Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.113262
Revised: September 21, 2025
Accepted: October 9, 2025
Published online: November 15, 2025
Processing time: 85 Days and 8.7 Hours
Gastrointestinal stromal tumors (GISTs) are generally characterized by driver mutations in KIT or PDGFRA. However, the molecular landscape of wild-type GISTs remains complex, posing significant therapeutic challenges. Recent evi
We retrospectively evaluated two patients with GIST, diagnosed between August 2021 and July 2022, harboring FGFR2 mutations through hybrid capture-based next-generation sequencing (NGS). We analyzed their clinicopathological characteristics, treatment response, and long-term follow-up data. Both patients, a 47-year-old man (case 1) and a 43-year-old woman (case 2), underwent successful surgical resection and received adjuvant imatinib therapy. They achieved sus
Our findings provided preliminary evidence that novel FGFR2 fusions might act as primary oncogenic drivers in a rare subset of KIT/PDGFRA wild-type GISTs. These cases highlight the importance for comprehensive genomic profiling and suggest that fibroblast growth factor receptor-targeted inhibitors could be a potential therapeutic strategy for advanced or imatinib-resistant diseases, warr
Core Tip: Herein, we report, for the first time, novel FGFR2 rearrangements FGFR2-CIT/intergenic-FGFR2 and FGFR2-CAMK2G/FGFR2-VCL fusions as potential primary oncogenic drivers in two patients with KIT/PDGFRA wild-type gastrointestinal stromal tumors (GISTs). Both patients achieved sustained remission following adjuvant imatinib, challenging the notion that non-canonical alterations preclude tyrosine kinase inhibitor response. These findings might expand the molecular spectrum of GISTs and suggest that FGFR2 fusions might define a distinct subtype with potential sensitivity to FGFR-targeted therapies, offering new avenues for precision treatment in imatinib-resistant or advanced disease cases.
- Citation: Hong YY, Shou CH, Yang WL, Wang XD, Zhang Q, Liu XS, Yu JR. FGFR2 fusions as novel oncogenic drivers in gastrointestinal stromal tumors: Two case reports and review of literature. World J Gastrointest Oncol 2025; 17(11): 113262
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/113262.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.113262
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract, originating from interstitial cells of Cajal or their precursors[1]. These tumors can arise anywhere along the gastr
Despite the remarkable success of TKI therapy in KIT/PDGFRA-mutant GISTs, a significant subset of tumors, known as wild-type GISTs, lacks these canonical mutations. These cases account for approximately 10%-15% of all GISTs and are often characterized by a heterogeneous molecular landscape, including mutations in SDHA/SDHB/SDHC/SDHD subunits, NF1, or genes within the RAS-mitogen-activated protein kinase (MAPK) signaling pathway[6,7]. The diverse molecular drivers and often limited response to standard imatinib treatment present substantial therapeutic challenges in patients with wild-type GISTs, highlighting a critical need for a deeper understanding about their underlying oncogenic mechanisms and identifying novel actionable targets[8,9].
The FGFR family of tyrosine kinases plays a crucial role in cell proliferation, survival, and differentiation. Aberrant activation of FGFR signaling, primarily through gene fusions, amplifications, or point mutations, is a well-established oncogenic event in patients with various solid tumors[10]. For instance, FGFR2 fusions are a key molecular subgroup in intrahepatic cholangiocarcinoma, where they serve as predictive biomarkers for response to FGFR-specific inhibitors such as pemigatinib and futibatinib[11,12]. Similarly, FGFR2 alterations have been implicated in the pathogenesis of gastric cancer and other malignancies, driving tumor growth and metastasis by activating downstream signaling pathways such as the RAS-MAPK and phosphatidylinositol 3-kinase/protein kinase B pathways[13,14]. Although the role of FGFR signaling in patients with other cancer types is well-documented, its contribution to the pathogenesis of GISTs remains uninvestigated, particularly in the context of primary driver events in patients with wild-type tumors[15].
In this two-case series, we aimed to bridge the above-mentioned knowledge gap by describing the clinicopathological features and treatment outcomes in two patients with GISTs driven by novel FGFR2 rearrangements. Both patients were diagnosed using comprehensive genomic profiling via next-generation sequencing (NGS), identifying unique FGFR2 fusions without canonical KIT or PDGFRA mutations. Our findings could provide novel insights into the molecular landscape of GISTs and underscore the critical importance of NGS for identifying rare, actionable mutations. We also discuss the potential therapeutic implications of FGFR2 alterations in patients with GISTs, laying the foundation for future investigations into FGFR-targeted therapies for this challenging molecular subset.
Case 1: A 47-year-old man presented with a 2-week history of dull, intermittent epigastric pain and a progressively enlarging palpable upper abdominal mass.
Case 2: A 43-year-old woman was referred to our hospital due to a substantial space-occupying lesion in the small intestine detected by computed tomography (CT) during a physical examination.
Case 1: The patient reported no associated symptoms such as dysphagia, early satiety, weight loss, or gastrointestinal bleeding.
Case 2: The patient was asymptomatic prior to the incidental finding.
Case 1: The patient had no significant medical history.
Case 2: Not specified.
Case 1: He had no known family history of cancer. Physical examination upon admission: A thorough physical examination revealed a firm, non-tender, and mobile mass in the left upper quadrant of the abdomen.
Case 2: Not specified.
Cases 1 and 2: Not specified.
Cases 1 and 2: Not specified.
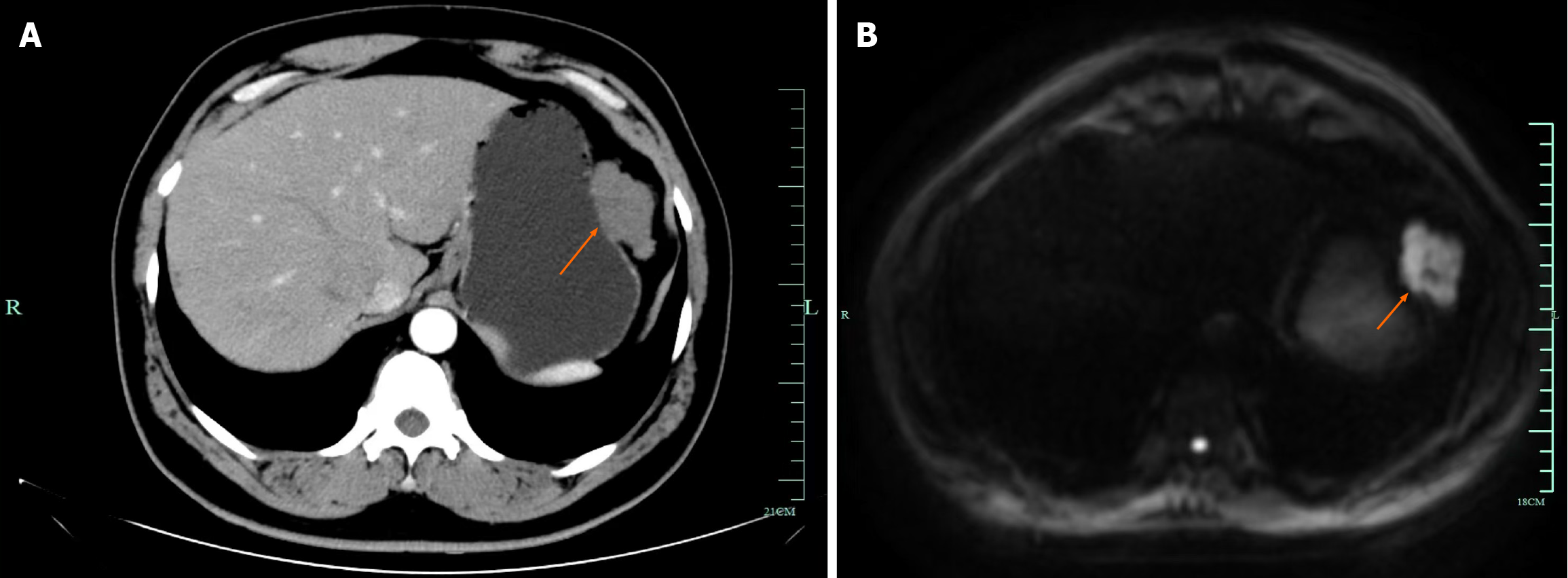
Case 1: A contrast-enhanced CT of the abdomen showed a large, well-circumscribed, exophytic mass originating from the greater curvature of the stomach, measuring approximately 5.0 cm × 6.0 cm (Figure 1A). Enhanced magnetic resonance imaging of the stomach revealed an irregular mass-like abnormal signal focus in the greater curvature of the gastric fundus, with a clear edge, approximately 53 mm × 34 mm in size. The mass showed high signal on T2-weighted imaging and diffusion weighted imaging, low signal on T1-weighted imaging, and significant enhancement post-contrast (Figure 1B). The imaging findings were suggestive of a mass in the gastric fundus on the greater curvature, considered a solid tumor (extrinsic growth type).
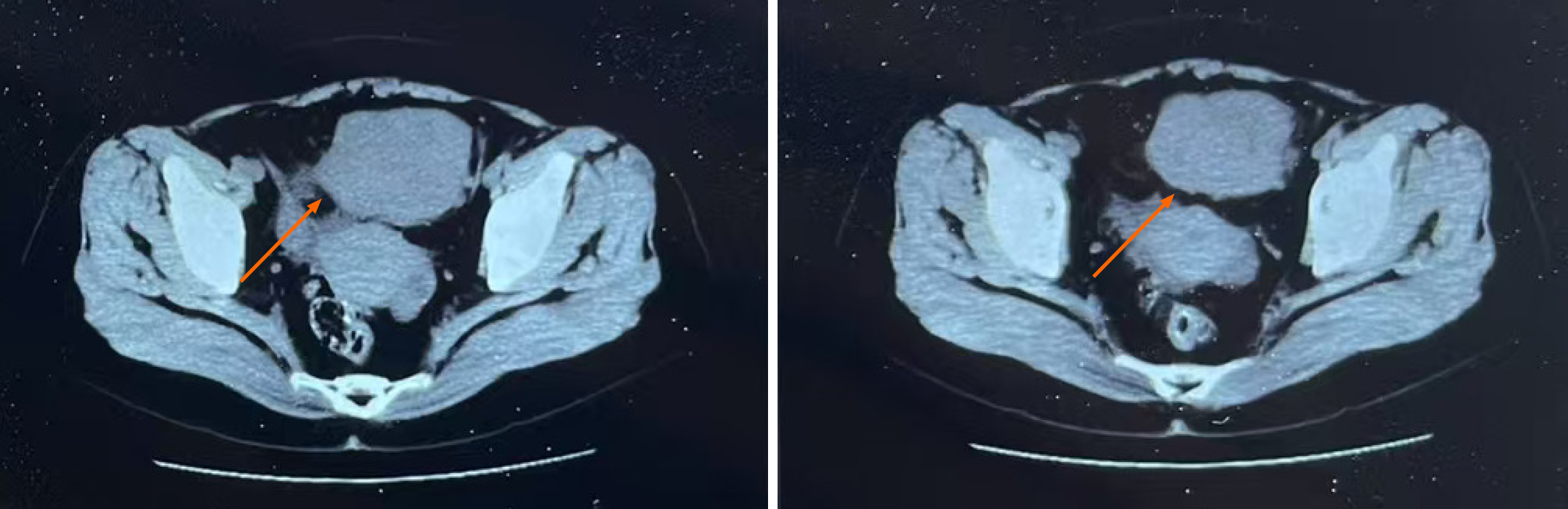
Case 2: CT scan revealed a solid mass lesion in the small intestine within the abdominal cavity (Figure 2).
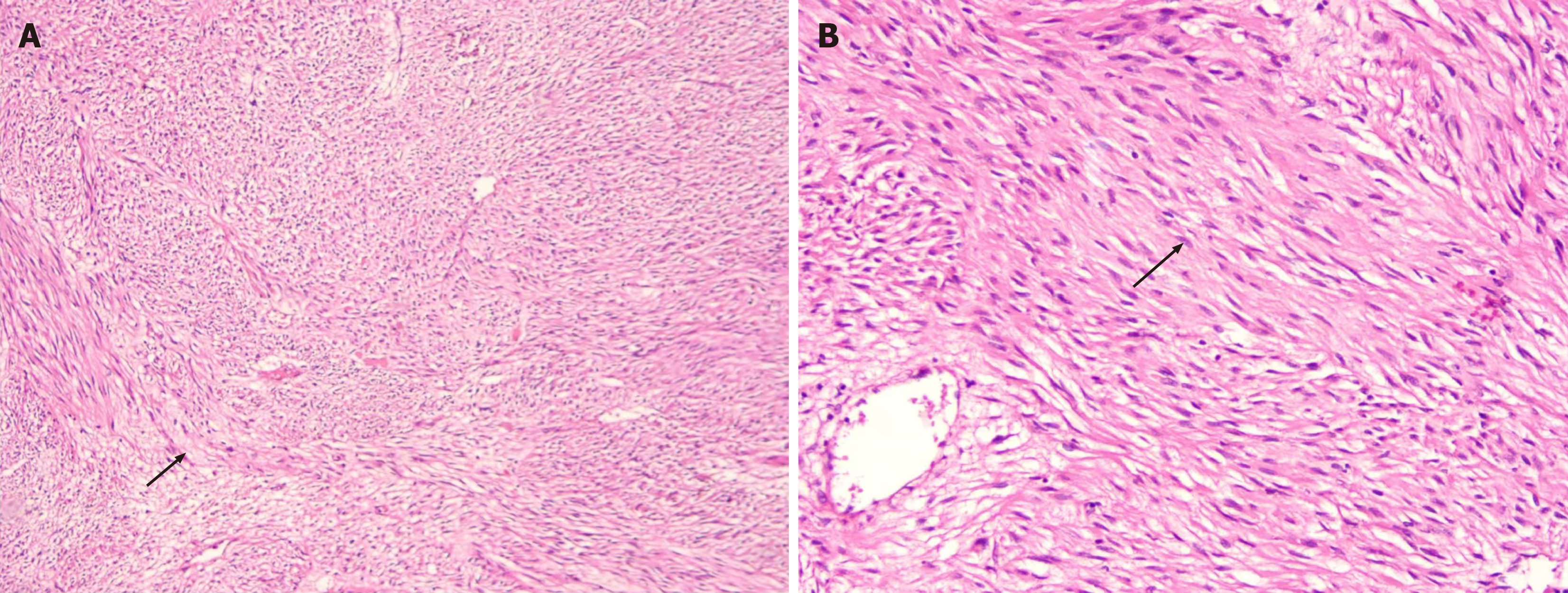
Pathological diagnosis confirmed a moderately risky GIST. Microscopic examination confirmed a spindle cell type GIST (Figure 3A). The tumor size was 5.8 cm × 5.5 cm × 4.0 cm, with a mitotic rate of < 5/5 mm2. Immunohistochemical results were: Creatine kinase (full specimen) (-), S-100 (-), smooth muscle actin (SMA) (-), anaplastic lymphoma kinase (-), SOX10 (-); Desmin (+), cluster of differentiation (CD) 34 (+), trimethylated histone H3 at lysine 27 (+); CD117 (partially +); DOG1 (strongly +); β-catenin(cytoplasmic +); SDHB expression was retained; Ki-67 proliferation index was approximately 2%.
Hybrid capture-based NGS was performed on the tumor tissue. This analysis confirmed that the tumor was wild-type, as no pathogenic mutations in the canonical KIT or PDGFRA genes were detected. Notably, the NGS panel revealed two novel gene fusions involving FGFR2: An FGFR2-CIT fusion and an intergenic-FGFR2 rearrangement. No other known driver mutations (e.g., in SDH or NF1 genes) were identified.
Postoperative histopathological examination of the resected specimen revealed multiple small intestinal masses (largest 9 cm, smallest 2 cm). Microscopically, the tumor was composed of spindle-shaped cells, with moderate atypia and a mitotic rate of approximately 4/5 mm2 (Figure 3B). The pathological diagnosis was high-risk multiple GISTs of the small intestine. Immunohistochemical results were: CD117 (+), DOG1 (+), SMA (-), S100 (slightly +), Ki67 (+, 5%), desmin (-). Similar to case 1, NGS performed on the tumor tissue did not detect any KIT or PDGFRA mutations. Instead, it revealed two distinct FGFR2 rearrangements: An FGFR2-CAMK2G fusion and an FGFR2-VCL fusion.
The patient underwent laparoscopic gastric tumor resection (R0 resection).
The patient underwent surgical resection of the small intestinal masses.
Following surgery, the patient was started on adjuvant therapy with imatinib mesylate at 400 mg daily. He tolerated the treatment well. During a median follow-up period of 28 months, regular surveillance showed no evidence of local recurrence or distant metastasis. The patient remains in sustained remission and continues to take imatinib.
Postoperatively, the patient was started on adjuvant imatinib mesylate at 400 mg daily. She showed good tolerance to the medication. During the last follow-up, 26 months after surgery, the patient was in continuous complete remission without signs of recurrence or metastasis. She continues to receive adjuvant imatinib therapy.
This report represents one of the first detailed clinical case series describing novel FGFR2 fusions as potential primary oncogenic drivers in patients with a rare subset of wild-type GISTs. The identification of FGFR2-CIT/intergenic-FGFR2 and FGFR2-CAMK2G/FGFR2-VCL fusions in these two cases significantly expands our understanding about the molecular landscape of GISTs and highlights a new potential therapeutic target for this challenging tumor type.
Most GISTs are driven by canonical gain-of-function mutations in KIT or PDGFRA[16,17]. However, approximately 10%-15% of GISTs are wild-type for these genes and are characterized by a heterogeneous molecular profile, including mutations in SDH subunits, NF1, or genes related to the RAS-MAPK signaling pathway[18,19]. Our discovery adds FGFR2 fusions to this growing list of drivers. The proposed mechanism of action, similar to that in other tumors, involves the 5’ partner genes (CIT, CAMK2G, VCL) providing dimerization domains that lead to ligand-independent, constitutive activation of the FGFR2 kinase domain, thereby promoting oncogenesis[20,21].
An intriguing and complex finding in our cases is the favorable response of both patients to adjuvant imatinib therapy, despite the absence of a classic KIT/PDGFRA mutations. Although imatinib is primarily a KIT/PDGFRA inhibitor, its clinical benefit here might be speculative. One hypothesis is a possible off-target inhibitory effect on the FGFR2 fusion protein or other critical downstream kinases[22-25]. Another possibility is that these tumors, despite their high-risk classification, possessed a relatively indolent biological behavior; the role of imatinib in their sustained remission is difficult to ascertain from this limited series[26-29]. This observation underscores the need for further mechanistic studies, such as in vitro drug sensitivity assays, to determine the precise reason for the imatinib response.
The identification of FGFR2 fusions opens a new avenue for targeted therapy, particularly in patients with advanced or imatinib-resistant disease[30-32]. In malignancies such as intrahepatic cholangiocarcinoma, FGFR2 fusions are established oncogenic drivers and predictive biomarkers for response to potent FGFR-specific inhibitors such as pemigatinib, futibatinib, and infigratinib[33-35]. Therefore, in patients with wild-type GIST who progress on standard TKIs, screening for FGFR2 alterations could be a promising strategy for guiding treatment with FGFR inhibitors.
This case series highlights the crucial role of comprehensive genomic profiling using NGS in modern GIST management. Conventional diagnostics are often insufficient to uncover rare molecular drivers in patients with wild-type GISTs[36]. Using NGS allowed us to identify these novel FGFR2 fusions, which would have otherwise been missed. This supports the consideration of NGS as a standard diagnostic component for all KIT/PDGFRA wild-type GISTs to enable precision-based therapeutic interventions[29,37].
Despite the compelling nature of our findings, this study has significant limitations. First, being a report of only two cases, our findings might lack generalizability; the prevalence of FGFR2 fusions in the wider wild-type GIST population remains unknown. Second, our study might lack functional validation[38]. Future research, including in vitro and in vivo experiments, is essential for confirming the oncogenicity of these specific FGFR2 fusions and to assess their sensitivity to various TKIs[39]. Third, the absence of orthogonal validation (e.g., fluorescence in situ hybridization or reverse transcription-polymerase chain reaction) for the NGS-detected fusions is a methodological limitation. Finally, the retrospective design and limited follow-up period prevent us from drawing conclusions on long-term survival.
This case series provides preliminary evidence that novel FGFR2 fusions could act as potential primary oncogenic drivers in a rare, molecularly distinct subset of wild-type GISTs. Our report highlights the critical value of comprehensive genomic profiling via NGS in identifying such rare, but clinically actionable, mutations. The sustained remission observed in our patients under adjuvant imatinib therapy warrants further investigation into the optimal treatment strategies for this molecular subtype. As precision oncology evolves, our findings suggest that FGFR-targeted therapies might represent a valuable future treatment strategy for managing patients with advanced or recurrent FGFR2-mutated GISTs, meriting further study in larger prospective cohorts.
| 1. | Hubbard SR, Miller WT. Receptor tyrosine kinases: mechanisms of activation and signaling. Curr Opin Cell Biol. 2007;19:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 387] [Cited by in RCA: 366] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 2. | Hubbard SR. Juxtamembrane autoinhibition in receptor tyrosine kinases. Nat Rev Mol Cell Biol. 2004;5:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 229] [Article Influence: 10.4] [Reference Citation Analysis (8)] |
| 3. | Simon MA. Receptor tyrosine kinases: specific outcomes from general signals. Cell. 2000;103:13-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Lasota J, Kowalik A, Felisiak-Golabek A, Zięba S, Wang ZF, Miettinen M. New Mechanisms of mTOR Pathway Activation in KIT-mutant Malignant GISTs. Appl Immunohistochem Mol Morphol. 2019;27:54-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Hoelzer D, Gökbuget N, Ottmann OG. Targeted therapies in the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia. Semin Hematol. 2002;39:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Yamamoto H, Tobo T, Nakamori M, Imamura M, Kojima A, Oda Y, Nakamura N, Takahira T, Yao T, Tsuneyoshi M. Neurofibromatosis type 1-related gastrointestinal stromal tumors: a special reference to loss of heterozygosity at 14q and 22q. J Cancer Res Clin Oncol. 2009;135:791-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Cohen S. Origins of growth factors: NGF and EGF. J Biol Chem. 2008;283:33793-33797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Gospodarowicz D, Bialecki H, Thakral TK. The angiogenic activity of the fibroblast and epidermal growth factor. Exp Eye Res. 1979;28:501-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 181] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Gospodarowicz D, Neufeld G, Schweigerer L. Fibroblast growth factor: structural and biological properties. J Cell Physiol Suppl. 1987;5:15-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 251] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Antoniades HN, Hunkapiller MW. Human platelet-derived growth factor (PDGF): amino-terminal amino acid sequence. Science. 1983;220:963-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 111] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1721] [Cited by in RCA: 1824] [Article Influence: 67.6] [Reference Citation Analysis (0)] |
| 12. | Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, Lee J, Yarden Y, Libermann TA, Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1913] [Cited by in RCA: 2021] [Article Influence: 48.1] [Reference Citation Analysis (0)] |
| 13. | Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3896] [Cited by in RCA: 3618] [Article Influence: 226.1] [Reference Citation Analysis (1)] |
| 14. | Diwanji D, Thaker T, Jura N. More than the sum of the parts: Toward full-length receptor tyrosine kinase structures. IUBMB Life. 2019;71:706-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 15. | Regad T. Targeting RTK Signaling Pathways in Cancer. Cancers (Basel). 2015;7:1758-1784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 223] [Cited by in RCA: 297] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 16. | Trenker R, Jura N. Receptor tyrosine kinase activation: From the ligand perspective. Curr Opin Cell Biol. 2020;63:174-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 17. | Robinson DR, Wu YM, Lin SF. The protein tyrosine kinase family of the human genome. Oncogene. 2000;19:5548-5557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 777] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 18. | Ségaliny AI, Tellez-Gabriel M, Heymann MF, Heymann D. Receptor tyrosine kinases: Characterisation, mechanism of action and therapeutic interests for bone cancers. J Bone Oncol. 2015;4:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 19. | Yamaoka T, Kusumoto S, Ando K, Ohba M, Ohmori T. Receptor Tyrosine Kinase-Targeted Cancer Therapy. Int J Mol Sci. 2018;19:3491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 210] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 20. | Zhang N, Li Y. Receptor tyrosine kinases: biological functions and anticancer targeted therapy. MedComm (2020). 2023;4:e446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 21. | Sigismund S, Avanzato D, Lanzetti L. Emerging functions of the EGFR in cancer. Mol Oncol. 2018;12:3-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 609] [Cited by in RCA: 1082] [Article Influence: 135.3] [Reference Citation Analysis (0)] |
| 22. | Thomas R, Weihua Z. Rethink of EGFR in Cancer With Its Kinase Independent Function on Board. Front Oncol. 2019;9:800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 140] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 23. | Farooqi AA, Siddik ZH. Platelet-derived growth factor (PDGF) signalling in cancer: rapidly emerging signalling landscape. Cell Biochem Funct. 2015;33:257-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 105] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 24. | Pandey P, Khan F, Upadhyay TK, Seungjoon M, Park MN, Kim B. New insights about the PDGF/PDGFR signaling pathway as a promising target to develop cancer therapeutic strategies. Biomed Pharmacother. 2023;161:114491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 25. | Zou X, Tang XY, Qu ZY, Sun ZW, Ji CF, Li YJ, Guo SD. Targeting the PDGF/PDGFR signaling pathway for cancer therapy: A review. Int J Biol Macromol. 2022;202:539-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 26. | Liu Y, Li Y, Wang Y, Lin C, Zhang D, Chen J, Ouyang L, Wu F, Zhang J, Chen L. Recent progress on vascular endothelial growth factor receptor inhibitors with dual targeting capabilities for tumor therapy. J Hematol Oncol. 2022;15:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 27. | Patel SA, Nilsson MB, Le X, Cascone T, Jain RK, Heymach JV. Molecular Mechanisms and Future Implications of VEGF/VEGFR in Cancer Therapy. Clin Cancer Res. 2023;29:30-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 290] [Article Influence: 96.7] [Reference Citation Analysis (0)] |
| 28. | Tiong KH, Mah LY, Leong CO. Functional roles of fibroblast growth factor receptors (FGFRs) signaling in human cancers. Apoptosis. 2013;18:1447-1468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 29. | Du S, Zhang Y, Xu J. Current progress in cancer treatment by targeting FGFR signaling. Cancer Biol Med. 2023;20:490-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 30. | Bahar ME, Kim HJ, Kim DR. Targeting the RAS/RAF/MAPK pathway for cancer therapy: from mechanism to clinical studies. Signal Transduct Target Ther. 2023;8:455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 468] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 31. | Pu X, Ye Q, Cai J, Yang X, Fu Y, Fan X, Wu H, Chen J, Qiu Y, Yue S. Typing FGFR2 translocation determines the response to targeted therapy of intrahepatic cholangiocarcinomas. Cell Death Dis. 2021;12:256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol. 2021;32:1111-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 33. | Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, Ni Z, Zhang B, Zhang D, Luo F, Chen H, Sun X, Feng JQ, Qi H, Chen L. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5:181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 363] [Cited by in RCA: 577] [Article Influence: 96.2] [Reference Citation Analysis (0)] |
| 34. | Katoh M, Loriot Y, Brandi G, Tavolari S, Wainberg ZA, Katoh M. FGFR-targeted therapeutics: clinical activity, mechanisms of resistance and new directions. Nat Rev Clin Oncol. 2024;21:312-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 103] [Reference Citation Analysis (0)] |
| 35. | Helsten T, Elkin S, Arthur E, Tomson BN, Carter J, Kurzrock R. The FGFR Landscape in Cancer: Analysis of 4,853 Tumors by Next-Generation Sequencing. Clin Cancer Res. 2016;22:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 629] [Article Influence: 57.2] [Reference Citation Analysis (0)] |
| 36. | De Luca A, Esposito Abate R, Rachiglio AM, Maiello MR, Esposito C, Schettino C, Izzo F, Nasti G, Normanno N. FGFR Fusions in Cancer: From Diagnostic Approaches to Therapeutic Intervention. Int J Mol Sci. 2020;21:6856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 37. | Katoh M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int J Mol Med. 2016;38:3-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 334] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 38. | Dienstmann R, Rodon J, Prat A, Perez-Garcia J, Adamo B, Felip E, Cortes J, Iafrate AJ, Nuciforo P, Tabernero J. Genomic aberrations in the FGFR pathway: opportunities for targeted therapies in solid tumors. Ann Oncol. 2014;25:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 321] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 39. | Cristinziano G, Porru M, Lamberti D, Buglioni S, Rollo F, Amoreo CA, Manni I, Giannarelli D, Cristofoletti C, Russo G, Borad MJ, Grazi GL, Diodoro MG, Giordano S, Sacconi A, Forcato M, Anastasi S, Leonetti C, Segatto O. FGFR2 fusion proteins drive oncogenic transformation of mouse liver organoids towards cholangiocarcinoma. J Hepatol. 2021;75:351-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |