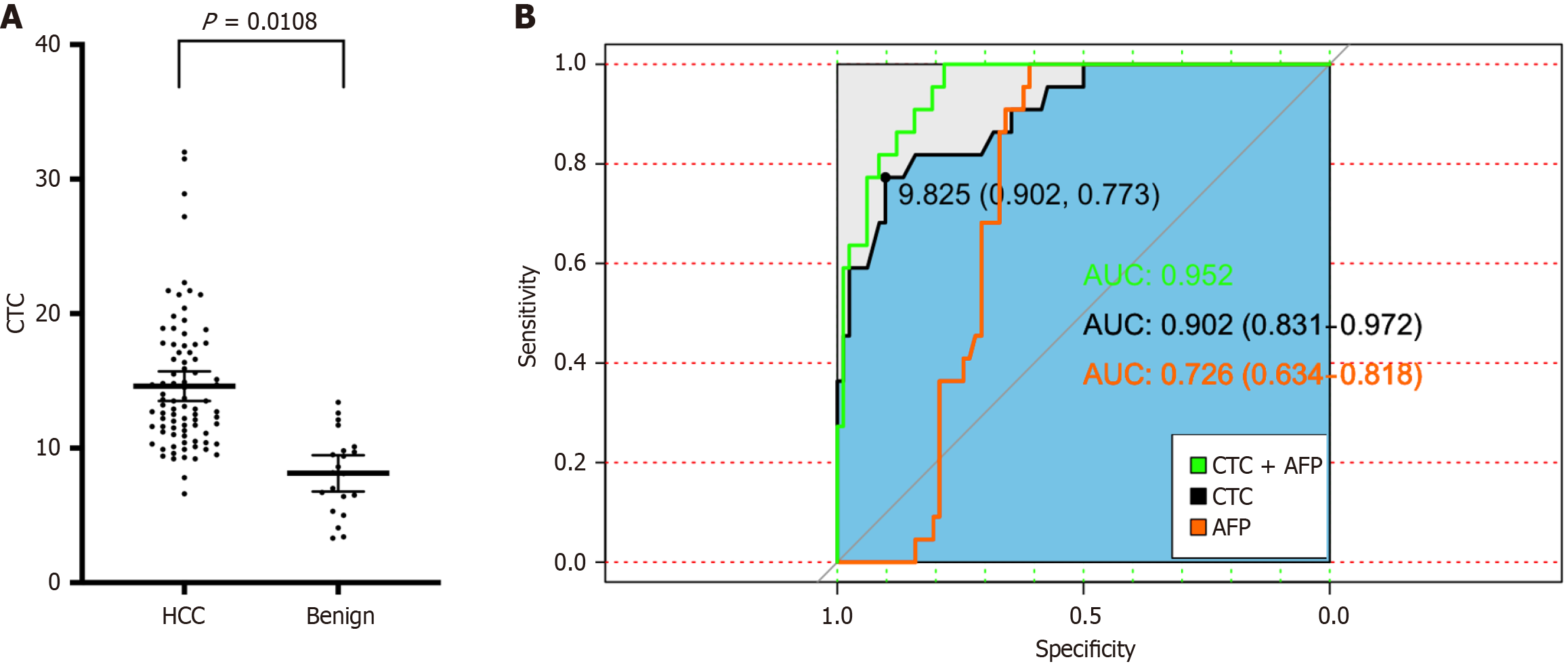
The FR + CTC counts were compared between the malignant group (before treatment) and the benign group. The average CTC count in patients with benign disease was 8.1 ± 3.0 FU/3 mL, which was significantly lower than that in patients with HCC (14.61 ± 5.01 FU/3 mL; P = 0.0108; Figure 1A). The diagnostic efficiency of the FR + CTC count in distinguishing HCC from benign HCC disease was based on ROC curves. The area under the ROC curve was 0.9. The optimal FR + CTC level cutoff value for differentiating patients with HCC from those with benign HCC disease was 9.825 FU/3 mL. The sensitivity and specificity were 0.902 and 0.773, respectively (Figure 1B). Moreover, the diagnostic performance of AFP level and FR + CTC count combined with AFP level was also evaluated. The areas under the ROC curves of the FR + CTC count combined with the AFP level and the AFP level alone were 0.952 (0.830-0.972) and 0.73 (0.639-0.821), res
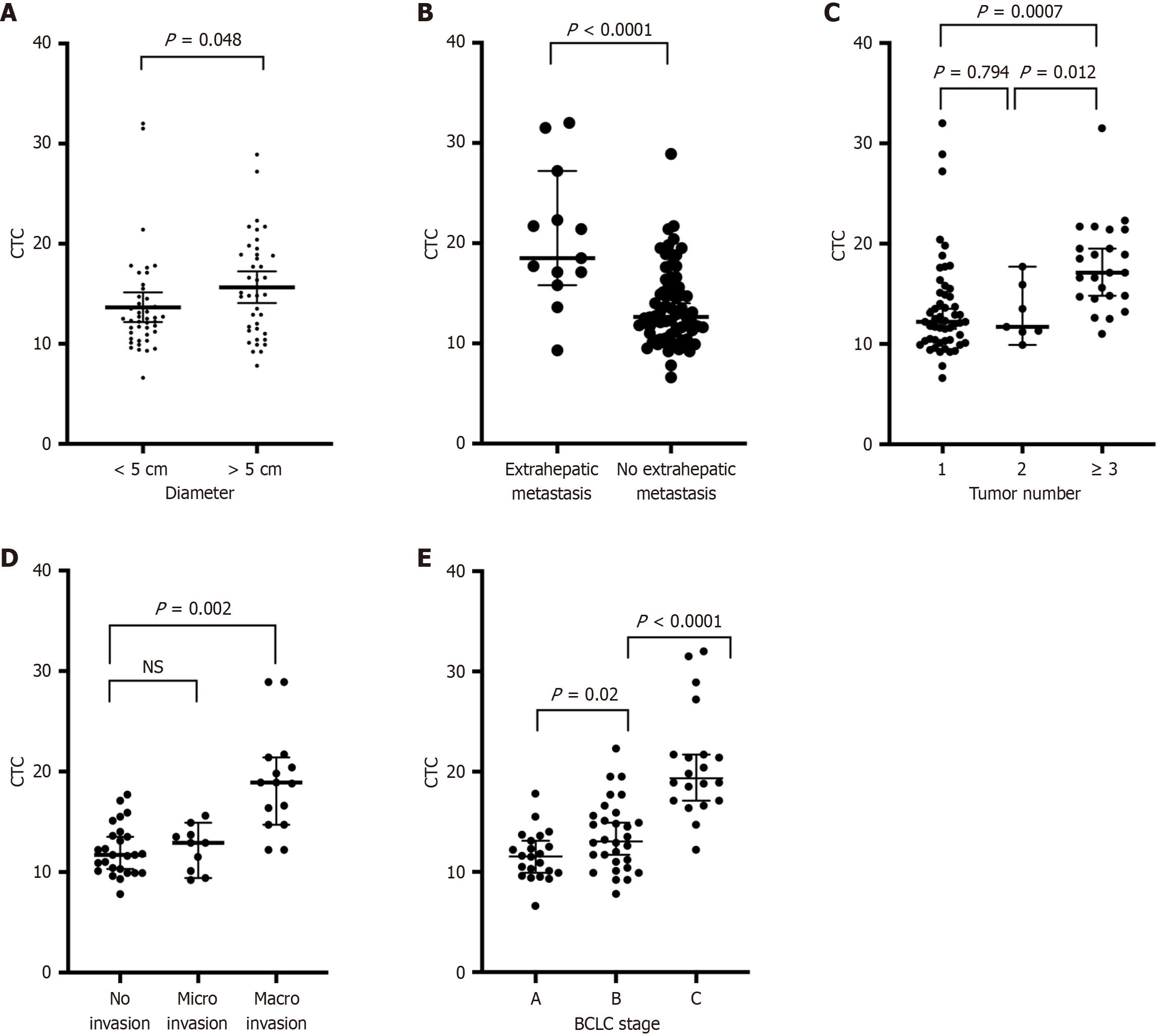
To continue our research, we compared the correlations between FR + CTC counts and clinicopathological characteristics in 84 HCC patients before treatment. All the information of the HCC patients is summarized in Table 2. We found that FR + CTC levels did not differ according to differentiation, satellite state or AFP level. However, significant differences in tumor diameter, extrahepatic metastatic state, tumor number, vascular invasion state, and BCLC stage were detected among the patients grouped by FR + CTC count (Figure 2). In HCC patients with tumor diameters greater than 5 cm, FR + CTC counts were elevated (15.8 FU/3 mL vs 12.7 FU/3 mL, P = 0.048; Figure 2A). In the subgroup of HCC patients with extrahepatic metastasis, the number of FR + CTCs was significantly greater than that in HCC patients with no extrahepatic metastasis (22.6 FU/3 mL vs 13.5 FU/3 mL, P < 0.001; Figure 2B). In the subgroup with more than 3 tumors, FR + CTC counts were higher than those in HCC patients with 1 or 2 tumors (17.77 FU/3 mL vs 13.03 FU/3 mL, P = 0.0007; 17.77 FU/3 mL vs 13.4 FU/3 mL, P = 0.003; Figure 2C). In the subgroup of patients with microvascular invasion, FR + CTC counts were slightly greater than those in patients with no invasion. However, the difference was not statistically significant (12.37 FU/3 mL vs 12.15 FU/3 mL, P = 0.8116; Figure 2D). FR + CTC counts were higher in the subgroup of HCC patients with macrovascular invasion than in patients with no vascular invasion (18.9 FU/3 mL vs 12.15 FU/3 mL, P < 0.0001). Overall, we found that FR + CTC counts were correlated with BCLC stage (11.62 vs 13.6 vs 20.4, P < 0.05; Figure 2E). However, satellite metastasis and AFP levels were not correlated with FR + CTC counts (Table 2).
| Characteristic | Number of patients | CTC units | P value |
| Sex | 0.92 | ||
| Female | 12 | 14.4 ± 5.8 | |
| male | 72 | 14.6 ± 4.9 | |
| Age | 0.62 | ||
| ≤ 50 | 31 | 14.2 ± 3.8 | |
| > 50 | 53 | 14.8 ± 5.5 | |
| Tumor diameter | 0.048a | ||
| < 5 cm | 45 | 12.7 ± 4.9 | |
| > 5 cm | 39 | 15.8 ± 5.0 | |
| Tumor stage | < 0.001c | ||
| BCLC (A + B) | 63 | 12.7 ± 3.2 | |
| BCLC (C) | 21 | 20.4 ± 5.3 | |
| Vascular invasion | 0.201 | ||
| No invasion | 58 | 14.1 ± 5.01 | |
| Micro-vascular invasion | 12 | 12.2 ± 2.09 | |
| Macro-vascular invasion | 14 | 18.8 ± 4.29 | 0.0023b |
| Satellite | 0.21 | ||
| No | 76 | 14.6 ± 5.1 | |
| yes | 8 | 12.5 ± 2.8 | |
| AFP | 0.636 | ||
| ≤ 8 | 34 | 14.3 ± 4.8 | |
| > 8 | 50 | 14.8 ± 5.2 | |
| Tumor number | 0.003b | ||
| 1 | 53 | 13.4 ± 5.0 | |
| > 1 | 31 | 16.6 ± 4.5 | |
| Extrahepatic metastasis | < 0.001c | ||
| No | 10 | 13.5 ± 3.9 | |
| Yes | 74 | 22.6 ± 6.6 |
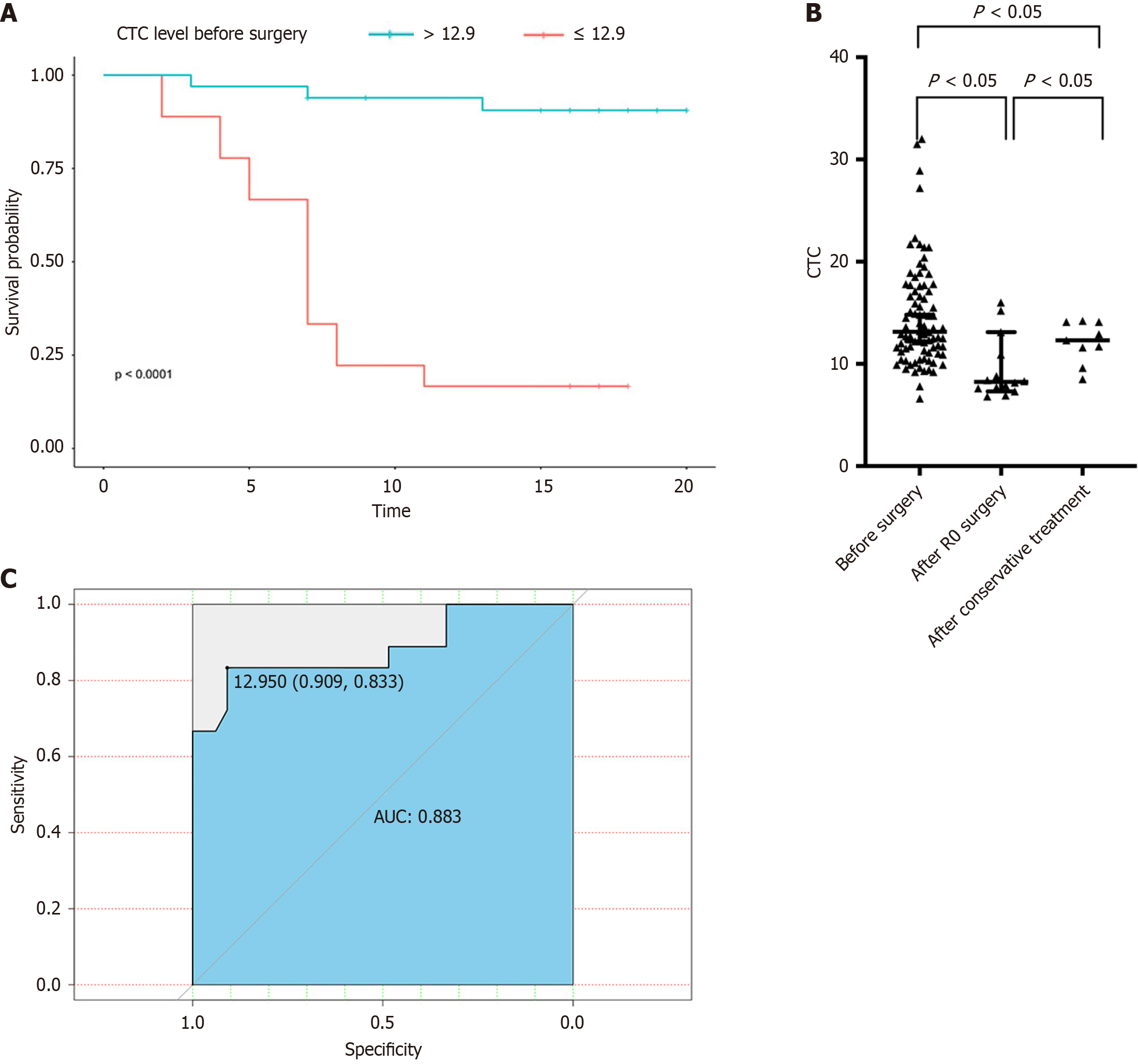
Next, we compared the CTC counts in patients with malignant disease before and after curative or noncurative treatment. We found that the CTC counts in HCC patients before treatment was greater than those in HCC patients after surgical treatment or TACE (14.6 FU/3 mL vs 9.5 FU/mL vs 12.1 FU/mL, P < 0.05; Figure 3A). Therefore, CTC counts might aid in the evaluation of treatment efficacy in HCC patients (Figure 3B).
In subsequent research, the ability of the FR + CTC counts to predict the recurrence of HCC patients after hepatectomy was determined via ROC curves of DFS time. The area under the ROC curve was 0.883 (Figure 3C). The optimal FR + CTC count cutoff value for stratifying HCC patients into different prognostic groups was 12.9 FU/3 mL. The sensitivity and specificity were 0.909 and 0.833, respectively. The DFS curve revealed that FR + CTC counts > 12.9 were associated with poor outcomes for patients with HCC after hepatectomy. The median DFS of HCC patients with FR + CTC counts > 12.9 was 7 months. For HCC patients with FR + CTC counts < 12.9, the median DFS has not been reached.
Univariate analysis of DFS time revealed that FR + CTC counts < 12.9 [hazard ratio (HR) = 0.057, 95% confidence interval (CI): 0.016-0.201], single tumors (HR =0.25, 95%CI: 0.096-0.65), extrahepatic metastasis (HR = 4.953, 95%CI: 1.118-21.94), and BCLC-C stage (HR = 4.978, 95%CI: 1.001-24.75) were correlated with tumor recurrence (Table 3). In the multivariate analysis, only FR + CTC counts < 12.9 (HR = 0.04, 95%CI: 0.01-0.17) and single tumors (HR = 0.19, 95%CI: 0.05-0.72) were correlated with a better prognosis (Table 4).
| Factors | Univariate analysis | ||
| HR (95%CI) | Significance | ||
| CTC level | > 12.9 | Reference1 | |
| < 12.9 | 0.057 (0.016-0.201) | < 0.01b | |
| Extrahepatic metastasis | No | Reference1 | |
| Yes | 4.953 (1.118-21.94) | 0.031a | |
| Tumor number | Multiple | Reference1 | |
| Single | 0.25 (0.096-0.65) | 0.004b | |
| Tumor diameter | > 5 cm | Reference1 | |
| < 5 cm | 0.44 (0.17-1.10) | 0.0812 | |
| BCLC stage | A | Reference1 | |
| B | 2.604 (0.95-7.18) | 0.064 | |
| C | 4.978 (1.001-24.75) | 0.049a | |
| Satellite | Yes | Reference1 | |
| No | 1.043 (0.6572-1.656) | 0.858 | |
| Differentiation | High | Reference1 | |
| Mediate | 1.256 (0.1403-11.25) | 0.458 | |
| Low | 2.213 (0.27-18.03) | 0.838 | |
| Age | < 50 | Reference1 | |
| > 50 | 0.512 (0.1674-1.55) | 0.237 | |
| AFP | > 400 | Reference1 | |
| < 400 | 0.57 (0.22-1.48) | 0.25 | |
| Sex | Male | Reference1 | |
| Female | 0.55 (0.17-1.662) | 0.287 | |
| Vascular invasion | Yes | Reference1 | |
| No | 1.132 (0.4-3.178) | 0.814 | |
The CTCs have potential value in diagnosing and monitoring recurrence in patients with various malignancies[13,14]. However, CTCs are extremely rare. The frequency of CTCs is as low as 1 per 106/107 leukocytes. Therefore, methods to detect CTCs must have high sensitivity and specificity. In recent years, many platforms have been developed to detect CTCs in peripheral blood[15]. The CellSearch system, which is among the most commonly used methods for identifying CTCs, is an immunology-based platform that uses epithelial cell adhesion molecule as the capture target[16]. However, for cancer types characterized by a loss of more epithelium-like CTCs, this detection method is not sensitive. Recently, several studies, including double-blind clinical trials, have proven the value of FR + CTCs labeled with folate-linked oligonucleotides and then quantified by reverse transcription PCR in detecting and assessing non-small cell carcinoma and pancreatic cancer[11]. As other reports have revealed, FRs are also highly expressed in the liver[17]. Early metastasis and recurrence are risk factors that negatively affect the prognosis of advanced HCC. Currently, potential biomarkers are not available to predict the risk of early metastasis and recurrence in HCC patients after surgery. AFP is currently the most common serum biomarker for detecting HCC and predicting tumor recurrence. However, its sensitivity and specificity are not sufficient, especially in patients who are AFP negative[8]. In the era of systemic treatment, if bio
In this study, we found that FR + CTC counts were elevated in patients with HCC compared with patients with benign liver diseases. A value of 9.825 FU/3 mL was confirmed as the cutoff value for distinguishing HCC from benign disease. In previous studies, the FR + CTC counts in healthy volunteers were reported to be lower than 6 FU/3 mL[18-20]. We reported that the cutoff value for classifying benign and malignant tumors was greater than that reported previously. After reviewing the data, we found that, the number of FR + CTCs was slightly greater in some patients with hepatolithiasis than that in patients with hemangioma and liver cirrhosis. This increase in CTCs might have caused inflammatory reactions in patients with hepatolithiasis. According to previous reports, FRs are expressed at high levels during inflammation[17]. This high expression may explain why the cutoff value was greater than the reported levels in healthy volunteers. Peripheral blood contains a small proportion of activated monocytes with functional FR expression[21]. In a subsequent study, the diagnostic efficacies of FR + CTC counts and those of FR + CTC counts combined with AFP levels were better than those of AFP levels alone (P = 0.0037; P = 0.0001). However, the area under the ROC curve was larger for the combination of FR + CTC counts and AFP levels than for AFP levels, but this difference was not significant. The combination of FR + CTC counts and AFP levels could improve the diagnostic efficiency for HCC. Although impro
Tumor stage is often used to determine prognosis after treatment. FR + CTC counts can also be used to predict prognosis. In this study, we explored the relationships among tumor biological characteristics, including tumor diameter, vascular invasion, differentiation stage, satellite metastasis, tumor number, extrahepatic metastasis, tumor stage, BCLC stage, and AFP level. We found that FR + CTC counts were correlated with tumor diameter, extrahepatic metastasis, tumor number, macrovascular invasion and BCLC stage. However, BCLC stage and extrahepatic metastasis were not significantly correlated with FR + CTC counts in multivariate Cox analysis despite being correlated in univariate Cox analysis. This difference is likely due to the limited sample size. In a subsequent study, we revealed that R0 resection or conservative treatment, including TACE or targeted medicine, decreased the number of FR + CTCs. These findings indicate that FR + CTC counts can be used to detect HCC in patients with malignant liver tumors. Moreover, the FR + CTC counts did not differ between HCC patients with normal AFP levels and HCC patients with abnormal AFP levels, which suggests that the FR + CTC count might serve as an independent serum biomarker.
Finally, survival analysis revealed that higher FR + CTC counts before treatment were correlated with shorter DFS after hepatectomy. The cutoff value of the ROC curves for distinguishing prognoses was 12.95 FU/3 mL. Both univariate and multivariate analyses confirmed that FR + CTC counts were correlated with DFS after hepatectomy. In contrast, the level of AFP, one of the most commonly used serum markers of HCC, was not correlated with the outcome of patients with HCC.
This retrospective clinical trial revealed that LT-PCR might be a useful and reliable tool for identifying FR + CTCs in the circulation of patients with HCC. The number of FR + CTCs correlated with tumor stage, vascular invasion state, extrahepatic metastatic state, tumor number and tumor diameter. We also reported that the number of FR + CTCs de
| 1. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4362] [Article Influence: 545.3] [Reference Citation Analysis (6)] |
| 2. | Lu J, Zhang XP, Zhong BY, Lau WY, Madoff DC, Davidson JC, Qi X, Cheng SQ, Teng GJ. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 3. | Rizzo A, Ricci AD, Brandi G. Trans-Arterial Chemoembolization Plus Systemic Treatments for Hepatocellular Carcinoma: An Update. J Pers Med. 2022;12:1788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 93] [Reference Citation Analysis (0)] |
| 4. | Guven DC, Erul E, Kaygusuz Y, Akagunduz B, Kilickap S, De Luca R, Rizzo A. Immune checkpoint inhibitor-related hearing loss: a systematic review and analysis of individual patient data. Support Care Cancer. 2023;31:624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 5. | Rizzo A, Santoni M, Mollica V, Logullo F, Rosellini M, Marchetti A, Faloppi L, Battelli N, Massari F. Peripheral neuropathy and headache in cancer patients treated with immunotherapy and immuno-oncology combinations: the MOUSEION-02 study. Expert Opin Drug Metab Toxicol. 2021;17:1455-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 131] [Reference Citation Analysis (0)] |
| 6. | Rizzo A, Ricci AD. Challenges and Future Trends of Hepatocellular Carcinoma Immunotherapy. Int J Mol Sci. 2022;23:11363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 75] [Reference Citation Analysis (0)] |
| 7. | Sahin TK, Rizzo A, Aksoy S, Guven DC. Prognostic Significance of the Royal Marsden Hospital (RMH) Score in Patients with Cancer: A Systematic Review and Meta-Analysis. Cancers (Basel). 2024;16:1835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 155] [Reference Citation Analysis (0)] |
| 8. | He S, Zhang DC, Wei C. MicroRNAs as biomarkers for hepatocellular carcinoma diagnosis and prognosis. Clin Res Hepatol Gastroenterol. 2015;39:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | He W, Wang H, Hartmann LC, Cheng JX, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proc Natl Acad Sci U S A. 2007;104:11760-11765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 10. | Zhou Q, Geng Q, Wang L, Huang J, Liao M, Li Y, Ding Z, Yang S, Zhao H, Shen Q, Pan C, Lou J, Lu S, Chen C, Luo Q. Value of folate receptor-positive circulating tumour cells in the clinical management of indeterminate lung nodules: A non-invasive biomarker for predicting malignancy and tumour invasiveness. EBioMedicine. 2019;41:236-243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 11. | Lou J, Ben S, Yang G, Liang X, Wang X, Ni S, Han B. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One. 2013;8:e80458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 12. | Li H, Li B, Pan Y, Zhang Y, Xiang J, Zhang Y, Sun Y, Yu X, He W, Hu H. Preoperative Folate Receptor-Positive Circulating Tumor Cell Level Is a Prognostic Factor of Long Term Outcome in Non-Small Cell Lung Cancer Patients. Front Oncol. 2020;10:621435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, Silberstein JL, Taylor MN, Maughan BL, Denmeade SR, Pienta KJ, Paller CJ, Carducci MA, Eisenberger MA, Luo J. Clinical Significance of Androgen Receptor Splice Variant-7 mRNA Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. J Clin Oncol. 2017;35:2149-2156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 386] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 14. | Goodman CR, Seagle BL, Friedl TWP, Rack B, Lato K, Fink V, Cristofanilli M, Donnelly ED, Janni W, Shahabi S, Strauss JB. Association of Circulating Tumor Cell Status With Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol. 2018;4:e180163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 118] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 15. | Lawrence R, Watters M, Davies CR, Pantel K, Lu YJ. Circulating tumour cells for early detection of clinically relevant cancer. Nat Rev Clin Oncol. 2023;20:487-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 213] [Reference Citation Analysis (0)] |
| 16. | Hamilton G, Rath B. Mesenchymal-Epithelial Transition and Circulating Tumor Cells in Small Cell Lung Cancer. Adv Exp Med Biol. 2017;994:229-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Chandrupatla DMSH, Jansen G, Mantel E, Low PS, Matsuyama T, Musters RP, Windhorst AD, Lammertsma AA, Molthoff CFM, van der Laken CJ. Imaging and Methotrexate Response Monitoring of Systemic Inflammation in Arthritic Rats Employing the Macrophage PET Tracer [(18)F]Fluoro-PEG-Folate. Contrast Media Mol Imaging. 2018;2018:8092781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Yu Y, Chen Z, Dong J, Wei P, Hu R, Zhou C, Sun N, Luo M, Yang W, Yao R, Gao Y, Li J, Yang G, He W, He J. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol. 2013;6:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 19. | Chen X, Zhou F, Li X, Yang G, Zhang L, Ren S, Zhao C, Deng Q, Li W, Gao G, Li A, Zhou C. Folate Receptor-Positive Circulating Tumor Cell Detected by LT-PCR-Based Method as a Diagnostic Biomarker for Non-Small-Cell Lung Cancer. J Thorac Oncol. 2015;10:1163-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 20. | Wang L, Wu C, Qiao L, Yu W, Guo Q, Zhao M, Yang G, Zhao H, Lou J. Clinical Significance of Folate Receptor-positive Circulating Tumor Cells Detected by Ligand-targeted Polymerase Chain Reaction in Lung Cancer. J Cancer. 2017;8:104-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 21. | Assaraf YG, Leamon CP, Reddy JA. The folate receptor as a rational therapeutic target for personalized cancer treatment. Drug Resist Updat. 2014;17:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 313] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 22. | Jelic S, Sotiropoulos GC; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2010;21 Suppl 5:v59-v64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 168] [Article Influence: 10.5] [Reference Citation Analysis (1)] |
| 23. | Choi J, Park Y, Kim JH, Kim HS. Evaluation of automated serum des-gamma-carboxyprothrombin (DCP) assays for detecting hepatocellular carcinoma. Clin Biochem. 2011;44:1464-1468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Wen Y, Han J, Chen J, Dong J, Xia Y, Liu J, Jiang Y, Dai J, Lu J, Jin G, Han J, Wei Q, Shen H, Sun B, Hu Z. Plasma miRNAs as early biomarkers for detecting hepatocellular carcinoma. Int J Cancer. 2015;137:1679-1690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 174] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 25. | Abdalla MA, Haj-Ahmad Y. Promising Candidate Urinary MicroRNA Biomarkers for the Early Detection of Hepatocellular Carcinoma among High-Risk Hepatitis C Virus Egyptian Patients. J Cancer. 2012;3:19-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 26. | Onishi M, Ochiya T, Tanaka Y. MicroRNA and liver cancer. Cancer Drug Resist. 2020;3:385-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/