Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.111339
Revised: July 28, 2025
Accepted: August 27, 2025
Published online: October 15, 2025
Processing time: 108 Days and 23 Hours
Gastrointestinal (GI) cancers exact a staggering global toll through high incidence, mortality, and treatment costs, yet clinical research continues to be hampered by inadequate patient stratification, challenging recruitment, suboptimal adherence, and time-consuming endpoint confirmations. Against this backdrop, artificial intelligence (AI) emerges as a powerful game-changer, offering streamlined trial design, predictive enrollment matching, dynamic endpoint assessment, and real-world data integration. This review synthesizes AI-driven advancements across the GI cancer research continuum. It covers precise patient stratification, auto
Core Tip: Artificial intelligence (AI) is reshaping gastrointestinal cancer research by improving patient stratification, streamlining clinical trial design, and enabling real-world data integration. This review highlights how AI can accelerate trial timelines, enhance precision, and support remote monitoring, while also addressing key challenges in data standardization and ethical implementation.
- Citation: Wang Z, Zhang RY, Ji C, Zhang JY, Yue BT, Wang F. Revolutionizing gastrointestinal cancer research with artificial intelligence: From precision patient stratification to real-world evidence. World J Gastrointest Oncol 2025; 17(10): 111339
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/111339.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.111339
Gastrointestinal (GI) cancers remain among the leading causes of cancer-related morbidity and mortality worldwide. In recent years, the global landscape of GI malignancies has undergone notable changes, particularly in East Asia, where both overall incidence and early-onset cases are rising[1,2]. Meanwhile, non-hereditary risk factors such as diet, microbiota, and environmental exposures are contributing increasingly to disease burden[3]. These trends call for a new disease management paradigm that integrates geographic epidemiology, molecular subtyping, and exposome-based profiling.
On the therapeutic front, chemoimmunotherapy has become a standard first-line treatment for advanced GI cancers[4,5]. However, despite initial efficacy, challenges remain in clinical translation, especially in trial recruitment, protocol adherence, and timely endpoint evaluation[6]. Moreover, cancer-specific immunotherapies—such as toripalimab in esophageal squamous cell carcinoma and nivolumab in gastric cancer—require further refinement in trial design and patient stratification strategies. Adaptive trial frameworks and targeted recruitment models are needed to enhance efficiency and clinical relevance.
Artificial intelligence (AI) has shown promise in transforming oncology drug development by improving data processing efficiency and accelerating mechanistic discovery[7,8]. Nevertheless, its integration into clinical research remains limited by homogeneous development pipelines, overlapping targets, and a disconnect from practical implementation[9]. To reduce clinical failure rates, future efforts must shift from black-box predictive models to mechanism-informed, interpretable frameworks[7,8]. Achieving this goal will require interdisciplinary collaboration and innovation chains aligned with clinical needs.
In clinical applications, AI is evolving beyond decision-support tools toward foundational infrastructure for healthcare delivery. Building effective systems will require multimodal data integration, interpretable and generalizable models, and embedded deployment within clinical workflows. By centering these systems on large language models (LLMs)[10], and integrating vertical-domain AI with process automation AI[11-13], a closed-loop pipeline can be formed—from clinical problem definition to model validation—with improved ethical governance and functional efficiency.
Despite growing momentum, practical challenges remain. High-performing AI models often rely on small, well-annotated datasets[14], whereas real-world validation depends on large, heterogeneous, and lower-quality datasets. Models such as TrialTranslator offer a framework to iteratively link prediction and validation[15]. Developing robust mappings between pathology and molecular features may help resolve this data quality gap. Furthermore, in diseases like pancreatic cancer, overcoming single-modality limitations is essential to realize a fully integrated, explainable AI system supported by real-world evidence (RWE).
Multimodal AI applications are also advancing beyond image-based classification. Deep learning has proven valuable in pathology standardization tasks, such as tumor grading and prognosis, yet its scope is constrained by single-dimensional data. By fusing molecular, imaging, and temporal clinical data, multimodal deep learning can support dynamic prediction and mechanistic inference. Recent developments—such as DrugCell for combination therapy design and MuMo for treatment optimization—highlight this potential[8,16]. Still, challenges remain in balancing model complexity with interpretability and translating retrospective validation into prospective utility.
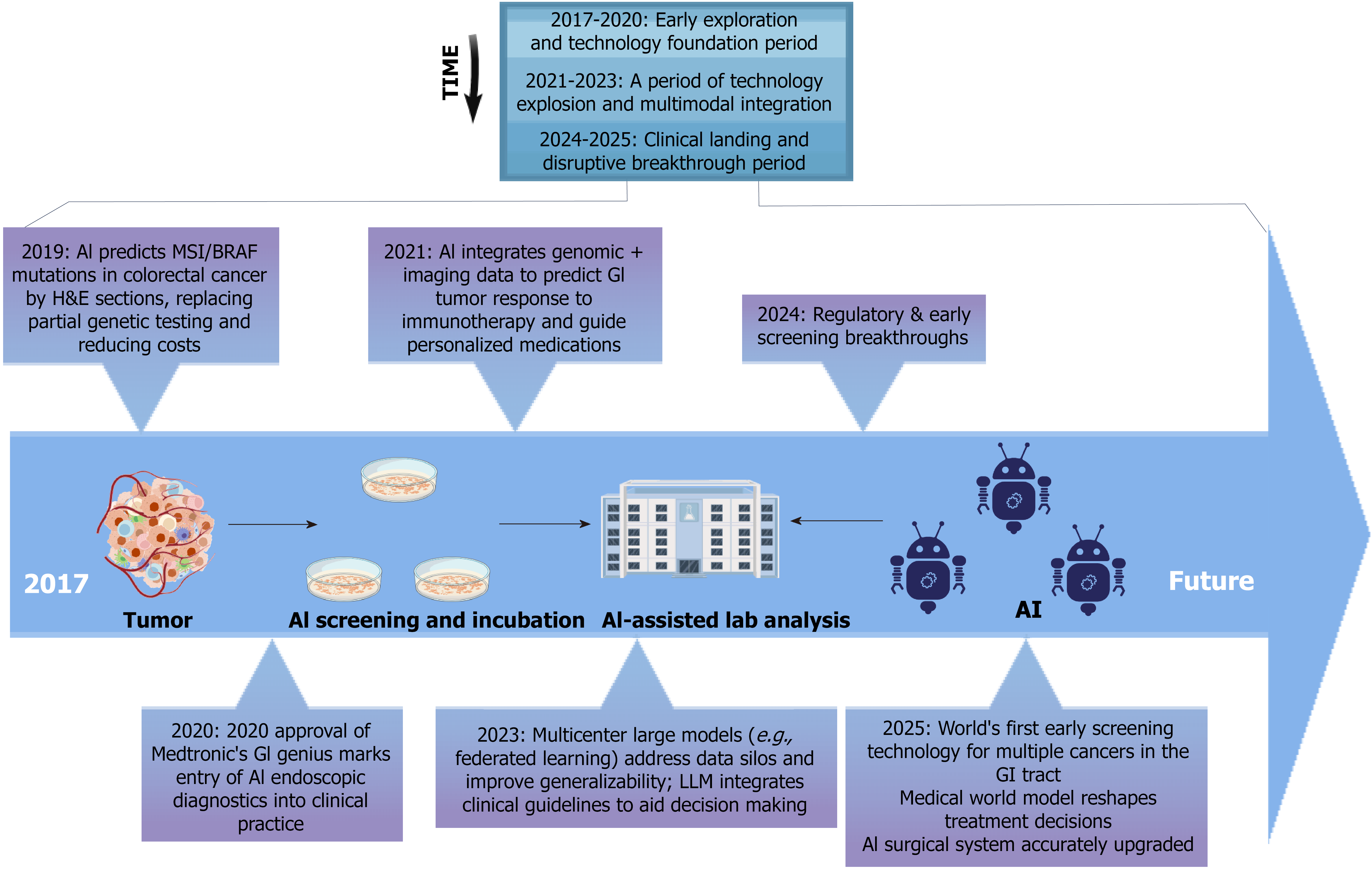
A timeline of key milestones from 2017 to 2025 (Figure 1) highlights the translational progress of AI in GI oncology from early development to clinical application. In this review, we provide a comprehensive overview of how AI is reshaping GI cancer research—from precision patient stratification and adaptive trial design to RWE integration. We also explore persistent challenges and future directions toward building interpretable, clinically integrated AI frameworks.
Integrating deep learning models with multimodal data—including imaging, pathology, clinical variables, and molecular profiles—has significantly improved the accuracy of patient stratification. Compared with traditional methods such as tumor-node-metastasis (TNM) staging and Eastern Cooperative Oncology Group performance status, which rely primarily on anatomical and functional assessments, AI-based approaches offer notable advantages. Conventional models struggle to capture tumor heterogeneity, whereas AI algorithms can extract complex features from diverse data sources to identify refined patient subgroups, thereby enhancing stratification precision.
For example, in the KEYNOTE-062 study, an AI model incorporating tumor mutational burden (TMB) demonstrated a stronger association with clinical outcomes in gastric cancer patients receiving first-line pembrolizumab ± chemotherapy, especially among those with high TMB levels. This AI-driven analysis provided more precise stratification than traditional methods, addressing their limitations in detecting response variability[17].
Similarly, the predictive value of PD-L1 expression is constrained by variability in evaluation criteria and tumor-type specificity. AI models that combine PD-L1 expression with tumor immune microenvironment characteristics and genomic biomarkers have shown superior predictive accuracy for immunotherapy response, improving the precision of treatment decisions[10,12].
In addition, AI has advanced HER2 stratification in gastric and gastroesophageal junction cancers, surpassing the limitations of conventional immunohistochemistry/fluorescence in situ hybridization techniques. These AI-based methods can more accurately identify patients with low HER2 expression who may still benefit from targeted therapy, thus overcoming classification gaps in current diagnostic tools[18,19].
AI also enhances early diagnosis and disease monitoring. When combined with liquid biopsy technologies—such as ctDNA methylation and cfDNA fragmentomics—AI can improve the sensitivity and specificity of early cancer detection and longitudinal surveillance, outperforming traditional imaging and tissue biopsy methods[20,21]. Moreover, integrating AI with radiomics allows for more nuanced tumor risk stratification and treatment response prediction, offering a non-invasive alternative to conventional tools and ultimately improving patient care[22,23].
Despite these promising developments, several challenges remain, including the lack of standardized data formats, protocol harmonization, and improved model interpretability. Furthermore, the reliance on retrospective studies and the absence of large-scale prospective validation limit the current clinical utility of these AI models. Future progress will depend on continued efforts in model standardization, multicenter collaboration, and rigorous prospective trials to fully unlock the potential of AI in personalized oncology[23,24].
Therapeutic strategies for certain GI cancers, such as gastroesophageal carcinoma, continue to improve. Key ad
AI has demonstrated clear advantages in the identification and modeling of alternative endpoints. In pancreatic cancer, a dual-response model combining CA19-9 reduction and tumor regression grade, constructed using recursive partitioning algorithms, has shown strong prognostic value by identifying survival-relevant subgroups[31]. However, CA19-9 alone remains insufficient for long-term prediction and must be integrated with molecular or imaging-based indicators to improve reliability.
Similar AI applications have emerged in gastric cancer. A deep learning model based on H&E-stained histological slides (CRSNet) achieved early prediction of pathological response, with area under the curves (AUCs) ranging from 0.936 to 0.923, effectively distinguishing major from minor responders to chemotherapy[32]. In addition, a radiomics-based nomogram derived from preoperative computed tomography (CT) demonstrated robust performance in predicting response to neoadjuvant chemotherapy in locally advanced cases, with external validation AUCs exceeding 0.80[33]. Radiomics features have also been used for non-invasive prediction of pathological residual disease and chemotherapy benefit[34].
Multimodal AI models further enhance predictive capability. The MuMo model, which integrates imaging, pathology, and clinical data, can differentiate survival outcomes among HER2-positive patients undergoing different treatment strategies[16]. The PMetNet model, built on preoperative CT imaging, identifies occult peritoneal metastases with high accuracy (AUC: 0.920-0.946)[35]. Similarly, a random forest model for predicting 90-day postoperative mortality has demonstrated superior performance compared to traditional clinical scoring systems[36].
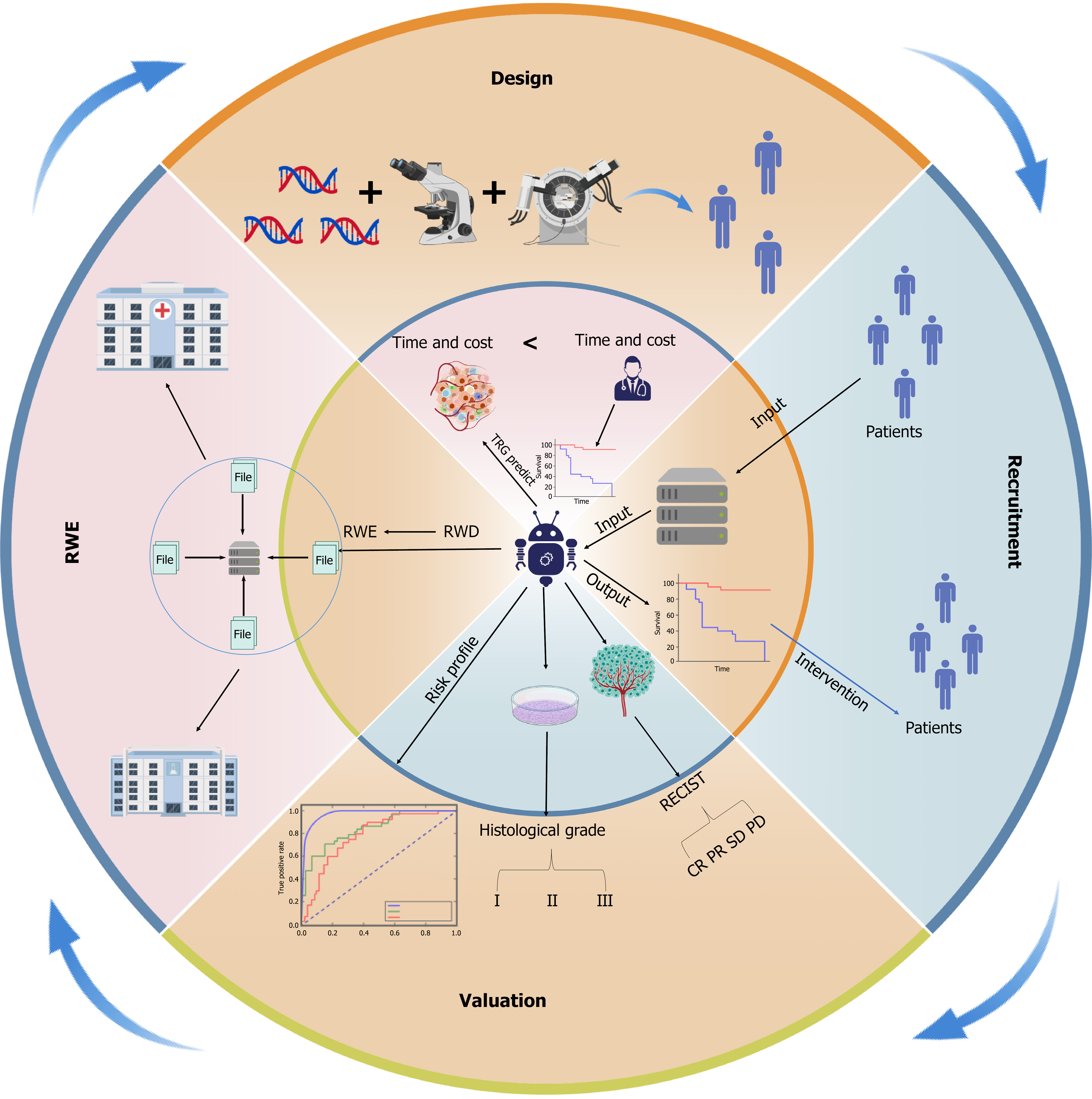
Despite their potential to enhance endpoint resolution and shorten trial timelines, the clinical implementation of AI-based models still requires standardized validation and improved interpretability. Figure 2 provides a visual summary of AI applications across the clinical trial workflow, highlighting how innovations in design and stratification lay the groundwork for improved recruitment, compliance, and outcome assessment.
As clinical trial success increasingly depends on precise and timely patient recruitment, AI-driven matching systems have emerged as essential tools to improve screening efficiency, accelerate enrollment, and reduce resource burdens.
Recent advancements in LLMs have demonstrated strong capabilities in clinical data extraction and bias evaluation. In a benchmarking study involving 107 complementary medicine trials, Moonshot-v1-128k and Claude-3.5-sonnet achieved accuracies of ≥ 95% in data extraction and bias scoring, which further improved to ≥ 97% with minimal human assistance, while significantly reducing processing time[37].
In pathology report structuring, ChatGPT-3.5 achieved an 89% accuracy rate in extracting histological classifications from over 1000 Lung cancer reports, outperforming traditional natural language processing (NLP) tools. However, it struggled with parsing specialized terminology and TNM staging rules. In pediatric osteosarcoma cases (n = 191), extraction accuracies for tumor grading and surgical margin status reached 98.6% and 100%, respectively, indicating more consistent performance in defined domains[38]. Additionally, a rule-based NLP pipeline for multi-cancer surgical pathology reports achieved 100% accuracy for ICD code extraction, with F1 scores of 0.88 and 0.90 for T and N staging, respectively, but only 0.24 for M staging, reflecting challenges in specific data fields[39].
Despite these advances, inefficiencies in patient recruitment remain a major barrier to trial success. An analysis of 7776 phase II-III oncology trials launched between 2005 and 2011 showed that around 20% were terminated prematurely, leading to the loss of nearly 48000 potential participants[40]. Moreover, approximately 32% of real-world patients were excluded due to strict eligibility criteria, and this subgroup tended to have worse prognoses[41]. To address this, the Trial Pathfinder framework used real-world data (RWD) to optimize inclusion criteria. This adjustment doubled the average number of eligible patients and reduced the overall hazard ratio by approximately 0.05, demonstrating the value of data-driven trial design[42].
AI has also shown promise in trial screening. TrialGPT achieved over 90% recall across more than 75000 clinical trials, with a patient–criteria matching accuracy of 87.3% and a 42.6% reduction in screening time[43]. Other studies indicate that AI performs comparably or better than human evaluators, while significantly reducing labor and time costs. However, challenges remain regarding fairness and generalizability, especially in under-resourced settings or diverse populations[44].
Importantly, AI-driven alerts—such as those based on imaging or genomic progression—delivered to oncologists alone have not significantly improved enrollment rates. This underscores the need to embed AI more deeply into clinical workflows and to align system outputs with patient preferences and institutional support mechanisms[45].
While these advances highlight the technical potential of AI in improving trial recruitment, their real-world implementation remains limited by multiple system-level barriers. Many hospitals lack standardized application programming interfaces or real-time data pipelines, restricting the ability of AI tools to access timely laboratory results, imaging data, and staging information—particularly in settings with legacy electronic health record (EHR) systems. Even when data access is available, substantial heterogeneity in data formats, terminologies, and documentation practices across EHR platforms undermines interoperability and matching accuracy. Furthermore, current AI-generated outputs often require downstream manual review and verification by clinical research staff before actionable decisions can be made, which limits automation and reduces overall workflow efficiency. In addition, data privacy regulations and fragmented data governance frameworks across institutions pose further obstacles to the deployment of centralized or federated AI systems. Addressing these issues will require the establishment of common data standards, robust technical infrastructure, and seamless integration pathways tailored to clinical trial operations.
As clinical trials become more complex and prolonged, maintaining high patient adherence has become a key factor in ensuring study quality and treatment validation. AI is shifting adherence support from passive follow-up to proactive risk identification and dynamic intervention.
In the preoperative setting, AI has shown promise in assessing nutritional risk. A hybrid model integrating the psoas muscle depth at the third lumbar vertebra on non-contrast CT, along with clinical indicators such as body mass index, lymphocyte count, and albumin level, achieved AUCs of 0.909 (95%CI: 0.869-0.948) in the training set and 0.857 (95%CI: 0.782-0.931) in the testing set. The corresponding accuracies were 0.845 and 0.861. Patients classified as high-risk for malnutrition had significantly worse overall survival (P = 0.005)[46].
Beyond surgical preparation, AI-based tools are being adopted for behavioral monitoring in cancer survivors. A study involving breast, prostate, and colorectal cancer survivors used mobile applications to track physical activity (PA). Most participants accepted digital interventions, though existing public PA apps failed to meet all user needs. Suggestions for personalized adaptation and feedback features were widely reported[47].
AI also supports treatment decision-making by predicting disease progression risks. A model built on easily accessible clinicopathological variables showed moderate performance in predicting lymph node metastasis in T2 colorectal cancer. However, false-negative rates remain a concern. Researchers recommended expanding multi-center datasets and separating colon from rectal cases to improve accuracy and generalizability[48].
In endoscopic surveillance, AI further enhances early detection and adherence. A computer-aided detection system applied to Barrett’s esophagus significantly increased image sensitivity from 74% to 88% (OR: 2.04; 95%CI: 1.73-2.42; P < 0.0001) and video sensitivity from 67% to 79% (OR: 2.35; 95%CI: 1.90-2.94; P < 0.0001), without compromising specificity. In both full and benchmark datasets, detection rates exceeded 95%[49].
Despite these advances, the deployment of AI-based adherence tools in clinical trials faces practical constraints. Many trial centers lack standardized protocols for integrating AI apps or wearables into study workflows. Data collected from mobile platforms often vary in format and quality, creating challenges in harmonization and regulatory documentation. Furthermore, patient access to smartphones, digital literacy, and willingness to use technology vary widely across populations, potentially introducing bias in adherence outcomes. These factors must be addressed to ensure equitable and effective AI-driven support in real-world trials.
AI is increasingly transforming the evaluation of therapeutic efficacy and safety in GI cancers by enhancing the precision, reproducibility, and scalability of clinical assessments. Traditional imaging and histopathological evaluations, though foundational, are often limited by interobserver variability and time constraints. AI-based contouring models have demonstrated high concordance with expert annotations in gross tumor volume delineation, while significantly reducing processing time. Notably, radiomics features derived from these models remain stable and predictive of pathologic complete response, even under automated conditions[50]. To overcome the narrow applicability of task-specific segmentation, generalized models like MedSAM have been trained across 30 tumor types and modalities. They exhibit robust cross-domain performance, although challenges persist in rare or low-quality data[51].
AI has also advanced volumetric tumor analysis. Techniques combining tumor spheroid models with indices such as the excessive perimeter index and multiscale entropy index allow for automated quantification of tumor invasiveness[52]. Deep learning has improved lesion detection in hepatic metastases > 20 mm, although performance remains suboptimal for nodules < 10 mm[53]. Similarly, automatic liver segmentation is effective for routine CT but less accurate in low-contrast or anatomically complex settings[54]. In colorectal cancer, AI-based CT interpretation remains under-validated across institutions. LLMs like GPT-4V show promise in low-data environments, matching dedicated neural networks in tasks such as polyp typing and lymph node detection, although their cross-platform robustness is yet to be established[55].
In digital pathology, deep learning models applied to whole-slide images (WSIs) can predict survival and chemotherapy benefit, independent of conventional variables[56]. The aiN score derived from H&E-stained gastric cancer slides correlates with survival more strongly than nodal staging[57]. Machine learning (ML) models have also been developed to predict recurrence in hepatocellular carcinoma and assess microsatellite instability (MSI) using WSIs[58,59]. Advanced graph neural networks convert WSIs into spatial graphs, enabling multi-omics predictions validated across centers[60]. Platforms like nuclei.io facilitate model refinement through human-in-the-loop strategies, though clinical implementation still demands further validation[61].
Alongside response evaluation, AI is increasingly used to predict adverse events and guide risk-based stratification. Models like DySPred, which leverage dynamic graph convolutional networks, have shown reliable performance in predicting immune-related toxicities, even with small datasets[62]. Deep learning applied to histopathological slides has improved survival prediction in colorectal cancer subgroups, while ensemble strategies enhance robustness[63]. However, image-only models show limited generalizability, underscoring the advantage of multimodal approaches that incorporate clinical variables.
The integration of molecular and histological data further strengthens predictive accuracy. For instance, immune-related lncRNA signatures correlate with PD-L1 expression and immune infiltration, informing treatment selection[64]. Prognostic scores derived from H&E slides outperform traditional indicators in stage II/III colorectal cancer[65,66], and the combinatory cancer hallmark–based gene signature identifies high-risk stage II patients who may benefit from adjuvant therapy[67]. AI models such as the "deep stromal score" and organoid-based predictive tools further support individualized treatment planning by linking tissue morphology and genomic responses[68,69].
In metastatic settings, multimodal frameworks like DERBY+ integrate positron emission tomography/CT, pathology, and clinical data to predict bevacizumab responsiveness and survival[70]. Other models use WSIs to quantify tumor-to-metastatic lymph node ratios as independent prognostic indicators[71]. In neoadjuvant contexts, AI models such as iSCLM and radiomics-deep learning hybrids assess tumor microenvironment characteristics and treatment response with high fidelity[72,73].
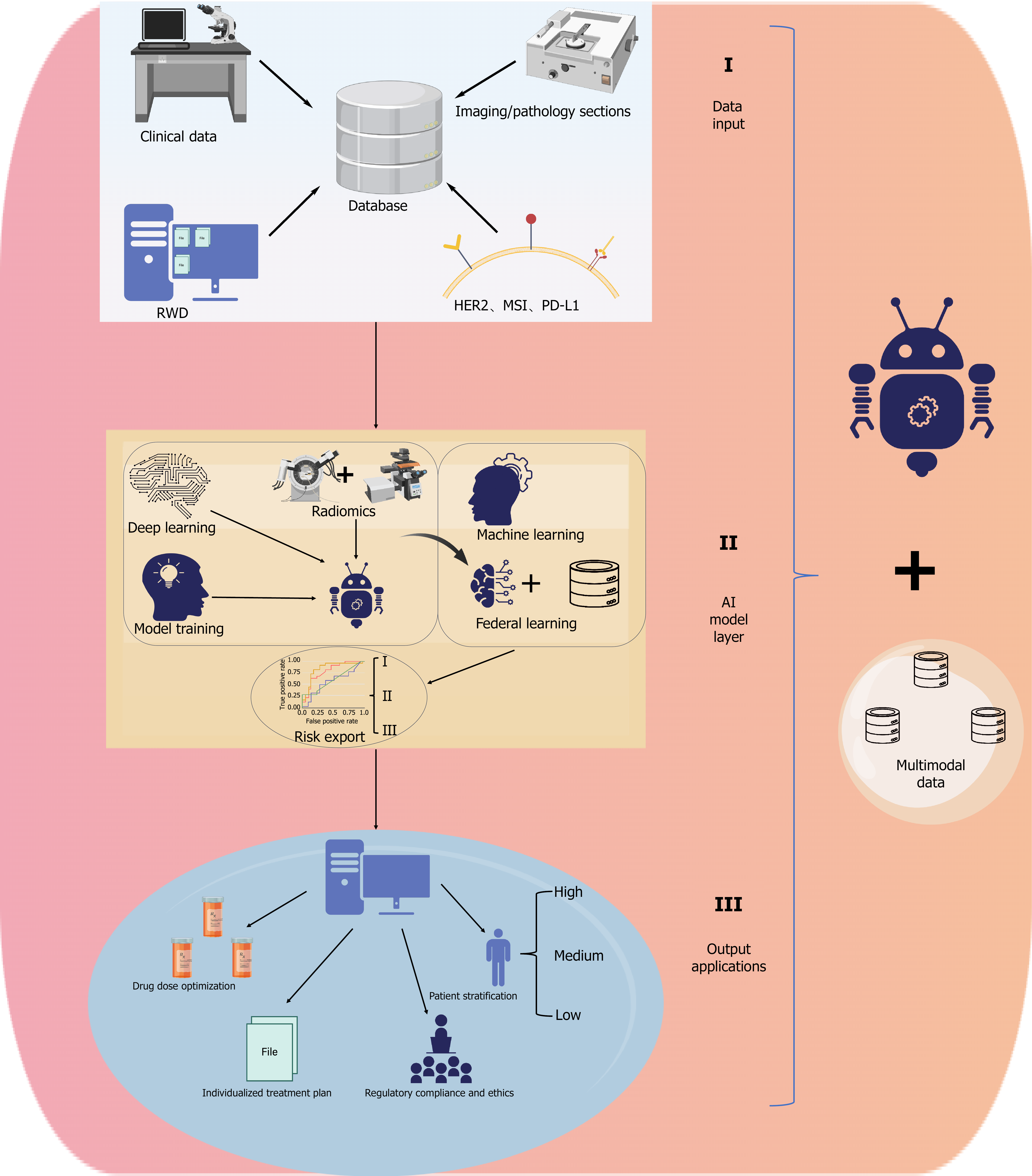
Collectively, AI is reshaping the evaluation paradigm and clinical decision-making in GI cancer research. It enhances the standardization of response assessment and risk prediction while providing forward-looking support for personalized treatment. Figure 3 illustrates a multi-tiered AI framework that systematically integrates multimodal data, modeling strategies, and clinical outputs, highlighting the translational pathway from data acquisition to real-world application.
Transforming RWD into RWE requires the integration of both structured and unstructured clinical information. In this process, NLP has become a key enabler, bridging free-text clinical narratives and structured datasets.
A representative example is the MSK-CHORD dataset from Memorial Sloan Kettering Cancer Center. This dataset includes unified annotations of pathology reports, medication records, patient-reported demographics, tumor registry entries, and genomic data from 24950 patients. Notably, NLP-derived features such as "site of disease" showed superior predictive performance—both in cross-validation and external multicenter cohorts—compared to models based solely on genomic or staging data[74].
Building on this, recent evaluations of patient-reported outcomes in EHRs suggest that most current NLP and ML methods still rely on rule-based parsing for unstructured text. However, given the increasing complexity of clinical care, there is a growing need to incorporate and validate neural network-based ML approaches to enhance processing efficiency and generalizability[75].
To further advance symptom-related research, NLP methods for extracting symptom information from free-text EHRs should focus on standardized symptom formats and patient-level heterogeneity. The development and dissemination of open-source algorithms, workflows, and standardized vocabularies are crucial for advancing symptom-focused research in oncology[76].
As AI becomes increasingly integrated into oncology, its role in connecting clinical trials with real-world practice is gaining attention. Beyond retrospective analyses and data curation, AI is now being explored as a dynamic tool for translational decision-making, particularly in drug development and precision medicine.
With the parallel expansion of large-scale oncology datasets and advanced computational methods, AI has demonstrated substantial promise in drug discovery. By integrating multi-source experimental data—such as genomic sequencing, imaging, and high-throughput screening—computational tools can broaden the pool of therapeutic candidates and accelerate discovery timelines. However, to realize these benefits, rigorous model design and ongoing evaluation are essential for achieving reliable human–machine collaboration in translational workflows[77].
In addition to drug discovery, AI is making significant progress in personalized oncology. By combining genomic features with digital health records, AI models have supported early diagnosis, survival prediction, and individualized treatment planning, especially through innovations in radiomics and deep learning. Still, key barriers remain. The scarcity of rare tumor data, the need for comprehensive clinical validation, and the lack of mature regulatory frameworks continue to limit the widespread application of AI in clinical oncology[78].
To illustrate how these AI-driven innovations have been applied across the clinical research continuum, Table 1 summarizes representative studies and tools from recent literature. This synthesis highlights the diversity of tumor types, algorithms, and translational outcomes involved in AI-powered GI oncology.
| Research or products | Application scenarios | Tumor type | Main technologies/algorithms | Findings/strengths | Ref. | |
| AI optimizes clinical trial design | Precise patient stratification | Multimodal integration | GIST | CE-CT WSI | Constructing a multimodal model to predict the RFS of GIST that outperforms unimodal models | Xiao et al[99], 2024 |
| Dynamic endpoint selection | AI predicts alternate endpoints | Pancreatic cancer | Kaplan-Meier, Cox regression and recursive partitioning analysis | By AI analysis, it was found that the combined assessment of CA19-9 response and MPR (TRG0-1) was a better predictor of pre-OS in patients with PDAC | [31] | |
| AI improves patient recruitment & adherence | Intelligent matching system | EHR + NLP parsing | None | NLP | The development of NLP process enables automated extraction of tumor staging and diagnostic information across cancer types with high accuracy | Abedian et al[39], 2021 |
| Patient adherence management | AI remote follow-up personalized intervention | Gastric cancer | CT, DL | DL based on hybrid models may be a potential tool for predicting malnutrition in gastric cancer patients | Huang et al[46], 2024 | |
| Application of AI in efficacy and safety assessment | Automated imaging/pathology evaluation | Image recognition | Colorectal cancer liver metastasis | Fully convolutional networks | The method can effectively improve the work efficiency, but it needs further improvement in segmentation accuracy and consistency | Vorontsov et al[53], 2019 |
| Digital pathology | Colon | WSI | This method can accurately predict molecular features such as gene mutations, copy number changes, MSI, and protein expression in colon cancer patients | Ding et al[60], 2022 | ||
| Adverse event forecasting | AI risk model | None | DySPred | The model demonstrated good stability, was able to predict toxicity trends in different populations and cancer types with small samples, and could be effectively compared with transcriptional changes of small molecule antitumor drugs | Yan et al[62], 2025 | |
| Value and limitations | Colorectal cancer | DL | Models perform well on specific datasets, but they do not generalize well enough to perform consistently well across many different clinical scenarios | Höhn et al[63], 2023 | ||
| RWE and AI | RWD into RWE | Fusion of multiple data sources, AI improves data cleaning efficiency | None | MSK-CHORD | Demonstrates the feasibility of automated annotation from unstructured notes and its utility in predicting patient prognosis | Jee et al[74], 2024 |
| AI bridging clinical trials & practices | Model generalizability validation | None | AI | AI has outstanding advantages in the processing of multiple sets of data and decision making for precise tumors, but still falls short in the areas of limited data on rare cancers, clinical validation needs, and regulatory issues | Vyas et al[78], 2025 | |
Because economic evidence specific to GI cancers is still limited, we broaden the scope to AI applications in solid tumors. Developing and maintaining AI systems—especially those involving multimodal data and continuous learning—requires substantial upfront investment, including costs for data curation, model training, and clinical integration. This leads to a key question raised by hospital administrators and policy-makers: Can the savings generated by AI offset its high development and operational expenses?
Real-world evaluations suggest that, under the right conditions, AI can deliver favorable cost-benefit outcomes. For example, in Europe, combined gastric and colorectal endoscopic screening becomes cost-effective if AI improves accuracy by just 1%, even in countries where gastric cancer incidence is low[79]. In breast cancer, AI outperforms polygenic risk scores for guiding personalized screening, reducing overdiagnosis and unnecessary procedures while remaining within accepted cost-effectiveness thresholds[80,81]. In liver cancer surveillance, adding AI to magnetic resonance imaging scans in cirrhotic patients results in earlier detection at an incremental cost of only €9888 per QALY—well below willingness-to-pay limits[82].
AI may also enhance economic efficiency by streamlining clinical trial operations. Though direct cost-effectiveness data in this context are scarce, the logic is clear: AI can accelerate patient recruitment, reduce screen failure rates, and improve trial targeting. This shortens timelines and lowers per-patient costs, especially in adaptive trial designs. These benefits, if realized at scale, may help justify the initial investment in AI infrastructure.
However, some applications reveal trade-offs. A large Dutch mHealth study showed that while AI-based apps detected more (pre)malignant skin lesions, they also led to more consultations for benign findings, raising per-case costs to €2567[83]. Similarly, in diagnostics for melanoma and diabetic retinopathy, AI offered only marginal gains in QALYs and cost savings, highlighting the importance of use-case specificity[84]. For lung cancer, AI was cost-effective in screening populations but not in symptomatic settings when full care pathway costs were considered[85].
These findings underscore a key insight for decision-makers: AI’s economic value depends not only on accuracy, but also on the context, workflow integration, and downstream clinical actions. Future AI development should incorporate cost-effectiveness modeling from early stages, with clear benchmarks for expected financial returns. Where appropriate, shared funding models and regulatory guidance can help align innovation with sustainable deployment.
The integration of AI into clinical oncology research is rapidly transforming how evidence is generated, interpreted, and applied. As AI technologies mature, they offer both unprecedented opportunities and complex challenges across data sharing, regulation, clinical collaboration, and ethical oversight. One major challenge lies in data standardization and privacy protection. Federated learning has emerged as a promising approach to enable cross-institutional modeling without transferring raw data, thereby mitigating data silos and privacy risks[86,87]. In medical imaging tasks, models trained with federated approaches have shown improved generalizability and accuracy in segmenting glioblastoma boundaries across institutions[88]. To further reduce communication overhead and enhance privacy, frameworks like ProxyFL and FedKD have incorporated differential privacy mechanisms[89]. For non-IID data, strategies such as FedPerGNN and federated learning task have introduced graph-based learning and task-relevance scoring to support personalized, fair modeling[90]. Additionally, federated learning has shown robustness in drug development by integrating small, biased datasets to improve molecular property prediction[91]. Homomorphic encryption further enables secure computation on encrypted genomic data[92], though concerns persist regarding vulnerability to low-visibility adversarial attacks, necessitating the development of robust defense strategies[93]. On the regulatory front, agencies such as the United States Food and Drug Administration (FDA) have recognized the need for a shift from one-time approval models to adaptive regulatory frameworks that accommodate AI/ML-based software's continual learning and updates. The FDA’s 2021 action plan proposed five focus areas: Updating the regulatory framework, establishing good ML practices, streamlining pre-specified change control processes, improving transparency and explainability, and adopting a patient-centered regulatory approach. These principles emphasize risk-based oversight tailored to the evolving nature of AI-based software as medical devices. Clinician-AI collaboration is also shaped by context-specific factors. In radiotherapy studies, physicians’ decisions to adopt AI recommendations were influenced by their domain expertise, tumor characteristics, treatment modalities, and model interpretability[94]. A randomized controlled trial showed that physicians aided by LLMs scored higher in open-ended reasoning tasks, though they required more time, and outcomes were similar to LLMs alone[95]. Tools such as MedFound have demonstrated diagnostic support capabilities, but how these models can be seamlessly integrated into clinical workflows remains unclear[96]. A meta-analysis found that generative AI achieved a diagnostic accuracy of only 52.1%, underperforming human experts and offering limited improvement over non-expert clinicians, highlighting the risk of misuse[97]. Moreover, in some trials, physicians using LLMs did not outperform unaided LLMs, emphasizing the need for improved user interface design and clinician training[98]. Overall, AI holds multifaceted value across the clinical research continuum—from enabling refined patient stratification and dynamic endpoint selection to enhancing safety monitoring and supporting RWE translation. As multi-center collaboration deepens and regulatory frameworks mature, AI is expected to play an increasingly integral role in bridging innovation and practice.
We express our gratitude to Dr. Tan for assisting in the preparation of the manuscript.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12615] [Article Influence: 6307.5] [Reference Citation Analysis (6)] |
| 2. | Huang J, Lucero-Prisno DE 3rd, Zhang L, Xu W, Wong SH, Ng SC, Wong MCS. Updated epidemiology of gastrointestinal cancers in East Asia. Nat Rev Gastroenterol Hepatol. 2023;20:271-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 172] [Reference Citation Analysis (0)] |
| 3. | Ben-Aharon I, van Laarhoven HWM, Fontana E, Obermannova R, Nilsson M, Lordick F. Early-Onset Cancer in the Gastrointestinal Tract Is on the Rise-Evidence and Implications. Cancer Discov. 2023;13:538-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 117] [Reference Citation Analysis (0)] |
| 4. | Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng J, Yang S, Fan Y, Shi J, Zhang X, Shen L, Shu Y, Wang C, Dai T, Mao T, Chen L, Guo Z, Liu B, Pan H, Cang S, Jiang Y, Wang J, Ye M, Chen Z, Jiang D, Lin Q, Ren W, Wang J, Wu L, Xu Y, Miao Z, Sun M, Xie C, Liu Y, Wang Q, Zhao L, Li Q, Huang C, Jiang K, Yang K, Li D, Liu Y, Zhu Z, Chen R, Jia L, Li W, Liao W, Liu HX, Ma D, Ma J, Qin Y, Shi Z, Wei Q, Xiao K, Zhang Y, Zhang Y, Chen X, Dai G, He J, Li J, Li G, Liu Y, Liu Z, Yuan X, Zhang J, Fu Z, He Y, Ju F, Liu Z, Tang P, Wang T, Wang W, Zhang J, Luo X, Tang X, May R, Feng H, Yao S, Keegan P, Xu RH, Wang F. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022;40:277-288.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 337] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 5. | Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC, Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, Chung IJ, Yamaguchi K, Kato K, Sym SJ, Kadowaki S, Tsuji K, Chen JS, Bai LY, Oh SY, Choda Y, Yasui H, Takeuchi K, Hirashima Y, Hagihara S, Boku N. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 590] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 6. | Gul RB, Ali PA. Clinical trials: the challenge of recruitment and retention of participants. J Clin Nurs. 2010;19:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 7. | Zhang K, Yang X, Wang Y, Yu Y, Huang N, Li G, Li X, Wu JC, Yang S. Artificial intelligence in drug development. Nat Med. 2025;31:45-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 136] [Article Influence: 136.0] [Reference Citation Analysis (1)] |
| 8. | Kuenzi BM, Park J, Fong SH, Sanchez KS, Lee J, Kreisberg JF, Ma J, Ideker T. Predicting Drug Response and Synergy Using a Deep Learning Model of Human Cancer Cells. Cancer Cell. 2020;38:672-684.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 286] [Article Influence: 47.7] [Reference Citation Analysis (0)] |
| 9. | Wang F, Ruan DY, Xu RH. Challenges and opportunities in oncology drug development and clinical research in China. Cell. 2024;187:1578-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 10. | Tayebi Arasteh S, Han T, Lotfinia M, Kuhl C, Kather JN, Truhn D, Nebelung S. Large language models streamline automated machine learning for clinical studies. Nat Commun. 2024;15:1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 49] [Reference Citation Analysis (0)] |
| 11. | Haug CJ, Drazen JM. Artificial Intelligence and Machine Learning in Clinical Medicine, 2023. N Engl J Med. 2023;388:1201-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 638] [Article Influence: 212.7] [Reference Citation Analysis (1)] |
| 12. | Hutson M. How AI is being used to accelerate clinical trials. Nature. 2024;627:S2-S5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 55] [Reference Citation Analysis (0)] |
| 13. | Poirion OB, Jing Z, Chaudhary K, Huang S, Garmire LX. DeepProg: an ensemble of deep-learning and machine-learning models for prognosis prediction using multi-omics data. Genome Med. 2021;13:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 14. | Nimgaonkar V, Krishna V, Krishna V, Tiu E, Joshi A, Vrabac D, Bhambhvani H, Smith K, Johansen JS, Makawita S, Musher B, Mehta A, Hendifar A, Wainberg Z, Sohal D, Fountzilas C, Singhi A, Rajpurkar P, Collisson EA. Development of an artificial intelligence-derived histologic signature associated with adjuvant gemcitabine treatment outcomes in pancreatic cancer. Cell Rep Med. 2023;4:101013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 15. | Orcutt X, Chen K, Mamtani R, Long Q, Parikh RB. Evaluating generalizability of oncology trial results to real-world patients using machine learning-based trial emulations. Nat Med. 2025;31:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 16. | Chen Z, Chen Y, Sun Y, Tang L, Zhang L, Hu Y, He M, Li Z, Cheng S, Yuan J, Wang Z, Wang Y, Zhao J, Gong J, Zhao L, Cao B, Li G, Zhang X, Dong B, Shen L. Predicting gastric cancer response to anti-HER2 therapy or anti-HER2 combined immunotherapy based on multi-modal data. Signal Transduct Target Ther. 2024;9:222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 17. | Lee KW, Van Cutsem E, Bang YJ, Fuchs CS, Kudaba I, Garrido M, Chung HC, Lee J, Castro HR, Chao J, Wainberg ZA, Cao ZA, Aurora-Garg D, Kobie J, Cristescu R, Bhagia P, Shah S, Tabernero J, Shitara K, Wyrwicz L. Association of Tumor Mutational Burden with Efficacy of Pembrolizumab±Chemotherapy as First-Line Therapy for Gastric Cancer in the Phase III KEYNOTE-062 Study. Clin Cancer Res. 2022;28:3489-3498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 18. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5823] [Cited by in RCA: 5528] [Article Influence: 345.5] [Reference Citation Analysis (3)] |
| 19. | Uzunparmak B, Haymaker C, Raso G, Masciari S, Wang L, Lin H, Gorur A, Kirby B, Cimo AM, Kennon A, Ding Q, Urschel G, Yuan Y, Feng G, Rizvi Y, Hussain A, Zhu C, Kim P, Abbadessa G, Subbiah V, Yap TA, Rodon J, Piha-Paul SA, Meric-Bernstam F, Dumbrava EE. HER2-low expression in patients with advanced or metastatic solid tumors. Ann Oncol. 2023;34:1035-1046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Shen SY, Singhania R, Fehringer G, Chakravarthy A, Roehrl MHA, Chadwick D, Zuzarte PC, Borgida A, Wang TT, Li T, Kis O, Zhao Z, Spreafico A, Medina TDS, Wang Y, Roulois D, Ettayebi I, Chen Z, Chow S, Murphy T, Arruda A, O'Kane GM, Liu J, Mansour M, McPherson JD, O'Brien C, Leighl N, Bedard PL, Fleshner N, Liu G, Minden MD, Gallinger S, Goldenberg A, Pugh TJ, Hoffman MM, Bratman SV, Hung RJ, De Carvalho DD. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature. 2018;563:579-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 666] [Article Influence: 83.3] [Reference Citation Analysis (0)] |
| 21. | Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, Jensen SØ, Medina JE, Hruban C, White JR, Palsgrove DN, Niknafs N, Anagnostou V, Forde P, Naidoo J, Marrone K, Brahmer J, Woodward BD, Husain H, van Rooijen KL, Ørntoft MW, Madsen AH, van de Velde CJH, Verheij M, Cats A, Punt CJA, Vink GR, van Grieken NCT, Koopman M, Fijneman RJA, Johansen JS, Nielsen HJ, Meijer GA, Andersen CL, Scharpf RB, Velculescu VE. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature. 2019;570:385-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 797] [Cited by in RCA: 973] [Article Influence: 139.0] [Reference Citation Analysis (0)] |
| 22. | Chen T, Ning Z, Xu L, Feng X, Han S, Roth HR, Xiong W, Zhao X, Hu Y, Liu H, Yu J, Zhang Y, Li Y, Xu Y, Mori K, Li G. Radiomics nomogram for predicting the malignant potential of gastrointestinal stromal tumours preoperatively. Eur Radiol. 2019;29:1074-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 23. | Dohan A, Gallix B, Guiu B, Le Malicot K, Reinhold C, Soyer P, Bennouna J, Ghiringhelli F, Barbier E, Boige V, Taieb J, Bouché O, François E, Phelip JM, Borel C, Faroux R, Seitz JF, Jacquot S, Ben Abdelghani M, Khemissa-Akouz F, Genet D, Jouve JL, Rinaldi Y, Desseigne F, Texereau P, Suc E, Lepage C, Aparicio T, Hoeffel C; PRODIGE 9 Investigators and PRODIGE 20 Investigators. Early evaluation using a radiomic signature of unresectable hepatic metastases to predict outcome in patients with colorectal cancer treated with FOLFIRI and bevacizumab. Gut. 2020;69:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 24. | Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, Sanduleanu S, Larue RTHM, Even AJG, Jochems A, van Wijk Y, Woodruff H, van Soest J, Lustberg T, Roelofs E, van Elmpt W, Dekker A, Mottaghy FM, Wildberger JE, Walsh S. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1825] [Cited by in RCA: 3944] [Article Influence: 438.2] [Reference Citation Analysis (0)] |
| 25. | Lin ZY, Zhang P, Chi P, Xiao Y, Xu XM, Zhang AM, Qiu XF, Wu JX, Yuan Y, Wang ZN, Qu XJ, Li X, Nie X, Yang M, Cai KL, Zhang WK, Huang Y, Sun Z, Hou ZG, Ma C, Cheng FZ, Tao KX, Zhang T. Neoadjuvant short-course radiotherapy followed by camrelizumab and chemotherapy in locally advanced rectal cancer (UNION): early outcomes of a multicenter randomized phase III trial. Ann Oncol. 2024;35:882-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 53] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 26. | Qin J, Xue L, Hao A, Guo X, Jiang T, Ni Y, Liu S, Chen Y, Jiang H, Zhang C, Kang M, Lin J, Li H, Li C, Tian H, Li L, Fu J, Zhang Y, Ma J, Wang X, Fu M, Yang H, Yang Z, Han Y, Chen L, Tan L, Dai T, Liao Y, Zhang W, Li B, Chen Q, Guo S, Qi Y, Wei L, Li Z, Tian Z, Kang X, Zhang R, Li Y, Wang Z, Chen X, Hou Z, Zheng R, Zhu W, He J, Li Y. Neoadjuvant chemotherapy with or without camrelizumab in resectable esophageal squamous cell carcinoma: the randomized phase 3 ESCORT-NEO/NCCES01 trial. Nat Med. 2024;30:2549-2557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 109] [Article Influence: 54.5] [Reference Citation Analysis (0)] |
| 27. | Xie C, Jing Z, Luo H, Jiang W, Ma L, Hu W, Zheng A, Li D, Ding L, Zhang H, Xie C, Lian X, Du D, Chen M, Bian X, Tan B, Xia B, Xie R, Liu Q, Wang L, Wu S. Chemoradiotherapy with extended nodal irradiation and/or erlotinib in locally advanced oesophageal squamous cell cancer: long-term update of a randomised phase 3 trial. Br J Cancer. 2020;123:1616-1624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Kato K, Machida R, Ito Y, Daiko H, Ozawa S, Ogata T, Hara H, Kojima T, Abe T, Bamba T, Watanabe M, Kawakubo H, Shibuya Y, Tsubosa Y, Takegawa N, Kajiwara T, Baba H, Ueno M, Takeuchi H, Nakamura K, Kitagawa Y; JCOG1109 investigators. Doublet chemotherapy, triplet chemotherapy, or doublet chemotherapy combined with radiotherapy as neoadjuvant treatment for locally advanced oesophageal cancer (JCOG1109 NExT): a randomised, controlled, open-label, phase 3 trial. Lancet. 2024;404:55-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 143] [Article Influence: 71.5] [Reference Citation Analysis (0)] |
| 29. | Veas Rodríguez J, Prieto A, Vilaprinyo E, Bonet M, Diez M, Salud A, Montal R. Surrogate endpoints in phase III randomized trials of advanced gastroesophageal cancer: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2024;201:104416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 30. | Sorbye H, Welin S, Langer SW, Vestermark LW, Holt N, Osterlund P, Dueland S, Hofsli E, Guren MG, Ohrling K, Birkemeyer E, Thiis-Evensen E, Biagini M, Gronbaek H, Soveri LM, Olsen IH, Federspiel B, Assmus J, Janson ET, Knigge U. Predictive and prognostic factors for treatment and survival in 305 patients with advanced gastrointestinal neuroendocrine carcinoma (WHO G3): the NORDIC NEC study. Ann Oncol. 2013;24:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 741] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 31. | Ahmad MU, Javadi CS, Chang JD, Forgó E, Delitto DJ, Dua MM, Fisher GA Jr, Heestand GM, Chang DT, Pollom E, Vitzthum LK, Kirane A, Lee B, Visser BC, Norton JA, Poultsides GA. Biochemical, Radiographic, or Pathologic Response to Neoadjuvant Chemotherapy in Resected Pancreatic Cancer: Which is Best? Ann Surg. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 32. | Zhou Z, Ren Y, Zhang Z, Guan T, Wang Z, Chen W, Luo T, Li G. Digital histopathological images of biopsy predict response to neoadjuvant chemotherapy for locally advanced gastric cancer. Gastric Cancer. 2023;26:734-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 33. | Cui Y, Zhang J, Li Z, Wei K, Lei Y, Ren J, Wu L, Shi Z, Meng X, Yang X, Gao X. A CT-based deep learning radiomics nomogram for predicting the response to neoadjuvant chemotherapy in patients with locally advanced gastric cancer: A multicenter cohort study. EClinicalMedicine. 2022;46:101348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 34. | Sun Z, Wang W, Huang W, Zhang T, Chen C, Yuan Q, Chen Y, Zhou K, Han Z, Feng H, Chen H, Liang X, Hu Y, Yu J, Liu H, Yu L, Xu Y, Li G, Jiang Y. Noninvasive imaging evaluation of peritoneal recurrence and chemotherapy benefit in gastric cancer after gastrectomy: a multicenter study. Int J Surg. 2023;109:2010-2024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Jiang Y, Liang X, Wang W, Chen C, Yuan Q, Zhang X, Li N, Chen H, Yu J, Xie Y, Xu Y, Zhou Z, Li G, Li R. Noninvasive Prediction of Occult Peritoneal Metastasis in Gastric Cancer Using Deep Learning. JAMA Netw Open. 2021;4:e2032269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 36. | Pera M, Gibert J, Gimeno M, Garsot E, Eizaguirre E, Miró M, Castro S, Miranda C, Reka L, Leturio S, González-Duaigües M, Codony C, Gobbini Y, Luna A, Fernández-Ananín S, Sarriugarte A, Olona C, Rodríguez-Santiago J, Osorio J, Grande L; Spanish EURECCA Esophagogastric Cancer Group. Machine Learning Risk Prediction Model of 90-day Mortality After Gastrectomy for Cancer. Ann Surg. 2022;276:776-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 37. | Lai H, Liu J, Bai C, Liu H, Pan B, Luo X, Hou L, Zhao W, Xia D, Tian J, Chen Y, Zhang L, Estill J, Liu J, Liao X, Shi N, Sun X, Shang H, Bian Z, Yang K, Huang L, Ge L; ADVANCED Working Group. Language models for data extraction and risk of bias assessment in complementary medicine. NPJ Digit Med. 2025;8:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 38. | Huang J, Yang DM, Rong R, Nezafati K, Treager C, Chi Z, Wang S, Cheng X, Guo Y, Klesse LJ, Xiao G, Peterson ED, Zhan X, Xie Y. A critical assessment of using ChatGPT for extracting structured data from clinical notes. NPJ Digit Med. 2024;7:106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 118] [Reference Citation Analysis (0)] |
| 39. | Abedian S, Sholle ET, Adekkanattu PM, Cusick MM, Weiner SE, Shoag JE, Hu JC, Campion TR Jr. Automated Extraction of Tumor Staging and Diagnosis Information From Surgical Pathology Reports. JCO Clin Cancer Inform. 2021;5:1054-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 40. | Stensland KD, McBride RB, Latif A, Wisnivesky J, Hendricks R, Roper N, Boffetta P, Hall SJ, Oh WK, Galsky MD. Adult cancer clinical trials that fail to complete: an epidemic? J Natl Cancer Inst. 2014;106:dju229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Gan CL, Stukalin I, Meyers DE, Dudani S, Grosjean HAI, Dolter S, Ewanchuk BW, Goutam S, Sander M, Wells C, Pabani A, Cheng T, Monzon J, Morris D, Basappa NS, Pal SK, Wood LA, Donskov F, Choueiri TK, Heng DYC. Outcomes of patients with solid tumour malignancies treated with first-line immuno-oncology agents who do not meet eligibility criteria for clinical trials. Eur J Cancer. 2021;151:115-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 42. | Liu R, Rizzo S, Whipple S, Pal N, Pineda AL, Lu M, Arnieri B, Lu Y, Capra W, Copping R, Zou J. Evaluating eligibility criteria of oncology trials using real-world data and AI. Nature. 2021;592:629-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 43. | Jin Q, Wang Z, Floudas CS, Chen F, Gong C, Bracken-Clarke D, Xue E, Yang Y, Sun J, Lu Z. Matching patients to clinical trials with large language models. Nat Commun. 2024;15:9074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 92] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 44. | Chow R, Midroni J, Kaur J, Boldt G, Liu G, Eng L, Liu FF, Haibe-Kains B, Lock M, Raman S. Use of artificial intelligence for cancer clinical trial enrollment: a systematic review and meta-analysis. J Natl Cancer Inst. 2023;115:365-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 45. | Mazor T, Farhat KS, Trukhanov P, Lindsay J, Galvin M, Mallaber E, Paul MA, Hassett MJ, Schrag D, Cerami E, Kehl KL. Clinical Trial Notifications Triggered by Artificial Intelligence-Detected Cancer Progression: A Randomized Trial. JAMA Netw Open. 2025;8:e252013. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 46. | Huang W, Wang C, Wang Y, Yu Z, Wang S, Yang J, Lu S, Zhou C, Wu E, Chen J. Predicting malnutrition in gastric cancer patients using computed tomography(CT) deep learning features and clinical data. Clin Nutr. 2024;43:881-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 47. | Roberts AL, Potts HW, Koutoukidis DA, Smith L, Fisher A. Breast, Prostate, and Colorectal Cancer Survivors' Experiences of Using Publicly Available Physical Activity Mobile Apps: Qualitative Study. JMIR Mhealth Uhealth. 2019;7:e10918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 48. | Ichimasa K, Foppa C, Kudo SE, Misawa M, Takashina Y, Miyachi H, Ishida F, Nemoto T, Lee JWJ, Yeoh KG, Paoluzzi Tomada E, Maselli R, Repici A, Terracciano LM, Spaggiari P, Mori Y, Hassan C, Spinelli A; early CRC group. Artificial Intelligence to Predict the Risk of Lymph Node Metastasis in T2 Colorectal Cancer. Ann Surg. 2024;280:850-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 49. | Fockens KN, Jong MR, Jukema JB, Boers TGW, Kusters CHJ, van der Putten JA, Pouw RE, Duits LC, Montazeri NSM, van Munster SN, Weusten BLAM, Alvarez Herrero L, Houben MHMG, Nagengast WB, Westerhof J, Alkhalaf A, Mallant-Hent RC, Scholten P, Ragunath K, Seewald S, Elbe P, Baldaque-Silva F, Barret M, Ortiz Fernández-Sordo J, Villarejo GM, Pech O, Beyna T, van der Sommen F, de With PH, de Groof AJ, Bergman JJ; Barrett's Oesophagus Imaging for Artificial Intelligence (BONS-AI) consortium. A deep learning system for detection of early Barrett's neoplasia: a model development and validation study. Lancet Digit Health. 2023;5:e905-e916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 50. | Zhang S, Li K, Sun Y, Wan Y, Ao Y, Zhong Y, Liang M, Wang L, Chen X, Pei X, Hu Y, Chen D, Li M, Shan H. Deep Learning for Automatic Gross Tumor Volumes Contouring in Esophageal Cancer Based on Contrast-Enhanced Computed Tomography Images: A Multi-Institutional Study. Int J Radiat Oncol Biol Phys. 2024;119:1590-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 51. | Ma J, He Y, Li F, Han L, You C, Wang B. Segment anything in medical images. Nat Commun. 2024;15:654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 267] [Cited by in RCA: 631] [Article Influence: 315.5] [Reference Citation Analysis (0)] |
| 52. | Chen Z, Ma N, Sun X, Li Q, Zeng Y, Chen F, Sun S, Xu J, Zhang J, Ye H, Ge J, Zhang Z, Cui X, Leong K, Chen Y, Gu Z. Automated evaluation of tumor spheroid behavior in 3D culture using deep learning-based recognition. Biomaterials. 2021;272:120770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 53. | Vorontsov E, Cerny M, Régnier P, Di Jorio L, Pal CJ, Lapointe R, Vandenbroucke-Menu F, Turcotte S, Kadoury S, Tang A. Deep Learning for Automated Segmentation of Liver Lesions at CT in Patients with Colorectal Cancer Liver Metastases. Radiol Artif Intell. 2019;1:180014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (1)] |
| 54. | Tang X, Jafargholi Rangraz E, Coudyzer W, Bertels J, Robben D, Schramm G, Deckers W, Maleux G, Baete K, Verslype C, Gooding MJ, Deroose CM, Nuyts J. Whole liver segmentation based on deep learning and manual adjustment for clinical use in SIRT. Eur J Nucl Med Mol Imaging. 2020;47:2742-2752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 55. | Ferber D, Wölflein G, Wiest IC, Ligero M, Sainath S, Ghaffari Laleh N, El Nahhas OSM, Müller-Franzes G, Jäger D, Truhn D, Kather JN. In-context learning enables multimodal large language models to classify cancer pathology images. Nat Commun. 2024;15:10104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 44] [Reference Citation Analysis (0)] |
| 56. | Wang X, Jiang Y, Yang S, Wang F, Zhang X, Wang W, Chen Y, Wu X, Xiang J, Li Y, Jiang X, Yuan W, Zhang J, Yu KH, Ward RL, Hawkins N, Jonnagaddala J, Li G, Li R. Foundation Model for Predicting Prognosis and Adjuvant Therapy Benefit From Digital Pathology in GI Cancers. J Clin Oncol. 2025;JCO2401501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Muti HS, Röcken C, Behrens HM, Löffler CML, Reitsam NG, Grosser B, Märkl B, Stange DE, Jiang X, Velduizen GP, Truhn D, Ebert MP, Grabsch HI, Kather JN. Deep learning trained on lymph node status predicts outcome from gastric cancer histopathology: a retrospective multicentric study. Eur J Cancer. 2023;194:113335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 58. | Saito A, Toyoda H, Kobayashi M, Koiwa Y, Fujii H, Fujita K, Maeda A, Kaneoka Y, Hazama S, Nagano H, Mirza AH, Graf HP, Cosatto E, Murakami Y, Kuroda M. Prediction of early recurrence of hepatocellular carcinoma after resection using digital pathology images assessed by machine learning. Mod Pathol. 2021;34:417-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 59. | Cao R, Yang F, Ma SC, Liu L, Zhao Y, Li Y, Wu DH, Wang T, Lu WJ, Cai WJ, Zhu HB, Guo XJ, Lu YW, Kuang JJ, Huan WJ, Tang WM, Huang K, Huang J, Yao J, Dong ZY. Development and interpretation of a pathomics-based model for the prediction of microsatellite instability in Colorectal Cancer. Theranostics. 2020;10:11080-11091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 177] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 60. | Ding K, Zhou M, Wang H, Zhang S, Metaxas DN. Spatially aware graph neural networks and cross-level molecular profile prediction in colon cancer histopathology: a retrospective multi-cohort study. Lancet Digit Health. 2022;4:e787-e795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 61. | Huang Z, Yang E, Shen J, Gratzinger D, Eyerer F, Liang B, Nirschl J, Bingham D, Dussaq AM, Kunder C, Rojansky R, Gilbert A, Chang-Graham AL, Howitt BE, Liu Y, Ryan EE, Tenney TB, Zhang X, Folkins A, Fox EJ, Montine KS, Montine TJ, Zou J. A pathologist-AI collaboration framework for enhancing diagnostic accuracies and efficiencies. Nat Biomed Eng. 2025;9:455-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 62. | Yan D, Bao S, Zhang Z, Sun J, Zhou M. Leveraging pharmacovigilance data to predict population-scale toxicity profiles of checkpoint inhibitor immunotherapy. Nat Comput Sci. 2025;5:207-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 63. | Höhn J, Krieghoff-Henning E, Wies C, Kiehl L, Hetz MJ, Bucher TC, Jonnagaddala J, Zatloukal K, Müller H, Plass M, Jungwirth E, Gaiser T, Steeg M, Holland-Letz T, Brenner H, Hoffmeister M, Brinker TJ. Colorectal cancer risk stratification on histological slides based on survival curves predicted by deep learning. NPJ Precis Oncol. 2023;7:98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 64. | Liu Z, Liu L, Weng S, Guo C, Dang Q, Xu H, Wang L, Lu T, Zhang Y, Sun Z, Han X. Machine learning-based integration develops an immune-derived lncRNA signature for improving outcomes in colorectal cancer. Nat Commun. 2022;13:816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 532] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 65. | Skrede OJ, De Raedt S, Kleppe A, Hveem TS, Liestøl K, Maddison J, Askautrud HA, Pradhan M, Nesheim JA, Albregtsen F, Farstad IN, Domingo E, Church DN, Nesbakken A, Shepherd NA, Tomlinson I, Kerr R, Novelli M, Kerr DJ, Danielsen HE. Deep learning for prediction of colorectal cancer outcome: a discovery and validation study. Lancet. 2020;395:350-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 406] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 66. | Kleppe A, Skrede OJ, De Raedt S, Hveem TS, Askautrud HA, Jacobsen JE, Church DN, Nesbakken A, Shepherd NA, Novelli M, Kerr R, Liestøl K, Kerr DJ, Danielsen HE. A clinical decision support system optimising adjuvant chemotherapy for colorectal cancers by integrating deep learning and pathological staging markers: a development and validation study. Lancet Oncol. 2022;23:1221-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 67. | Gao S, Tibiche C, Zou J, Zaman N, Trifiro M, O'Connor-McCourt M, Wang E. Identification and Construction of Combinatory Cancer Hallmark-Based Gene Signature Sets to Predict Recurrence and Chemotherapy Benefit in Stage II Colorectal Cancer. JAMA Oncol. 2016;2:37-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 68. | Kather JN, Krisam J, Charoentong P, Luedde T, Herpel E, Weis CA, Gaiser T, Marx A, Valous NA, Ferber D, Jansen L, Reyes-Aldasoro CC, Zörnig I, Jäger D, Brenner H, Chang-Claude J, Hoffmeister M, Halama N. Predicting survival from colorectal cancer histology slides using deep learning: A retrospective multicenter study. PLoS Med. 2019;16:e1002730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 555] [Cited by in RCA: 535] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 69. | Kong J, Lee H, Kim D, Han SK, Ha D, Shin K, Kim S. Network-based machine learning in colorectal and bladder organoid models predicts anti-cancer drug efficacy in patients. Nat Commun. 2020;11:5485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 140] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 70. | Zhou S, Sun D, Mao W, Liu Y, Cen W, Ye L, Liang F, Xu J, Shi H, Ji Y, Wang L, Chang W. Deep radiomics-based fusion model for prediction of bevacizumab treatment response and outcome in patients with colorectal cancer liver metastases: a multicentre cohort study. EClinicalMedicine. 2023;65:102271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 71. | Wang X, Chen Y, Gao Y, Zhang H, Guan Z, Dong Z, Zheng Y, Jiang J, Yang H, Wang L, Huang X, Ai L, Yu W, Li H, Dong C, Zhou Z, Liu X, Yu G. Predicting gastric cancer outcome from resected lymph node histopathology images using deep learning. Nat Commun. 2021;12:1637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 72. | Gao P, Xiao Q, Tan H, Song J, Fu Y, Xu J, Zhao J, Miao Y, Li X, Jing Y, Feng Y, Wang Z, Zhang Y, Yao E, Xu T, Mei J, Chen H, Jiang X, Yang Y, Wang Z, Gao X, Zheng M, Zhang L, Jiang M, Long Y, He L, Sun J, Deng Y, Wang B, Zhao Y, Ba Y, Wang G, Zhang Y, Deng T, Shen D, Wang Z. Interpretable multi-modal artificial intelligence model for predicting gastric cancer response to neoadjuvant chemotherapy. Cell Rep Med. 2024;5:101848. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 73. | Jiang Y, Zhou K, Sun Z, Wang H, Xie J, Zhang T, Sang S, Islam MT, Wang JY, Chen C, Yuan Q, Xi S, Li T, Xu Y, Xiong W, Wang W, Li G, Li R. Non-invasive tumor microenvironment evaluation and treatment response prediction in gastric cancer using deep learning radiomics. Cell Rep Med. 2023;4:101146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 74. | Jee J, Fong C, Pichotta K, Tran TN, Luthra A, Waters M, Fu C, Altoe M, Liu SY, Maron SB, Ahmed M, Kim S, Pirun M, Chatila WK, de Bruijn I, Pasha A, Kundra R, Gross B, Mastrogiacomo B, Aprati TJ, Liu D, Gao J, Capelletti M, Pekala K, Loudon L, Perry M, Bandlamudi C, Donoghue M, Satravada BA, Martin A, Shen R, Chen Y, Brannon AR, Chang J, Braunstein L, Li A, Safonov A, Stonestrom A, Sanchez-Vela P, Wilhelm C, Robson M, Scher H, Ladanyi M, Reis-Filho JS, Solit DB, Jones DR, Gomez D, Yu H, Chakravarty D, Yaeger R, Abida W, Park W, O'Reilly EM, Garcia-Aguilar J, Socci N, Sanchez-Vega F, Carrot-Zhang J, Stetson PD, Levine R, Rudin CM, Berger MF, Shah SP, Schrag D, Razavi P, Kehl KL, Li BT, Riely GJ, Schultz N; MSK Cancer Data Science Initiative Group. Automated real-world data integration improves cancer outcome prediction. Nature. 2024;636:728-736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 82] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 75. | Sim JA, Huang X, Horan MR, Stewart CM, Robison LL, Hudson MM, Baker JN, Huang IC. Natural language processing with machine learning methods to analyze unstructured patient-reported outcomes derived from electronic health records: A systematic review. Artif Intell Med. 2023;146:102701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 33] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 76. | Koleck TA, Dreisbach C, Bourne PE, Bakken S. Natural language processing of symptoms documented in free-text narratives of electronic health records: a systematic review. J Am Med Inform Assoc. 2019;26:364-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 243] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 77. | Kim J, Kusko R, Zeskind B, Zhang J, Escalante-Chong R. A primer on applying AI synergistically with domain expertise to oncology. Biochim Biophys Acta Rev Cancer. 2021;1876:188548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Vyas A, Kumar K, Sharma A, Verma D, Bhatia D, Wahi N, Yadav AK. Advancing the frontier of artificial intelligence on emerging technologies to redefine cancer diagnosis and care. Comput Biol Med. 2025;191:110178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 79. | Libanio D, Antonelli G, Marijnissen F, Spaander MC, Hassan C, Dinis-Ribeiro M, Areia M. Combined gastric and colorectal cancer endoscopic screening may be cost-effective in Europe with the implementation of artificial intelligence: an economic evaluation. Eur J Gastroenterol Hepatol. 2024;36:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 80. | Mital S, Nguyen HV. Cost-effectiveness of using artificial intelligence versus polygenic risk score to guide breast cancer screening. BMC Cancer. 2022;22:501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 81. | Hill H, Roadevin C, Duffy S, Mandrik O, Brentnall A. Cost-Effectiveness of AI for Risk-Stratified Breast Cancer Screening. JAMA Netw Open. 2024;7:e2431715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 31] [Reference Citation Analysis (0)] |
| 82. | Maas L, Contreras-Meca C, Ghezzo S, Belmans F, Corsi A, Cant J, Vos W, Bobowicz M, Rygusik M, Laski DK, Annemans L, Hiligsmann M; EuCanImage Working Group; *EuCanImage Working Group. Cost-effectiveness analysis of artificial intelligence (AI) in earlier detection of liver lesions in cirrhotic patients at risk of hepatocellular carcinoma in Italy. J Med Econ. 2025;28:1023-1036. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 83. | Smak Gregoor AM, Sangers TE, Bakker LJ, Hollestein L, Uyl-de Groot CA, Nijsten T, Wakkee M. An artificial intelligence based app for skin cancer detection evaluated in a population based setting. NPJ Digit Med. 2023;6:90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 84. | Gomez Rossi J, Rojas-Perilla N, Krois J, Schwendicke F. Cost-effectiveness of Artificial Intelligence as a Decision-Support System Applied to the Detection and Grading of Melanoma, Dental Caries, and Diabetic Retinopathy. JAMA Netw Open. 2022;5:e220269. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 85. | Geppert J, Auguste P, Asgharzadeh A, Ghiasvand H, Patel M, Brown A, Jayakody S, Helm E, Todkill D, Madan J, Stinton C, Gallacher D, Taylor-Phillips S, Chen YF. Software with artificial intelligence-derived algorithms for detecting and analysing lung nodules in CT scans: systematic review and economic evaluation. Health Technol Assess. 2025;29:1-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 86. | Sadilek A, Liu L, Nguyen D, Kamruzzaman M, Serghiou S, Rader B, Ingerman A, Mellem S, Kairouz P, Nsoesie EO, MacFarlane J, Vullikanti A, Marathe M, Eastham P, Brownstein JS, Arcas BAY, Howell MD, Hernandez J. Privacy-first health research with federated learning. NPJ Digit Med. 2021;4:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 87. | Pati S, Baid U, Edwards B, Sheller M, Wang SH, Reina GA, Foley P, Gruzdev A, Karkada D, Davatzikos C, Sako C, Ghodasara S, Bilello M, Mohan S, Vollmuth P, Brugnara G, Preetha CJ, Sahm F, Maier-Hein K, Zenk M, Bendszus M, Wick W, Calabrese E, Rudie J, Villanueva-Meyer J, Cha S, Ingalhalikar M, Jadhav M, Pandey U, Saini J, Garrett J, Larson M, Jeraj R, Currie S, Frood R, Fatania K, Huang RY, Chang K, Balaña C, Capellades J, Puig J, Trenkler J, Pichler J, Necker G, Haunschmidt A, Meckel S, Shukla G, Liem S, Alexander GS, Lombardo J, Palmer JD, Flanders AE, Dicker AP, Sair HI, Jones CK, Venkataraman A, Jiang M, So TY, Chen C, Heng PA, Dou Q, Kozubek M, Lux F, Michálek J, Matula P, Keřkovský M, Kopřivová T, Dostál M, Vybíhal V, Vogelbaum MA, Mitchell JR, Farinhas J, Maldjian JA, Yogananda CGB, Pinho MC, Reddy D, Holcomb J, Wagner BC, Ellingson BM, Cloughesy TF, Raymond C, Oughourlian T, Hagiwara A, Wang C, To MS, Bhardwaj S, Chong C, Agzarian M, Falcão AX, Martins SB, Teixeira BCA, Sprenger F, Menotti D, Lucio DR, LaMontagne P, Marcus D, Wiestler B, Kofler F, Ezhov I, Metz M, Jain R, Lee M, Lui YW, McKinley R, Slotboom J, Radojewski P, Meier R, Wiest R, Murcia D, Fu E, Haas R, Thompson J, Ormond DR, Badve C, Sloan AE, Vadmal V, Waite K, Colen RR, Pei L, Ak M, Srinivasan A, Bapuraj JR, Rao A, Wang N, Yoshiaki O, Moritani T, Turk S, Lee J, Prabhudesai S, Morón F, Mandel J, Kamnitsas K, Glocker B, Dixon LVM, Williams M, Zampakis P, Panagiotopoulos V, Tsiganos P, Alexiou S, Haliassos I, Zacharaki EI, Moustakas K, Kalogeropoulou C, Kardamakis DM, Choi YS, Lee SK, Chang JH, Ahn SS, Luo B, Poisson L, Wen N, Tiwari P, Verma R, Bareja R, Yadav I, Chen J, Kumar N, Smits M, van der Voort SR, Alafandi A, Incekara F, Wijnenga MMJ, Kapsas G, Gahrmann R, Schouten JW, Dubbink HJ, Vincent AJPE, van den Bent MJ, French PJ, Klein S, Yuan Y, Sharma S, Tseng TC, Adabi S, Niclou SP, Keunen O, Hau AC, Vallières M, Fortin D, Lepage M, Landman B, Ramadass K, Xu K, Chotai S, Chambless LB, Mistry A, Thompson RC, Gusev Y, Bhuvaneshwar K, Sayah A, Bencheqroun C, Belouali A, Madhavan S, Booth TC, Chelliah A, Modat M, Shuaib H, Dragos C, Abayazeed A, Kolodziej K, Hill M, Abbassy A, Gamal S, Mekhaimar M, Qayati M, Reyes M, Park JE, Yun J, Kim HS, Mahajan A, Muzi M, Benson S, Beets-Tan RGH, Teuwen J, Herrera-Trujillo A, Trujillo M, Escobar W, Abello A, Bernal J, Gómez J, Choi J, Baek S, Kim Y, Ismael H, Allen B, Buatti JM, Kotrotsou A, Li H, Weiss T, Weller M, Bink A, Pouymayou B, Shaykh HF, Saltz J, Prasanna P, Shrestha S, Mani KM, Payne D, Kurc T, Pelaez E, Franco-Maldonado H, Loayza F, Quevedo S, Guevara P, Torche E, Mendoza C, Vera F, Ríos E, López E, Velastin SA, Ogbole G, Soneye M, Oyekunle D, Odafe-Oyibotha O, Osobu B, Shu'aibu M, Dorcas A, Dako F, Simpson AL, Hamghalam M, Peoples JJ, Hu R, Tran A, Cutler D, Moraes FY, Boss MA, Gimpel J, Veettil DK, Schmidt K, Bialecki B, Marella S, Price C, Cimino L, Apgar C, Shah P, Menze B, Barnholtz-Sloan JS, Martin J, Bakas S. Federated learning enables big data for rare cancer boundary detection. Nat Commun. 2022;13:7346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 163] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 88. | Lu MY, Chen RJ, Kong D, Lipkova J, Singh R, Williamson DFK, Chen TY, Mahmood F. Federated learning for computational pathology on gigapixel whole slide images. Med Image Anal. 2022;76:102298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 89. | Kalra S, Wen J, Cresswell JC, Volkovs M, Tizhoosh HR. Decentralized federated learning through proxy model sharing. Nat Commun. 2023;14:2899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 90. | Wu C, Wu F, Lyu L, Qi T, Huang Y, Xie X. A federated graph neural network framework for privacy-preserving personalization. Nat Commun. 2022;13:3091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 91. | Xiong Z, Cheng Z, Lin X, Xu C, Liu X, Wang D, Luo X, Zhang Y, Jiang H, Qiao N, Zheng M. Facing small and biased data dilemma in drug discovery with enhanced federated learning approaches. Sci China Life Sci. 2022;65:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 92. | Kim M, Harmanci AO, Bossuat JP, Carpov S, Cheon JH, Chillotti I, Cho W, Froelicher D, Gama N, Georgieva M, Hong S, Hubaux JP, Kim D, Lauter K, Ma Y, Ohno-Machado L, Sofia H, Son Y, Song Y, Troncoso-Pastoriza J, Jiang X. Ultrafast homomorphic encryption models enable secure outsourcing of genotype imputation. Cell Syst. 2021;12:1108-1120.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 93. | Kumar KN, Mohan CK, Cenkeramaddi LR. The Impact of Adversarial Attacks on Federated Learning: A Survey. IEEE Trans Pattern Anal Mach Intell. 2024;46:2672-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 94. | Niraula D, Cuneo KC, Dinov ID, Gonzalez BD, Jamaluddin JB, Jin JJ, Luo Y, Matuszak MM, Ten Haken RK, Bryant AK, Dilling TJ, Dykstra MP, Frakes JM, Liveringhouse CL, Miller SR, Mills MN, Palm RF, Regan SN, Rishi A, Torres-Roca JF, Yu HM, El Naqa I. Intricacies of human-AI interaction in dynamic decision-making for precision oncology. Nat Commun. 2025;16:1138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 95. | Goh E, Gallo RJ, Strong E, Weng Y, Kerman H, Freed JA, Cool JA, Kanjee Z, Lane KP, Parsons AS, Ahuja N, Horvitz E, Yang D, Milstein A, Olson APJ, Hom J, Chen JH, Rodman A. GPT-4 assistance for improvement of physician performance on patient care tasks: a randomized controlled trial. Nat Med. 2025;31:1233-1238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 96. | Liu X, Liu H, Yang G, Jiang Z, Cui S, Zhang Z, Wang H, Tao L, Sun Y, Song Z, Hong T, Yang J, Gao T, Zhang J, Li X, Zhang J, Sang Y, Yang Z, Xue K, Wu S, Zhang P, Yang J, Song C, Wang G. A generalist medical language model for disease diagnosis assistance. Nat Med. 2025;31:932-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 48] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 97. | Takita H, Kabata D, Walston SL, Tatekawa H, Saito K, Tsujimoto Y, Miki Y, Ueda D. A systematic review and meta-analysis of diagnostic performance comparison between generative AI and physicians. NPJ Digit Med. 2025;8:175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 49] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 98. | Goh E, Gallo R, Hom J, Strong E, Weng Y, Kerman H, Cool JA, Kanjee Z, Parsons AS, Ahuja N, Horvitz E, Yang D, Milstein A, Olson APJ, Rodman A, Chen JH. Large Language Model Influence on Diagnostic Reasoning: A Randomized Clinical Trial. JAMA Netw Open. 2024;7:e2440969. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 238] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 99. | Xiao X, Han X, Sun Y, Zheng G, Miao Q, Zhang Y, Tan J, Liu G, He Q, Zhou J, Zheng Z, Jiang G, Song H. Development and interpretation of a multimodal predictive model for prognosis of gastrointestinal stromal tumor. NPJ Precis Oncol. 2024;8:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/