Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.110997
Revised: August 9, 2025
Accepted: September 10, 2025
Published online: October 15, 2025
Processing time: 94 Days and 22.9 Hours
The gastric microbiome is closely associated with gastric cancer, and single-region 16S rRNA sequencing has limitations in analyzing its characteristics, necessitating the search for a better sequencing method.
To evaluate the effectiveness of multi-region 16S rRNA gene sequencing in studying the microbiome of gastric cancer tissues.
Patients with gastric cancer (n = 118) who underwent surgery at Liyang People's Hospital from January 2022 to December 2024 were enrolled. Fifty-nine paraffin-embedded and 59 fresh tissue samples were obtained. The ZymoBIOMICSTM microbial community standard and Escherichia coli ATCC 25922 were used as positive controls. Multi-region and single-region 16S rRNA gene sequencing were performed. Species identification, detection rates at varying microbial abun
Multi-region 16S rRNA sequencing identified more species (eight species and eight genera) in the positive controls compared with single-region sequencing (one species and six genera). Detection rates at concentrations of 103, 102, and 10 CFU/mg were significantly higher using multi-region sequencing (P < 0.05). Multi-region sequencing also revealed significantly higher OTU counts and alpha diversity indices (Shannon, Simpson, and Chao1) in gastric cancer tissues (P < 0.05).
Compared with single-region sequencing, multi-region 16S rRNA gene sequencing demonstrates superior species resolution and detection sensitivity, providing a more comprehensive profile of microbial diversity in gastric cancer tissues.
Core Tip: This study demonstrated that multi-region 16S rRNA gene sequencing provides significantly improved species resolution and detection rates compared with traditional single-region method. In both positive controls and gastric cancer tissue samples, it revealed richer and more diverse microbial communities. These findings suggest that compared to single-region sequencing, multi-region sequencing is a more accurate and effective approach for profiling the microbiome in gastric cancer research.
- Citation: Wu TT, Zhou X, Huang Q, Yang Q. Effectiveness of multi-region 16S rRNA gene sequencing in studying the microbiome of gastric cancer tissues. World J Gastrointest Oncol 2025; 17(10): 110997
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/110997.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.110997
Gastric cancer ranks as the fifth most common cancer globally, with millions of new cases diagnosed each year, the majority occurring in developing countries[1,2]. Its development has been associated with a combination of genetic, environmental, dietary, and lifestyle factors. Gastric cancer is the third leading cause of cancer-related mortality worldwide. Prognosis remains poor, particularly in advanced stages, where the five-year survival rate is less than 30%[3,4]. Understanding the pathogenesis of gastric cancer and identifying effective strategies for its prevention and treatment hold substantial clinical and societal importance.
Recently, the microbiome within the tumor microenvironment has been recognized as a key factor influencing the onset, progression, and therapeutic response of gastric cancer. For instance, Helicobacter pylori infection has been strongly established as a class I carcinogen for gastric cancer. In addition, other commensal bacteria, including Clostridium and Streptococcus, have been associated with aggressive disease phenotypes[5,6].
Currently, 16S rRNA gene sequencing, a high-throughput, highly sensitive technique, is widely used in tumor microbiome research[7,8]. However, traditional 16S rRNA sequencing typically targets only one or a few specific tumor regions. This approach limits taxonomic resolution to the genus level and fails to fully capture the complexity and diversity of the gastric cancer tissue microbiome[9,10]. In this study, we aimed to perform a comprehensive analysis of the gastric cancer microbiome using a multi-region 16S rRNA gene sequencing approach to provide new insights for the prevention and treatment of gastric cancer.
A total of 118 patients with gastric cancer who underwent surgical treatment at Liyang People's Hospital from January 2022 to December 2024 were selected for this study. Fifty-nine paraffin-embedded tissue samples and 59 fresh tissue samples (cryopreserved at -80 °C after surgical excision) were collected. All samples were collected under sterile conditions and immediately processed or cryopreserved to preserve quality and integrity. The cohort included 68 males and 50 females, aged 42-78 years, with an average age of 61.54 ± 7.32 years. Based on TNM staging, 53 patients were classified as stage I/II, and 65 patients were stage III/IV. According to the Lauren classification, 72 cases were of the intestinal type and 46 cases were of the diffuse type.
Inclusion criteria: (1) Pathological diagnosis of gastric cancer[11]; (2) Availability of complete medical records and surgical documentation; and (3) Provision of signed informed consent.
Exclusion criteria: (1) Concurrent diagnosis of other malignancies; (2) History of radiotherapy, chemotherapy, or immunotherapy; (3) Presence of severe comorbidities involving the heart, lungs, liver, or kidneys; and (4) Incomplete medical records or missing data.
DNA extraction from paraffin-embedded tissue samples: Three 10-μm thick paraffin sections were cut and placed into 1.5-mL centrifuge tubes. One milliliter of xylene was added to each tube, followed by vortexing for 10 minutes and centrifugation at 12000 × g for 5 minutes. The supernatant was discarded, and this xylene treatment was repeated twice. The residue was then dehydrated through a graded ethanol series of 100%, 90%, and 70%, with each step involving 5 minutes of vortexing, followed by centrifugation and supernatant removal. After dehydration, 180 μL of buffer ATL (QIAGEN, Germany) and 20 μL of proteinase K (20 mg/mL; QIAGEN, Germany) were added to the sample. The mixture was incubated overnight for 16 hours at 56 °C in a D1100 metal bath (Labnet Dry Bath Incubator, United States) with continuous oscillation. Following the QIAamp DNA FFPE Kit instructions (QIAGEN, Germany), DNA was subsequently eluted with 50 μL of AE buffer.
DNA extraction from fresh tissue samples: Fresh gastric cancer tissue (50 mg) was obtained from a -80 °C ultra-low temperature freezer and placed in a pre-cooled stainless steel mortar. The tissue was rapidly ground into powder with liquid nitrogen. The powdered sample was then transferred into 2-mL bead tubes (containing 0.1 mm glass beads; QIAGEN, Germany) and kept on ice. Solution CD1 (800 μL) was added to the bead tubes, and the tubes were vortexed for 10 minutes to ensure complete suspension. The samples were incubated at 65 °C in a metal bath for 10 minutes, during which they were vortexed intermittently three times for 30 seconds each to promote cell membrane lysis. Following incubation, the tubes were centrifuged at 10000 × g for 5 minutes at 4 °C. The supernatant (600 μL) was carefully transferred to new 2-mL collection tubes, avoiding any precipitate.
To the collected supernatant, 250 μL of Solution CD2 was added. The mixture was vortexed for 5 seconds and left to stand at room temperature for 5 minutes. It was then loaded onto spin filters (QIAGEN, Germany) and centrifuged at 10000 × g for 1 minute. The resulting filtrate was discarded. Each spin filter received 500 μL of solution CD3 (ethanol wash), followed by centrifugation at 10000 × g for 30 seconds. The filtrate was discarded, and this wash step was repeated once. The spin filters were subsequently returned to the collection tubes and centrifuged at 13000 × g for 1 minute to remove residual ethanol. Finally, the spin filters were placed into sterile 1.5-mL centrifuge tubes. A volume of 50 μL of solution C6 (10 mmol/L Tris-HCl, pH 8.5) was added to each filter and allowed to stand at room temperature for 3 minutes. The eluate was collected by centrifugation at 10000 × g for 1 minute. A second elution was performed in the same manner, and the two eluates were combined to obtain a final volume of 100 μL.
DNA quality control: The Qubit 4.0 fluorometer (Thermo Fisher, United States) and dsDNA HS assay kit were used to detect DNA concentration, with a detection limit of 0.1 ng/μL. The NanoDrop One spectrophotometer (Thermo Fisher, United States) measured DNA purity, with qualification criteria of A260/A280 = 1.8-2.0 and A260/A230 > 1.5. DNA integrity was assessed using the G2939BA 2100 bioanalyzer (Agilent, United States) and high sensitivity DNA kit; samples showing a main peak > 500 bp were considered qualified.
16S rRNA gene multi-region library construction and analysis: Five pairs of primers were designed for the variable regions V2, V3, V5, V6, and V8 (Table 1), with amplified fragment lengths of 120-210 bp. The first-round PCR mixture consisted of 12.5 μL of KAPA HiFi HotStart ReadyMix (Roche, Switzerland), 3 μL of primer mix, 2 μL of DNA template (5 ng/μL), and 7.5 μL of nuclease-free water, forming a total volume of 25 μL. Reaction was performed on Thermo Fisher Veriti 96-well PCR machine under the following conditions: Pre-denaturation at 98 °C for 2 minutes; followed by 30 cycles of 98 °C for 10 seconds, 62 °C for 15 seconds, and 72 °C for 35 seconds; with a final extension at 72 °C for 5 minutes. Reactions were held at 4 °C after completion. PCR products were purified using Agencourt AMPure XP beads (Beckman Coulter, United States) at a 1:1 volume ratio (25 μL), washed twice with 80% ethanol, and eluted in 30 μL of nuclease-free water.
| Group | n | Bacterial concentration | ||
| 103 CFU/mg | 102 CFU/mg | 10 CFU/mg | ||
| Multi-region sequencing | 59 | 92.86 ± 3.52 | 76.43 ± 5.15 | 34.24 ± 4.87 |
| Single-region sequencing | 59 | 45.65 ± 6.27 | 18.96 ± 4.74 | 2.38 ± 1.19 |
| t value | - | 50.413 | 67.219 | 45.681 |
| P value | - | < 0.001 | < 0.001 | < 0.001 |
The second-round PCR mixture consisted of 25 μL of KAPA HiFi HotStart ReadyMix, 5 μL of Illumina Nextera XT Index Kit primers (Illumina, United States), and 20 μL of the purified first-round product, resulting in a total reaction volume of 50 μL. The PCR conditions were as follows: Pre-denaturation at 95 °C for 3 minutes; 8 cycles of 95 °C for 30 seconds, 60 °C for 30 seconds, and 72 °C for 30 seconds; followed by a final extension at 72 °C for 5 minutes. High-throughput sequencing was performed on the NovaSeq 6000 platform (Illumina, United States) using PE150 mode, with a data output of 2 Gb/sample. Primer sequences were removed using Cutadapt (v3.5), and chimeric and low-quality sequences were filtered out using DADA2 (v1.24). Operational taxonomic units (OTUs) were clustered at 97% similarity and annotated with the SILVA database (v138). Alpha-diversity analysis was conducted using MicrobiomeAnalyst (v2.0), including the calculation of Shannon, Simpson, and Chao1 indices.
Traditional single-region 16S rRNA gene sequencing method: The single V4 region was selected for sequencing, using the forward and reverse primers 5′-GTGYCAGCMGCCGCGGTAA-3′ and 5′-GGACTACNVGGGTWTCTAAT-3′, respectively. The amplified fragment was 250 bp in length. The same protocol as that used for the multi-region 16S rRNA gene sequencing method was followed.
Positive controls: The ZymoBIOMICSTM standard microbial community (containing eight strains of known abundance) and Escherichia coli ATCC 25922 were used as positive controls. Both multi-region and traditional single-region 16S rRNA gene sequencing methods were used for detection and validation.
The analysis included the following comparisons: (1) Comparison of species detection between multi- and single-region 16S rRNA gene sequencing in positive controls, as well as under different microbial abundances; and (2) Comparison of the number of OTUs and microbiome alpha diversity indices in gastric cancer tissues as detected by multi-region vs single-region 16S rRNA gene sequencing.
SPSS software (version 22.0) was used for the analysis. Measurement data that conformed to a normal distribution are expressed as mean ± SD and analyzed using a t-test. Statistical significance was set at P < 0.05.
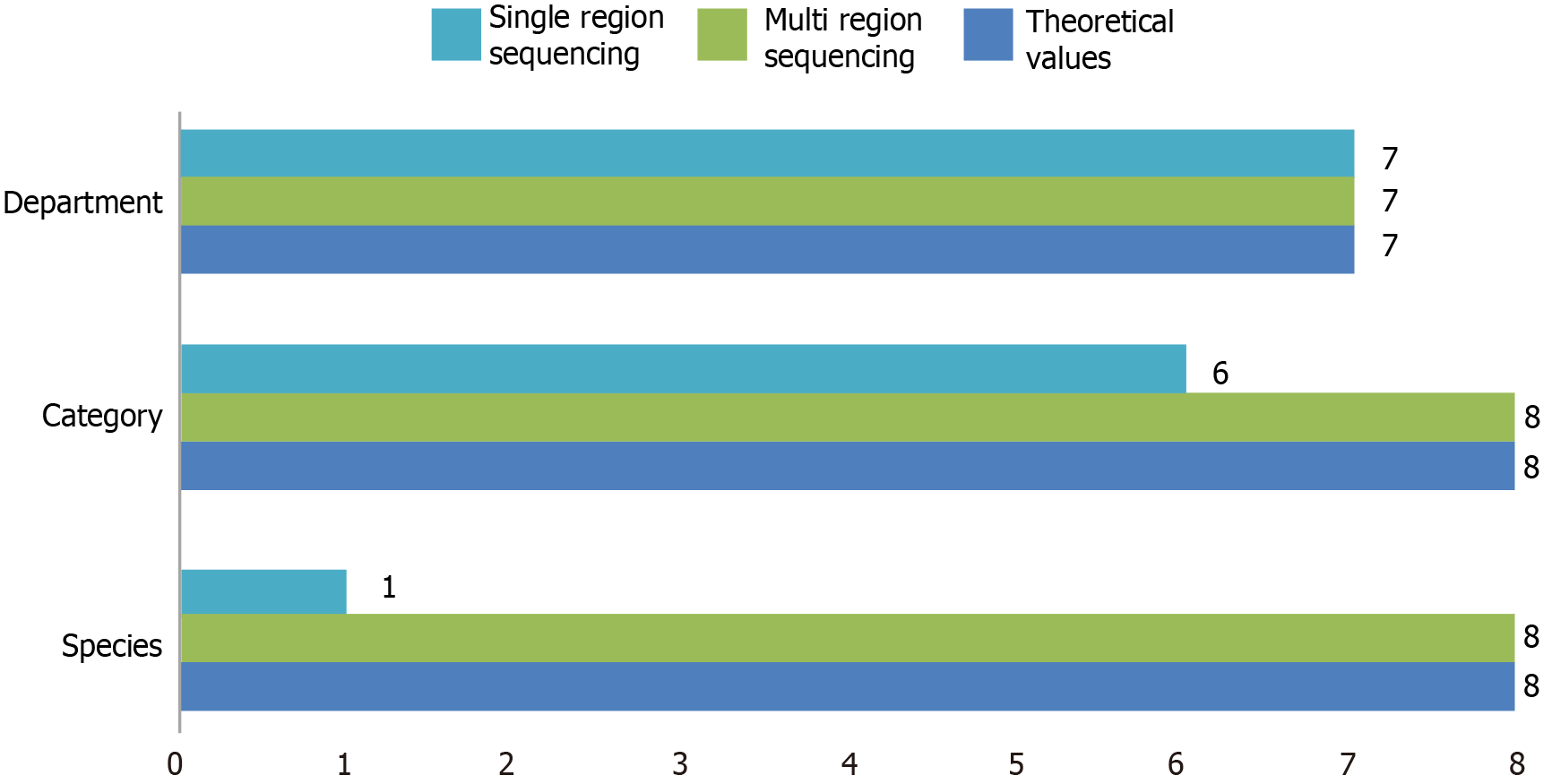
For the detection of positive controls, multi-region 16S rRNA gene sequencing (eight species and eight genera detected) demonstrated significantly greater species resolution compared with traditional single-region 16S rRNA gene sequencing (one species and six genera detected; Figure 1).
At bacterial concentrations of 103 CFU/mg, 102 CFU/mg, and 10 CFU/mg in positive controls, the detection rates achieved by multi-region 16S rRNA gene sequencing were all significantly higher than those achieved by traditional single-region 16S rRNA gene sequencing, with statistically significant differences observed (P < 0.05; Table 1).
The number of OTUs detected at the genus and species levels in paraffin-embedded and fresh gastric cancer tissue samples using multi-region 16S rRNA gene sequencing was significantly higher than that detected using traditional single-region 16S rRNA gene sequencing (P < 0.05; Table 2).
| Group | n | Genus | Species | ||
| Paraffinized tissue | Fresh tissue | Paraffinized tissue | Fresh tissue | ||
| Multi-region sequencing | 59 | 2.67 ± 0.58 | 2.33 ± 1.53 | 1.03 ± 0.35 | 0.67 ± 0.18 |
| Single-region sequencing | 59 | 74.21 ± 7.81 | 51.00 ± 16.09 | 32.33 ± 5.51 | 28.67 ± 9.07 |
| t value | - | 70.224 | 25.392 | 46.861 | 25.937 |
| P value | - | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
The Shannon, Simpson, and Chao1 indices of the microbiome detected in paraffin-embedded and fresh gastric cancer tissue samples by multi-region 16S rRNA gene sequencing were significantly higher than those detected by traditional single-region 16S rRNA gene sequencing (P < 0.05; Table 3).
| Group | n | Shannon | Simpson | Chao1 | |||
| Paraffinized tissue | Fresh tissue | Paraffinized tissue | Fresh tissue | Paraffinized tissue | Fresh tissue | ||
| Multi-region sequencing | 59 | 1.18 ± 0.12 | 0.68 ± 0.18 | 0.63 ± 0.08 | 0.37 ± 0.07 | 14.67 ± 3.21 | 3.33 ± 3.21 |
| Single-region sequencing | 59 | 3.08 ± 0.39 | 2.88 ± 0.40 | 0.89 ± 0.15 | 0.88 ± 0.24 | 199.67 ± 22.50 | 115.83 ± 31.95 |
| t value | - | 35.672 | 38.917 | 12.621 | 15.804 | 63.194 | 25.071 |
| P value | - | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 | < 0.001 |
Gastric cancer is a major disease that poses a threat to human health worldwide. Its pathogenesis is complex and involves multiple factors, with particular attention given to alterations in the microbial community[12,13]. Studies have shown that gastric cancer tissue is not a sterile environment; the colonizing microbiota can directly or indirectly influence tumor initiation, progression, and therapeutic response by regulating host immune responses, metabolic pathways, and epigenetic modifications[14,15]. However, traditional microbiome research methods have significant limitations. For example, classical 16S rRNA gene sequencing lacks the taxonomic resolution needed to accurately identify pathogenic bacteria at the species level[15,16]. Metagenomic sequencing can improve resolution but is limited by low sensitivity in low-biomass samples and high associated costs[17,18]. Therefore, developing a new technology that combines high resolution, high sensitivity, and low cost is urgently required for gastric cancer microbiome research.
Our results showed that, in detecting positive controls, multi-region 16S rRNA gene sequencing exhibited significantly better species resolution (detecting eight species and eight genera) than traditional single-region 16S rRNA gene sequencing did (detecting one species and six genera). The authors attribute this improvement to the core principle of 16S rRNA gene sequencing, which is based on the highly conserved and variable characteristics of the bacterial and archaeal small subunit ribosomal RNA (16S rRNA) gene. When combined with high-throughput sequencing technology, targeted amplification and analysis of these variable regions enable accurate analysis of microbial community composition and diversity[19,20], providing an efficient and economical tool for microbiome research. To address the technical limitations of traditional single-region 16S rRNA gene sequencing, this study innovatively applied a multi-region approach. Specific primers targeting five variable regions (V2, V3, V5, V6, and V8) were designed and combined with two-step PCR amplification and Illumina NovaSeq high-throughput sequencing. This strategy provided comprehensive genetic information, thereby improving species resolution and allowing for a detailed analysis of the gastric cancer tissue microbiome[21,22]. In contrast, traditional single-region 16S rRNA gene sequencing selects only one variable region for sequencing, resulting in relatively low species resolution and limited accuracy in identifying pathogenic bacteria at the species level[23,24]. Compared with traditional single-region V4 sequencing, the multi-region method achieved a 100% detection rate at the species level in the ZymoBIOMICSTM standard bacterial community, whereas single-region sequencing detected only 12.5%. This result is consistent with that of a study by Niikura et al[25], which indicated that multi-region amplification reduces classification bias caused by sequence conservation in a single region. Moreover, Park et al[26] showed that the V2 and V3 regions are particularly effective in distinguishing gram-positive bacteria, while the V5 and V6 regions are more sensitive in identifying gram-negative bacteria.
The results also showed that detection rates using multi-region 16S rRNA gene sequencing at bacterial concentrations of 103 CFU/mg, 102 CFU/mg, and 10 CFU/mg in positive controls were all significantly higher than those achieved with traditional single-region 16S rRNA gene sequencing (P < 0.05). Maintaining high sensitivity under conditions of low microbial abundance is particularly important for samples such as gastric cancer tissues, which typically contain relatively low microbial biomass. Traditional single-region 16S rRNA gene sequencing exhibits significantly decreased detection rates as microbial abundance declines, potentially resulting in the loss of important microbial information and hindering a comprehensive and accurate understanding of the gastric cancer microbiome. This study demonstrated that under different microbial abundances in positive control detection, multi-region 16S rRNA gene sequencing significantly achieved higher detection rates than traditional single-region sequencing. This result was particularly observed in the detection of low-abundance bacterial communities at 102 CFU/mg and 10 CFU/mg, where multi-region sequencing showed higher sensitivity. This finding is of great significance for early screening and diagnosis of gastric cancer, given that microbial communities within the tumor microenvironment are often present at low abundance and are easily overlooked by traditional sequencing methods. For instance, the detection rate of single-region sequencing in samples with a low microbial abundance of 10 CFU/mg was only 2.38%, whereas multi-region sequencing increased the detection rate to 34.24%. This result is consistent with that of Zhang et al[27], who reported that multiplex primer designs targeting different variable regions can effectively balance PCR bias. Furthermore, Spiegelhauer et al[28] proposed that the V6 region has a higher sensitivity to low-abundance gram-negative bacteria, and the V8 region is more compatible with degraded DNA in paraffin-embedded samples. These factors may contribute to the superior performance of multi-region 16S rRNA gene sequencing in samples with low microbial abundance. Additionally, multi-region 16S rRNA sequencing can amplify short fragments of 120-210 bp, effectively addressing the issue of DNA fragmentation in paraffin-embedded samples and enhancing detection stability. This technical advantage is valuable for the secondary analysis of archived samples.
At the same time, the results showed that compared with traditional single-region 16S rRNA gene sequencing, multi-region 16S rRNA gene sequencing detected significantly more OTUs at the genus and species levels, along with higher Shannon, Simpson, and Chao1 indices, in paraffin-embedded and fresh gastric cancer tissue samples (P < 0.05). The authors believe that, in gastric cancer tissues, the number of species-level OTUs detected using the multi-region approach was 32.3 times greater than that detected using traditional single-region sequencing. Notably, in samples with low microbial abundance (10 CFU/mg), the detection rate increased from approximately 2.1% to 36.8%, suggesting that multi-region sequencing can capture rarer species and more comprehensively analyze microbiome complexity. This is mainly attributed to the synergistic effects of multiplex primers in the multi-region method, which broaden the detection range and enhance sensitivity to low-abundance microorganisms[29]. Additionally, multi-region 16S rRNA gene sequencing significantly outperformed single-region sequencing in alpha diversity metrics, such as the Shannon, Simpson, and Chao1 indices. For example, the Shannon index in paraffin samples increased from 3.08 (single-region) to 4.26 (multi-region), indicating that the multi-region approach more accurately reflects microbial community evenness and richness. This phenomenon was also reported in the study by Liu et al[30] and may be explained by the ability of multi-region sequencing to reduce the “dominant species masking effect” caused by amplification of a single region, in which signals from high-abundance species overshadow those of low-abundance species. By reducing this effect, the multi-region method enables more accurate detection and analysis of low-abundance species. This advantage is particularly important in gastric cancer microbiome research, where microbial community structures are complex and variable, and low-abundance microbes may play key roles in tumorigenesis and disease progression.
Moreover, this study found that the performance of multi-region 16S rRNA gene sequencing was consistent in both paraffin-embedded and fresh tissue samples and superior to that of traditional single-region sequencing. This result further confirms the reliability and stability of the multi-region approach, providing strong technical support for its application in gastric cancer microbiome research.
Although this study yielded promising results, several limitations remain, including the use of a single sample source, relatively high technical costs, and long analysis times. Future research will aim to expand the sample size, including gastric cancer samples from different regions and pathological types, optimize experimental protocols, reduce costs, and shorten analysis times to promote the clinical translation and application of this technology.
This study had several limitations. First, all gastric cancer samples were obtained from a single center (Liyang People’s Hospital), which may have introduced regional microbial biases and limited the generalizability of the results to populations in other geographic areas or those with different ethnic backgrounds. Second, subgroup analyses based on histological classification (intestinal vs diffuse) and TNM stage were not performed. Consequently, the clinical utility of multi-region 16S rRNA sequencing across various tumor subtypes remains unclear. Third, although the high cost of metagenomic sequencing is well recognized, a direct comparison was not conducted. This omission limits the ability to evaluate differences in taxonomic resolution between the two methods and to define the technical role of multi-region sequencing within current microbial profiling approaches. Finally, although positive controls were used to assess detection performance, false-positive rates were not examined, nor was contamination by non-target microorganisms systematically ruled out. These gaps may have led to an overestimation of microbial diversity.
In conclusion, the multi-region 16S rRNA gene sequencing method demonstrated higher species-level resolution and detection rates in gastric cancer microbiome research and more comprehensively reflected the microbial diversity present in gastric cancer tissues.
| 1. | Merali N, Chouari T, Sweeney C, Halle-Smith J, Jessel MD, Wang B, O' Brien J, Suyama S, Jiménez JI, Roberts KJ, Velliou E, Sivakumar S, Rockall TA, Demirkan A, Pedicord V, Deng D, Giovannetti E, Annels NE, Frampton AE. The microbial composition of pancreatic ductal adenocarcinoma: a systematic review of 16S rRNA gene sequencing. Int J Surg. 2024;110:6771-6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 2. | Booth ME, Wood HM, Travis MA; Genomics England Research Consortium, Quirke P, Grabsch HI. The relationship between the gastric cancer microbiome and clinicopathological factors: a metagenomic investigation from the 100,000 genomes project and The Cancer Genome Atlas. Gastric Cancer. 2025;28:358-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 3. | Abate M, Walch H, Arora K, Vanderbilt CM, Fei T, Drebin H, Shimada S, Maio A, Kemel Y, Stadler ZK, Schmeltz J, Sihag S, Ku GY, Gu P, Tang L, Vardhana S, Berger MF, Brennan MF, Schultz ND, Strong VE. Unique Genomic Alterations and Microbial Profiles Identified in Patients With Gastric Cancer of African, European, and Asian Ancestry: A Novel Path for Precision Oncology. Ann Surg. 2023;278:506-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Abate M, Vos E, Gonen M, Janjigian YY, Schattner M, Laszkowska M, Tang L, Maron SB, Coit DG, Vardhana S, Vanderbilt C, Strong VE. A Novel Microbiome Signature in Gastric Cancer: A Two Independent Cohort Retrospective Analysis. Ann Surg. 2022;276:605-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 5. | Retnakumar RJ, Nath AN, Nair GB, Chattopadhyay S. Gastrointestinal microbiome in the context of Helicobacter pylori infection in stomach and gastroduodenal diseases. Prog Mol Biol Transl Sci. 2022;192:53-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 6. | Hunter C, Dia K, Boykins J, Perry K, Banerjee N, Cuffee J, Armstrong E, Morgan G, Banerjee HN, Banerjee A, Bhattacharya S. An investigation for phylogenetic characterization of human Pancreatic cancer microbiome by 16SrDNA Sequencing and Bioinformatics techniques. Res Sq. 2024;rs.3.rs-4140368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Kim MJ, Kim HN, Jacobs JP, Yang HJ. Combined DNA Methylation and Gastric Microbiome Marker Predicts Helicobacter pylori-Negative Gastric Cancer. Gut Liver. 2024;18:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Nakatsu G, Ko D, Michaud M, Franzosa EA, Morgan XC, Huttenhower C, Garrett WS. Virulence factor discovery identifies associations between the Fic gene family and Fap2(+) fusobacteria in colorectal cancer microbiomes. mBio. 2025;16:e0373224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 9. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3252] [Article Influence: 542.0] [Reference Citation Analysis (6)] |
| 10. | Guan SW, Lin Q, Wu XD, Yu HB. Weighted gene coexpression network analysis and machine learning reveal oncogenome associated microbiome plays an important role in tumor immunity and prognosis in pan-cancer. J Transl Med. 2023;21:537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Sambruni G, Macandog AD, Wirbel J, Cagnina D, Catozzi C, Dallavilla T, Borgo F, Fazio N, Fumagalli-Romario U, Petz WL, Manzo T, Ravenda SP, Zeller G, Nezi L, Schaefer MH. Location and condition based reconstruction of colon cancer microbiome from human RNA sequencing data. Genome Med. 2023;15:32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Nikitina D, Lehr K, Vilchez-Vargas R, Jonaitis LV, Urba M, Kupcinskas J, Skieceviciene J, Link A. Comparison of genomic and transcriptional microbiome analysis in gastric cancer patients and healthy individuals. World J Gastroenterol. 2023;29:1202-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (2)] |
| 13. | Mannion A, Sheh A, Shen Z, Dzink-Fox J, Piazuelo MB, Wilson KT, Peek R, Fox JG. Shotgun Metagenomics of Gastric Biopsies Reveals Compositional and Functional Microbiome Shifts in High- and Low-Gastric-Cancer-Risk Populations from Colombia, South America. Gut Microbes. 2023;15:2186677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | In H, Perati SR, Usyk M, Yang J, Sarkar S, Rana B, Wang F, Oh A, Adams A, Diggs LP, Sollecito C, Burk RD. Oral Microbiome Signatures as Potential Biomarkers for Gastric Cancer Risk Assessment. J Gastrointest Surg. 2024;101933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Oh S, Kim J, Shin CM, Lee HJ, Lee HS, Park KU. Metagenomic characterization of oral microbiome signatures to predict upper gastrointestinal and pancreaticobiliary cancers: a case-control study. J Transl Med. 2025;23:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Mathebela P, Damane BP, Mulaudzi TV, Mkhize-Khwitshana ZL, Gaudji GR, Dlamini Z. Influence of the Microbiome Metagenomics and Epigenomics on Gastric Cancer. Int J Mol Sci. 2022;23:13750. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 17. | Szymczak A, Ferenc S, Majewska J, Miernikiewicz P, Gnus J, Witkiewicz W, Dąbrowska K. Application of 16S rRNA gene sequencing in Helicobacter pylori detection. PeerJ. 2020;8:e9099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Peng R, Liu S, You W, Huang Y, Hu C, Gao Y, Jia X, Li G, Xu Z, Chen Y. Gastric Microbiome Alterations Are Associated with Decreased CD8+ Tissue-Resident Memory T Cells in the Tumor Microenvironment of Gastric Cancer. Cancer Immunol Res. 2022;10:1224-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 19. | Yang W, Zhao Y, Ge Q, Wang X, Jing Y, Zhao J, Liu G, Huang H, Cheng F, Wang X, Ye Y, Song W, Liu X, Du J, Sheng J, Cao X. Genetic mutation and tumor microbiota determine heterogenicity of tumor immune signature: Evidence from gastric and colorectal synchronous cancers. Front Immunol. 2022;13:947080. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Vadhwana B, Tarazi M, Boshier PR, Hanna GB. Evaluation of the Oesophagogastric Cancer-Associated Microbiome: A Systematic Review and Quality Assessment. Cancers (Basel). 2023;15:2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Oosterlinck B, Ceuleers H, Arras W, De Man JG, Geboes K, De Schepper H, Peeters M, Lebeer S, Skieceviciene J, Hold GL, Kupcinskas J, Link A, De Winter BY, Smet A. Mucin-microbiome signatures shape the tumor microenvironment in gastric cancer. Microbiome. 2023;11:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | You HS, Park JY, Seo H, Kim BJ, Kim JG. Increasing correlation between oral and gastric microbiota during gastric carcinogenesis. Korean J Intern Med. 2024;39:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | Ai B, Mei Y, Liang D, Wang T, Cai H, Yu D. Uncovering the special microbiota associated with occurrence and progression of gastric cancer by using RNA-sequencing. Sci Rep. 2023;13:5722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 24. | Qian ST, Zhao HY, Xie FF, Liu QS, Cai DL. Streptococcus anginosus in the development and treatment of precancerous lesions of gastric cancer. World J Gastrointest Oncol. 2024;16:3771-3780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (1)] |
| 25. | Niikura R, Hayakawa Y, Nagata N, Miyoshi-Akiayama T, Miyabayashi K, Tsuboi M, Suzuki N, Hata M, Arai J, Kurokawa K, Abe S, Uekura C, Miyoshi K, Ihara S, Hirata Y, Yamada A, Fujiwara H, Ushiku T, Woods SL, Worthley DL, Hatakeyama M, Han YW, Wang TC, Kawai T, Fujishiro M. Non-Helicobacter pylori Gastric Microbiome Modulates Prooncogenic Responses and Is Associated With Gastric Cancer Risk. Gastro Hep Adv. 2023;2:684-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 26. | Park CH, Lee AR, Lee YR, Eun CS, Lee SK, Han DS. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter. 2019;24:e12547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 27. | Zhang P, Xu J, Zhou Y. The relationship between gastric microbiome features and responses to neoadjuvant chemotherapy in gastric cancer. Front Microbiol. 2024;15:1357261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 28. | Spiegelhauer MR, Kupcinskas J, Johannesen TB, Urba M, Skieceviciene J, Jonaitis L, Frandsen TH, Kupcinskas L, Fuursted K, Andersen LP. Transient and Persistent Gastric Microbiome: Adherence of Bacteria in Gastric Cancer and Dyspeptic Patient Biopsies after Washing. J Clin Med. 2020;9:1882. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Chen J, Nie S, Qiu X, Zheng S, Ni C, Yuan Y, Gong Y. Leveraging existing 16S rRNA microbial data to identify diagnostic biomarker in Chinese patients with gastric cancer: a systematic meta-analysis. mSystems. 2023;8:e0074723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Liu D, Zhang R, Chen S, Sun B, Zhang K. Analysis of gastric microbiome reveals three distinctive microbial communities associated with the occurrence of gastric cancer. BMC Microbiol. 2022;22:184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (1)] |