Published online Jun 15, 2024. doi: 10.4251/wjgo.v16.i6.2284
Revised: April 2, 2024
Accepted: April 24, 2024
Published online: June 15, 2024
Processing time: 168 Days and 22.7 Hours
T1 colorectal cancer (CRC), defined by tumor invasion confined to the submucosa, has historically been managed by surgery. Improved understanding of recurrence and lymph node metastases risk, coupled with advances in endoscopic resection techniques, have led to an increasing capacity for organ-sparing local excision. Minimally invasive management of T1 CRC begins with optical evaluation of the lesion to diagnose invasive disease and quantify depth of invasion, which informs therapeutic decision making. Modality selection between various available endoscopic resection techniques depends upon lesion characteristics, technique risk-benefit profiles, and location-specific implications. Following endoscopic resection, established histopathology features determine the risk of recurrence and subsequent management including surveillance or adjuvant surgical excision. The management of non-operative candidates deviates from conventional recommendations with emerging treatment strategies in select populations.
Core Tip: Advances in minimally invasive endoscopic resection techniques, including endoscopic mucosal resection, endoscopic submucosal dissection, endoscopic full-thickness resection and transanal endoscopic surgery, have revolutionized the management of T1 colorectal cancer (CRC); allowing for organ preservation while mitigating the associated morbidity of colorectal surgery. Herein we outline the pre-resection, resection and post-resection phases of care for T1 CRC including emerging techniques and adjuvant strategies for non-operative candidates.
- Citation: Jiang SX, Zarrin A, Shahidi N. T1 colorectal cancer management in the era of minimally invasive endoscopic resection. World J Gastrointest Oncol 2024; 16(6): 2284-2294
- URL: https://www.wjgnet.com/1948-5204/full/v16/i6/2284.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i6.2284
T1 colorectal cancer (CRC), as described by the Vienna Classification and the American Joint Committee on Cancer, refers to lesions with neoplastic invasion confined to the submucosa[1,2]. This does not include high grade dysplasia or carcinoma in situ; as in these scenarios neoplasia is confined to the mucosa, which is devoid of lymphatics and therefore metastatic potential[3]. With prevalence estimates as high as 5% within population-based screening, T1 CRC represents an important well-characterized clinical entity associated with established risks of recurrence and lymph node metastases (LNM)[4].
Historically, radical surgery was the default management strategy for T1 CRC. However, in low-risk T1 CRC, surgery has modest additional oncologic benefit but with significant mortality and morbidity including permanent ostomy formation. In a population-based cohort of 5170 patients with T1 CRC who underwent surgery, 30-d mortality was 1.7% and 8.3% had severe adverse events requiring re-intervention[5]. Therefore, with a growing number of patients with advanced age and comorbid disease, an alternative approach to treatment is needed[6].
Minimally invasive endoscopic resection offers a safe, effective, and organ-sparing alternative. Modalities include endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), endoscopic full thickness resection (EFTR), and transanal endoscopic surgery (TES; Table 1). A retrospective analysis of 1069 T1 CRC that underwent EMR, ESD and conventional snare resections demonstrated a 5.5% rate of adverse events and no procedure-associated mortality[7]. Minimally invasive endoscopic resection also has comparable efficacy to surgery; in a meta-analysis comparing minimally invasive endoscopic resection and surgery for T1 CRC, the 5-year recurrence-free survival was similar at 96.0% and 96.7%, respectively[8].
| Endoscopic technique | Application in T1 CRC | Outcomes | Advantages | Disadvantages |
| EMR | Recommended for lesions < 20 mm due to risk of piecemeal resection | En-bloc resection: 85.2%[44]; R0 resection: 83.9%[44] | Widely available, efficient, less resource intensive, high technical success in expert centers | Limited en-bloc resection rate with increasing size |
| ESD | Recommended for T1 CRC without signs of deep submucosal invasion | En-bloc resection: 98.7%[45]; R0 resection: 97.4%[45] | High en-bloc resection, technical success, and clinical success rate | Resource intensive and requires specific training |
| EFTR | Primary and secondary resection of T1 CRC | Technical success: 87.0%[48]; R0 resection: 85%[48] | High en-bloc and R0 resection rate, particularly for deep invasion and submucosal fibrosis | Depends on local expertise and technology availability. Risk of appendicitis and heightened risk of delayed perforation |
| TES: TEM, TAMIS | Rectal T1 CRC | TEM: En-bloc resection: 97.0%[57]; R0 resection: 93.0%[57] | Full thickness-resection. High en-bloc and R0 resection rate, particularly for deep invasion | For rectal lesions only. Resource intensive and requires specific training. May affect planes for completion total mesorectal excision |
Advances in minimally invasive endoscopic resection techniques alongside an improved understanding of metastatic risk have led to a paradigm shift in the management of T1 CRC; with minimally invasive endoscopic resection now considered first-line. Herein we describe the risk stratification of T1 CRC, optical evaluation characteristics and performance, resection modalities, post-resection management, and treatment of non-operative candidates with review of emerging therapeutic strategies.
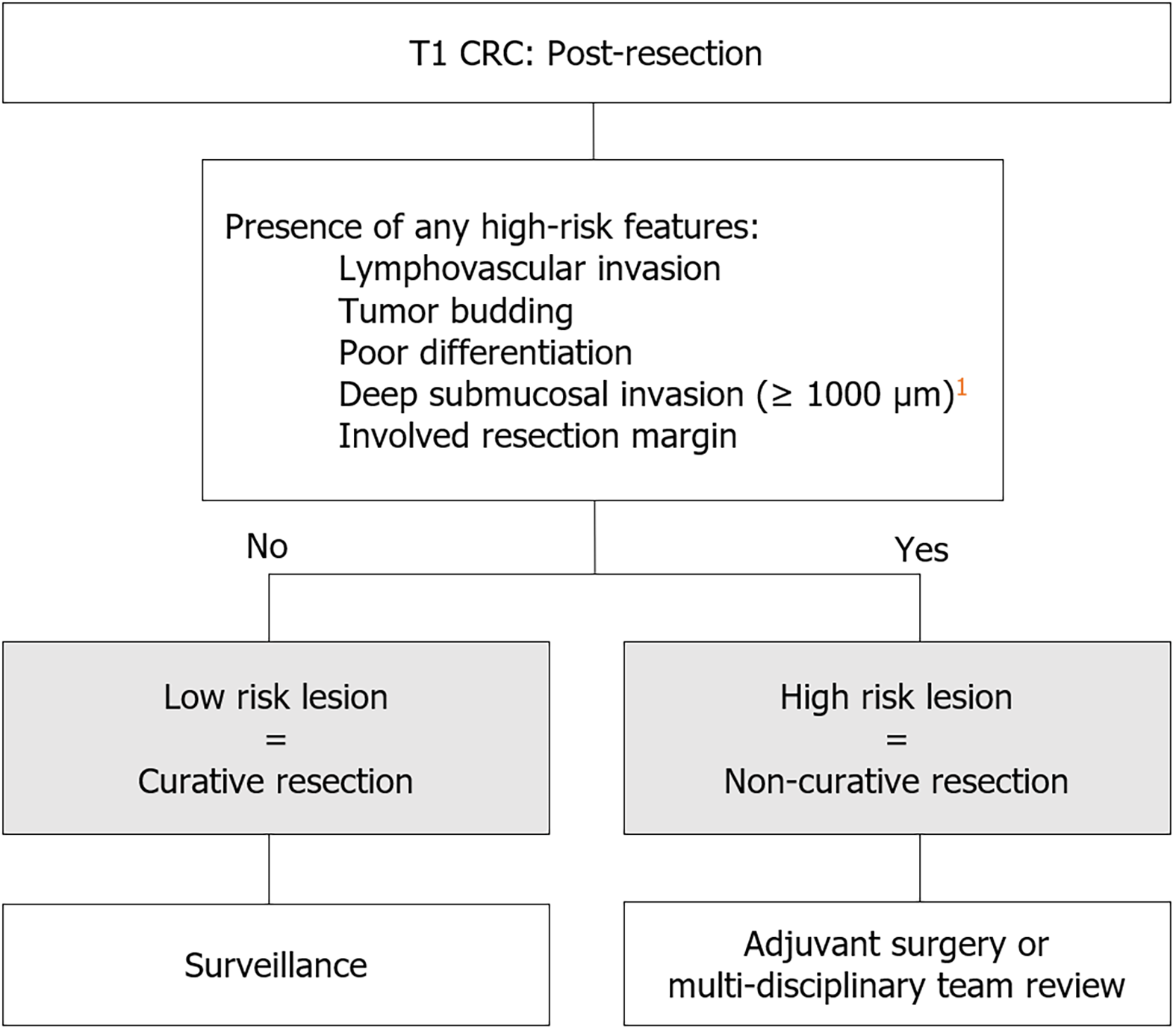
Low risk T1 CRC is defined by established histopathology risk factors for local and distant recurrent disease. International consensus guidelines define low risk T1 CRC by the absence of lymphovascular invasion (LVI), poor differentiation (PD), tumor budding (TB), and deep submucosal invasion (DSI; ≥ 1000 µm), as well as en-bloc resection with negative histologic margins (R0 resection). Conversely, the presence of any of these features denotes high risk T1 CRC and requires completion surgery[9-11]. In a meta-analysis including 71 studies with 5167 patients, the pooled incidence of local and distant recurrence for T1 CRC was 3.3%[12]. However, when stratified by high risk and low risk T1 CRC, pooled incidence of local and distant recurrence was 7.0% and 0.7% respectively[12]. Given the low risk of recurrent disease, compared to the adverse event profile of completion surgery, endoscopic resection followed by surveillance for low risk T1 CRC is now recommended[10,11].
The above features which define high risk T1 CRC are associated with differing clinical relevance, particularly depth of invasion. Prior meta-analyses report increased LNM in the presence of DSI, with a relative risk of 5.2[13,14]. However, it was noted that the effect of DSI may be due to concurrent high-risk features; in their absence, T1 CRC with DSI in isolation had a frequency of LNM as low as 1.2%[15]. Similarly, a recent meta-analysis of 67 studies and 21238 patients found that DSI was not an independent risk factor for LNM after adjusting for other high risk criteria[16]. Studies have proposed alternative assessments including deeper cut-offs of ≥ 1800 or 2500 µm[15,17], or total area of submucosal invasion as a proportion of tumor stroma[17].Therefore, it is reasonable to consider expanding current low-risk criteria to allow for DSI.
The definition of a negative margin on histopathology is also in question. Recent guidelines from the US Multi-Society Task Force suggest a distance from the margin of at least 1 mm[9], whereas the Japanese Society for Cancer of the Colon and Rectum define safe margin as > 0 mm[10]. Further, measurement of the margin may be confounded by specimen handling and cautery effect[12]. For low risk T1 CRC, residual disease remains infrequent with either definition. Gijsbers et al[18] compared margins of 0.1-1 mm and > 1 mm in 522 low risk T1 CRC and found no significant difference in the frequency of residual disease at 2.9% and 0.6% respectively. Conversely, residual tumor rate of high risk T1 CRC is 6%-16% in studies with margins < 1 mm[19,20]. While histopathology remains definitive, endoscopic evaluation to define margin adequacy remains notable; local recurrence or residual neoplastic tissue in the surgical specimen was only found in 0%-3% of T1 CRC with macroscopically complete resection, even if the original local excision had R1/Rx status[19-21]. Further study is needed to delineate an optimized definition of a negative histologic margin, particularly for low-risk T1 CRC to avoid unnecessary surgery.
PD, TB, and LVI are well-established histopathologic features. PD and TB have an odds ratio (OR) for LNM of 2.14 and 2.83, respectively, in the prior meta-analysis including 21,238 patients[16,22,23]. LVI is commonly reported as a combined entity, which is associated with an OR for LNM of 3.16. However, when considered separately lymphatic and vascular invasion are seen in 20% and 14% of T1 CRC with LNM, and lymphatic invasion has a higher OR for LNM[13,14,24].
Predictive models and artificial intelligence systems to determine the risk of LNM in T1 CRC are being developed that incorporate clinical and histopathological parameters, with good performance against current guidelines[25,26].
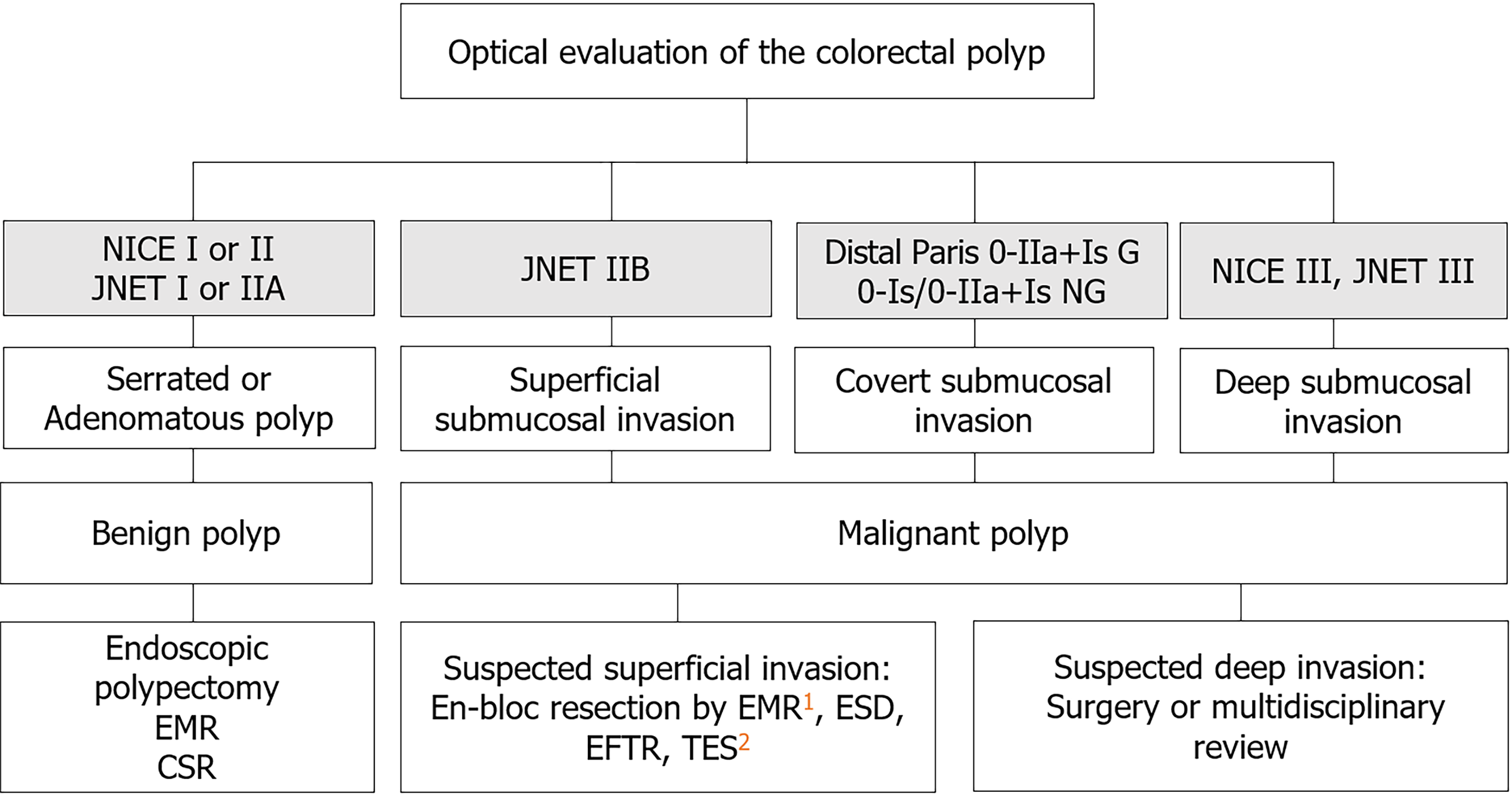
Optical evaluation refers to classifying the lesion by location, size, and lesion morphology alongside interrogating the surface pit and microvascular pattern to predict: (1) Lesion histopathology (adenomatous vs serrated); (2) the presence of invasive disease; and (3) the depth of invasion.
Multiple optical features have been evaluated as a predictive tool for invasive disease and to stratify depth of invasion[27]. This includes increasing lesion size, distal location (rectosigmoid), gross morphological features (GMF) and abnormal pit/microvascular surface patterns as evaluated by image enhanced endoscopy [e.g., narrow band imaging (NBI), magnifying chromoendoscopy (MCE)]. Using NBI, submucosal invasion appears as a loss of the regular microvascular pattern, such as NBI International Colorectal Endoscopic classification III or Japan NBI Expert Team classification IIB/III. Using MCE, an amorphous pit pattern may be seen, corresponding with Kudo Pit Pattern Vi/Vn. Finally GMF are identified with high definition white light endoscopy and include non-granular morphology, depression (Paris 0-IIc), ulceration, presence of a large nodule, spontaneous bleeding, white spots, exudate, and non-lifting sign)[28-35]. In a meta-analysis of 31568 lesions, optical prediction of T1 CRC was superior using NBI and MCE features. Sensitivity and specificity was 85% and 94% for NBI, 90% and 96% for MCE, compared to sensitivity of 21%-46% for GMF. Similarly, sensitivity and specificity for prediction of DSI was 77% and 98% for NBI, 81% and 95% for MCE, and 18%-68% and 80%-98% for GMF[35].
Optical evaluation by endoscopists, even without magnification, has demonstrated high negative predictive value (NPV) and reasonable positive predictive value (PPV). In a prospective study, advanced endoscopists using GMF and NBI predicted T1 CRC with PPV of 68% and NPV of 96%; DSI was predicted with PPV of 86% and NPV of 96%[36]. Subsequently, a real-world study including non-expert endoscopists retained a high NPV of 98% for DSI, though PPV was 41%[31]. While this is potentially attributable to the low prevalence of deeply invasive lesions, it suggests over-estimation of DSI.
While DSI is challenged as an independent risk factor for LNM, the presence of other high risk T1 CRC criteria are associated with similar optical features including, protuberance within the depression, expansiveness, and loss of mucosal pattern[37].
For large non-pedunculated colorectal polyps, submucosal invasion may be present without any optical features, thus termed “covert” submucosal invasive cancer. In an analysis of 2277 lesions, Burgess et al[30] found that location, morphology, and granularity were associated with covert submucosal invasion. Rectosigmoid Paris 0-IIA+IS granular lesions and Paris 0-IS/0-IIA+IS non-granular lesions of any location were associated with a > 10% risk of submucosal invasion; these lesions should be treated as T1 CRC and removed en-bloc (Figure 1 and Table 2).
| Optical evaluation | Corresponding histopathology | Suspicion of malignancy | Recommended management |
| NICE I, JNET I | Serrated Polyp | Low | Endoscopic polypectomy, EMR, CSR |
| NICE II, JNET II | Adenomatous Polyp | Low | If suspected superficial invasion: en-bloc resection by EMR1, ESD, EFTR, TES2 If suspected deep invasion: surgery or multidisciplinary review |
| JNET IIB | Superficial submucosal invasion | Yes, superficial | |
| NICE III, JNET III | Deep submucosal invasion | Yes, deep | |
| Paris 0-IIa+Is granular lesions in distal colorectum | Covert submucosal invasion | Yes | |
| Paris 0-Is/0-IIa+Is nongranular lesions in distal colorectum | Covert submucosal invasion | Yes |
Initial endoscopic en-bloc resection modalities for T1 CRC were EMR and ESD. High-quality EMR involves submucosal injection followed by use of a snare with electrocautery to capture and resect the lesion in question, paying careful attention to capture a generous margin of normal mucosa. In contrast, ESD uses an electrosurgical knife to dissect along the submucosal plane beneath the lesion[38]. Multiple studies evaluating colorectal neoplasia have shown that ESD has superior en-bloc, R0 resection, and recurrence, but longer procedural times and more adverse events compared to EMR[39-41]. Specifically in early CRC, EMR demonstrated en-bloc and R0 resection rates of 85.2% and 83.9% respectively[42], limiting use to lesions smaller than 20 mm given the potential for deep mural injury/perforation[10]. Comparatively, ESD for superficial T1 CRC had an en-bloc resection rate of 98.7% and R0 rate of 97.4%[43]. Thus, incorporating ESD in a selective resection algorithm accounting for T1 CRC can improve oncologic outcomes compared to universal use of EMR[44]. However, in cases of submucosal fibrosis and DSI, R0 resection of ESD is reduced to 64.7%[43].
Endoscopic full-thickness resection was developed to address the deficiencies of ESD. In the colorectum, this is most commonly performed using the full-thickness resection device; where the lesion is pulled into the application cap, incorporating the muscularis propria, with clip closure of the colorectum and ultimately, full-thickness resection of the lesion[45]. Specific to T1 CRC, the Dutch EFTR registry of 330 lesions demonstrated 85% R0 resection, 87% technical success, 8.1% adverse event rate, and 99.3% complete histopathology specimens for both primary and secondary scar resection[46]. The German EFTR registry of 156 T1 CRC had similar results, including 99.4% of lesions with complete risk stratification, with 43.9% re-classified as low-risk T1 CRC[47]. Earlier EFTR studies reported lower R0 resection for lesions > 20 mm[45,48], but can be addressed with a “hybrid-EFTR” technique for larger, non-lifting lesions[49,50]. Availability of EFTR technology and experience with EFTR limits widespread use, but there is increasing uptake in Western countries.
For early rectal cancer, TES is another option, comprised of transanal endoscopic microsurgery (TEM) and transanal minimally invasive surgery (TAMIS). TEM utilizes a specialized anoscope that facilitates both insufflation and passage of an endoscope and surgical instruments to enable full thickness excision[51]. TAMIS is facilitated by a single transanal port, but uses standard laparoscopic instruments, camera, and insufflator, thus making it a more accessible modality than TEM[52]. Compared to TAMIS, TEM is considered to have less risk of fragmentation but is more time and resource intensive, with a steeper learning curve, and a similar recurrence rate when performed within high-volume centers[53,54]. Compared to ESD, TEM has similar rate of en-bloc, R0 resection, and adverse events, but significantly longer procedure time and length of stay[55,56].There is a signal that TEM and TAMIS may affect subsequent surgery with higher rate of incomplete mesorectal specimens and longer operative time compared to primary total mesorectal excision (TME), however this has not been shown to affect recurrence or survival[57]. Overall, there appears to be similar risk-benefit profiles for TEM, TAMIS and ESD, thus leaving technique selection largely determined by local availability and expertise.
While high risk T1 CRC generally requires subsequent completion surgery due to LNM risk, T1 CRC meeting low risk criteria is recommended to undergo surveillance; which may potentially include both endoscopic and radiographic surveillance (Figure 2 and Table 3). Western guidelines agree that the first endoscopic surveillance should occur in 3-6 months post-resection[11,58], however Japanese guidelines suggest surveillance in 1 year[9]. Proponents for a longer interval surveillance argue a low likelihood of missed synchronous lesions in endoscopically manageable T1 CRC and low rate of local recurrence[59].
| High-risk T1 CRC | Low-risk T1 CRC | |
| High Risk Histopathology Features (lymphovascular invasion, tumor budding, poor differentiation, deep submucosal invasion (≥ 1000 µm)1, positive resection margin) | Presence of one or more histopathology features | Absence of all high-risk histopathology features |
| Resection status | Non-curative | Curative |
| Recommended management | Adjuvant surgery or multidisciplinary review | Surveillance |
Following the first surveillance endoscopy, guidelines diverge. The European Society of Gastrointestinal Endoscopy recommends standard CRC surveillance after 1, 3, and 5 years, for both surgically and endoscopically cured CRC[11]. Comparatively, the American Gastroenterology Association recommendations are specific to endoscopic resection and location of CRC. For colon cancers, the second surveillance colonoscopy is advised after 6 months, then after 1 year. For rectal cancers, flexible sigmoidoscopy is suggested every 6 months up to 5 years, with concomitant regular endoscopic ultrasound or pelvic magnetic resonance imaging surveillance up to 5 years[60], attributed to the higher risk of recurrence in rectal cancers[61]. Following publication of these guidelines, a multi-center cohort study of 336 patients with T1 CRC (84 rectal cancers) underwent radiological surveillance, finding an overall 5-year cumulative incidence of 2.4% for distant recurrence and no distant metastases in low risk T1 CRC[60]. As experience with endoscopic resection of low risk T1 CRC continues to grow, guidelines surrounding surveillance will likely be updated accordingly (Table 4).
| Guideline | First surveillance | Subsequent surveillance | |
| Japanese Society for Cancer of the Colon and Rectum 2019[10] | Colonoscopy at 6-12 months | No specific comment | |
| European Society of Gastrointestinal Endoscopy 2019[11] | Colonoscopy at 3-6 months | 1, 3, and 5 yr | |
| American Gastroenterology Association 2021[60] | Colon | Colonoscopy at 3-6 months | 6 months and 1 yr |
| Rectum | Flexible sigmoidoscopy at 3-6 months and colonoscopy at 1 yr | Flexible sigmoidoscopy every 6 months up to 5 yr, with concomitant EUS or pelvic magnetic resonance imaging every 3-6 months for 2 yr, then every 6 months to complete 5 yr. May consider CT chest, abdomen, and pelvis for 3-5 yr | |
While surgery remains standard of care for T1 CRC with suspected DSI, there is emerging evidence for endoscopic management. In lesions with deep invasion, endoscopic resection can retain both diagnostic value for histopathologic risk stratification and potential for definitive therapy. A study of 126 T1 CRC with a focal DSI surface pattern treated by ESD found R0 resection in 76.7% and curative resection in 26.6% meeting low risk criteria[62]. This may partly reflect the moderate PPV of optical evaluation for DSI[31], but highlights the changing role of minimally invasive endoscopic resection for more definitive diagnosis. Thus, particularly for patients who are poor surgical candidates, ESD may still be considered given its safety profile.
Initially developed to address the failure of ESD in attaining deep margins for rectal lesions with submucosal fibrosis, endoscopic intermuscular dissection (EID) facilitates dissection of T1 rectal cancer with DSI and leaves the rectal wall intact in case of potential subsequent TME, providing proposed advantages over ESD and TES, respectively. In a prospective cohort study of 67 patients with deeply invasive T1 rectal cancer, EID achieved R0 resection in 81% and curative resection in 45%, with no major adverse events[63].
Through modification of existing EMR/ESD techniques, endoscopic submucosal resection and snare-based full thickness resection have also been used for deeply invasive T1 CRC which are unresectable with conventional techniques[64,65].
Combined endoscopic laparoscopic surgery allow for less invasive and precise full-thickness resection of early colon cancers that are too large for endoscopic resection[66]. Colonoscopy-assisted laparoscopic wedge resection (CAL-WR) has been shown to have R0 resection rate of approximately 90% and adverse rate of 2% for early colon cancer. Further, there were no reported adverse events due to CAL-WR in subsequent completion surgery, which occurred in 29% and were largely due to ≥ T2 colon cancer[67]. CAL-WR has similar efficacy and safety as a secondary technique following incomplete endoscopic resection of T1 colon cancer[68] (Table 5).
| Emerging technique | Description | Application in T1 CRC | Outcomes and evidence |
| Endoscopic submucosal dissection for suspected focal deep submucosal invasion | En-bloc endoscopic resection for lesions with optical evaluation suggesting focal deep submucosal invasion | For patients preferring or only eligible for conservative management, who would otherwise be referred to first-line surgery | Retrospective study of colorectal neoplasia with focal deep invasion found R0 resection of 77% and curative resection in 27%[62] |
| Endoscopic intermuscular dissection | Dissection between inner (circular) and outer (longitudinal) muscularis propria | For rectal cancers, particularly with a concern for deep submucosal invasion | Prospective cohort study of T1 rectal cancer demonstrated technical success of 96%, R0 resection of 81%, and curative resection of 45%[63] |
| Colonoscopy-assisted laparoscopic wedge resection | Laparoscopic resection and closure of colonic lesions under direct intraluminal endoscopic guidance | For colon cancers, particularly with deep submucosal invasion | Case series of patients with high grade dysplasia or T1 colon cancer demonstrated R0 resection of 89%[67] |
| Neoadjuvant and adjuvant chemoradiation1 | Use of chemoradiation or chemotherapy alone before or after resection to increase efficacy of local excision1 | For downstaging early rectal cancer or for prevention of recurrence following local excision of high risk T1 CRC | NEO trial (phase II) of early rectal cancer showed 57% downstaging, 79% organ preservation, and 90% 2-yr local regional relapse free survival[73]. Systematic review subgroup analysis of T1 CRC treated with adjuvant chemoradiation showed local recurrence rate of 3.9%[75] |
Study of long-term oncologic outcomes and cost-effectiveness have yet to be reported for these novel techniques; however, if the need for surgery can be reduced similar to that of large non-pedunculated colorectal polyps, there is potential for significant improvement in patient outcomes and resource utilization.
Chemoradiotherapy has been used in the neoadjuvant setting to downstage early-stage rectal cancer for local excision, largely TES. Multiple trials have found that the rate of downstaging to T0-1 from T2-3 with neoadjuvant chemoradiotherapy is approximately 50%-65%[69-72]. Following neoadjuvant chemoradiotherapy, there was no significant difference in 5 year recurrence or cancer-related survival between subsequent TES and TME[69,70]. As pre-operative radiation can increase surgical adverse events[72], neoadjuvant chemotherapy alone prior to TES was recently studied in the phase II NEO trial and has shown 57% downstaging, 79% organ preservation rate, and 90% 2-year locoregional relapse free survival[73]. While the watch-and-wait approach for those with complete clinical response following neoadjuvant therapy can enable patients to avoid radical surgery[74], use of local excision maintains organ preservation while addressing local disease, evidenced by the 23% of patients with microscopic residual disease on TES specimens who may appear to have complete clinical response[73].
Adjuvant chemoradiotherapy has also shown efficacy following local excision for high risk T1 rectal cancers. A systematic review examined a high risk T1 CRC subgroup initially treated with local excision, and found a local recurrence rate of 4.1% with secondary TME and 3.9% with adjuvant chemoradiotherapy[75]. The decision for adjuvant chemoradiation is best determined by multidisciplinary review, taking into account patient preferences and candidacy for surgery or chemoradiotherapy.
While completion surgery is still recommended following non-curative T1 CRC resection, in patients at high risk for surgery, a conservative approach may be justified. A single center retrospective cohort of 180 patients with non-curative ESD who underwent additional surgery or endoscopic surveillance alone had no significant difference in 5-year overall survival, disease-free survival, cancer-specific survival or cumulative recurrence[76]. In a multicenter cohort of 207 patients with non-curative ESD, conservative management was associated with a recurrence rate of 1.2% and no disease-specific deaths at a median follow up of 28 months, but had higher overall mortality compared to secondary surgery due to non-CRC causes[77]. This finding was reflected in a meta-analysis of 2961 patients with high risk T1 CRC treated by local resection or surgery. Disease-specific survival was similar at 5 years and net benefit of surgery became significant only after 10 years post-resection[78]. Overall survival in non-curative, non-operative patients is likely driven by comorbidities and advanced age, thus limiting long term benefit of further surgical intervention. Conservative management may be a preferable strategy in patients with limited life expectancy, and at high risk of morbidity and mortality from radical surgery.
Minimally invasive endoscopic resection techniques provide safe, and effective strategies for T1 CRC. Advances in resection techniques and treatment strategies offer further options for effective local excision, particularly for those of increased age and comorbid populations, in whom surgery is not preferred. With ongoing refinement, endoscopic management will continue to transform early CRC management.
| 1. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1575] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 2. | Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more "personalized" approach to cancer staging. CA Cancer J Clin. 2017;67:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2341] [Cited by in RCA: 4668] [Article Influence: 518.7] [Reference Citation Analysis (4)] |
| 3. | Rex DK, Hassan C, Bourke MJ. The colonoscopist's guide to the vocabulary of colorectal neoplasia: histology, morphology, and management. Gastrointest Endosc. 2017;86:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Chang LC, Shun CT, Lin BR, Sanduleanu S, Hsu WF, Wu MS, Chiu HM. Recurrence Outcomes Less Favorable in T1 Rectal Cancer than in T1 Colon Cancer. Oncologist. 2021;26:e1548-e1554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Vermeer NCA, Backes Y, Snijders HS, Bastiaannet E, Liefers GJ, Moons LMG, van de Velde CJH, Peeters KCMJ; Dutch T1 Colorectal Cancer Working Group. National cohort study on postoperative risks after surgery for submucosal invasive colorectal cancer. BJS Open. 2019;3:210-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 67] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 6. | Chowdhury SR, Chandra Das D, Sunna TC, Beyene J, Hossain A. Global and regional prevalence of multimorbidity in the adult population in community settings: a systematic review and meta-analysis. EClinicalMedicine. 2023;57:101860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 418] [Reference Citation Analysis (0)] |
| 7. | van de Ven SEM, Backes Y, Hilbink M, Seerden TCJ, Kessels K, de Vos Tot Nederveen Cappel WH, Groen JN, Wolfhagen FHJ, Geesing JMJ, Borg FT, van Bergeijk J, Spanier BWM, Mundt MW, Pullens HJM, Boonstra JJ, Opsteeg B, van Lent AUG, Schrauwen RWM, Laclé MM, Moons LMG, Terhaar Sive Droste JS; Dutch T1 CRC Working Group. Periprocedural adverse events after endoscopic resection of T1 colorectal carcinomas. Gastrointest Endosc. 2020;91:142-152.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Yeh JH, Tseng CH, Huang RY, Lin CW, Lee CT, Hsiao PJ, Wu TC, Kuo LT, Wang WL. Long-term Outcomes of Primary Endoscopic Resection vs Surgery for T1 Colorectal Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2020;18:2813-2823.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (1)] |
| 9. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;159:1916-1934.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (2)] |
| 10. | Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kinugasa Y, Murofushi K, Nakajima TE, Oka S, Tanaka T, Taniguchi H, Tsuji A, Uehara K, Ueno H, Yamanaka T, Yamazaki K, Yoshida M, Yoshino T, Itabashi M, Sakamaki K, Sano K, Shimada Y, Tanaka S, Uetake H, Yamaguchi S, Yamaguchi N, Kobayashi H, Matsuda K, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1024] [Cited by in RCA: 1424] [Article Influence: 237.3] [Reference Citation Analysis (3)] |
| 11. | Hassan C, Wysocki PT, Fuccio L, Seufferlein T, Dinis-Ribeiro M, Brandão C, Regula J, Frazzoni L, Pellise M, Alfieri S, Dekker E, Jover R, Rosati G, Senore C, Spada C, Gralnek I, Dumonceau JM, van Hooft JE, van Cutsem E, Ponchon T. Endoscopic surveillance after surgical or endoscopic resection for colorectal cancer: European Society of Gastrointestinal Endoscopy (ESGE) and European Society of Digestive Oncology (ESDO) Guideline. Endoscopy. 2019;51:266-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 12. | Dang H, Dekkers N, le Cessie S, van Hooft JE, van Leerdam ME, Oldenburg PP, Flothuis L, Schoones JW, Langers AMJ, Hardwick JCH, van der Kraan J, Boonstra JJ. Risk and Time Pattern of Recurrences After Local Endoscopic Resection of T1 Colorectal Cancer: A Meta-analysis. Clin Gastroenterol Hepatol. 2022;20:e298-e314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 13. | Bosch SL, Teerenstra S, de Wilt JH, Cunningham C, Nagtegaal ID. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy. 2013;45:827-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 311] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 14. | Choi JY, Jung SA, Shim KN, Cho WY, Keum B, Byeon JS, Huh KC, Jang BI, Chang DK, Jung HY, Kong KA; Korean ESD Study Group. Meta-analysis of predictive clinicopathologic factors for lymph node metastasis in patients with early colorectal carcinoma. J Korean Med Sci. 2015;30:398-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Nakadoi K, Tanaka S, Kanao H, Terasaki M, Takata S, Oka S, Yoshida S, Arihiro K, Chayama K. Management of T1 colorectal carcinoma with special reference to criteria for curative endoscopic resection. J Gastroenterol Hepatol. 2012;27:1057-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 158] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 16. | Zwager LW, Bastiaansen BAJ, Montazeri NSM, Hompes R, Barresi V, Ichimasa K, Kawachi H, Machado I, Masaki T, Sheng W, Tanaka S, Togashi K, Yasue C, Fockens P, Moons LMG, Dekker E. Deep Submucosal Invasion Is Not an Independent Risk Factor for Lymph Node Metastasis in T1 Colorectal Cancer: A Meta-Analysis. Gastroenterology. 2022;163:174-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 17. | Brockmoeller S, Toh EW, Kouvidi K, Hepworth S, Morris E, Quirke P. Improving the management of early colorectal cancers (eCRC) by using quantitative markers to predict lymph node involvement and thus the need for major resection of pT1 cancers. J Clin Pathol. 2022;75:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Gijsbers KM, van der Schee L, van Veen T, van Berkel AM, Boersma F, Bronkhorst CM, Didden PD, Haasnoot KJC, Jonker AM, Kessels K, Knijn N, van Lijnschoten I, Mijnals C, Milne AN, Moll FCP, Schrauwen RWM, Schreuder RM, Seerden TJ, Spanier MBWM, Terhaar Sive Droste JS, Witteveen E, de Vos Tot Nederveen Cappel WH, Vleggaar FP, Laclé MM, Ter Borg F, Moons LMG; Dutch T1 CRC Working Group. Impact of ≥ 0.1-mm free resection margins on local intramural residual cancer after local excision of T1 colorectal cancer. Endosc Int Open. 2022;10:E282-E290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Kobayashi H, Higuchi T, Uetake H, Iida S, Ishikawa T, Ishiguro M, Sugihara K. Resection with en bloc removal of regional lymph node after endoscopic resection for T1 colorectal cancer. Ann Surg Oncol. 2012;19:4161-4167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Backes Y, de Vos Tot Nederveen Cappel WH, van Bergeijk J, Ter Borg F, Schwartz MP, Spanier BWM, Geesing JMJ, Kessels K, Kerkhof M, Groen JN, Wolfhagen FHJ, Seerden TCJ, van Lelyveld N, Offerhaus GJA, Siersema PD, Lacle MM, Moons LMG. Risk for Incomplete Resection after Macroscopic Radical Endoscopic Resection of T1 Colorectal Cancer: A Multicenter Cohort Study. Am J Gastroenterol. 2017;112:785-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Boenicke L, Fein M, Sailer M, Isbert C, Germer CT, Thalheimer A. The concurrence of histologically positive resection margins and sessile morphology is an important risk factor for lymph node metastasis after complete endoscopic removal of malignant colorectal polyps. Int J Colorectal Dis. 2010;25:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Hamilton SR, Aaltonen LA. Pathology and Genetics of Tumours of the Digestive System. 3rd. Lyon: IARC Press, 2000. |
| 23. | Lugli A, Kirsch R, Ajioka Y, Bosman F, Cathomas G, Dawson H, El Zimaity H, Fléjou JF, Hansen TP, Hartmann A, Kakar S, Langner C, Nagtegaal I, Puppa G, Riddell R, Ristimäki A, Sheahan K, Smyrk T, Sugihara K, Terris B, Ueno H, Vieth M, Zlobec I, Quirke P. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30:1299-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 575] [Cited by in RCA: 789] [Article Influence: 87.7] [Reference Citation Analysis (0)] |
| 24. | Nishimura T, Oka S, Tanaka S, Asayama N, Nagata S, Tamaru Y, Kuwai T, Yamashita K, Ninomiya Y, Kitadai Y, Arihiro K, Kuraoka K, Kaneko M, Shimamoto F, Chayama K. Clinical significance of immunohistochemical lymphovascular evaluation to determine additional surgery after endoscopic submucosal dissection for colorectal T1 carcinoma. Int J Colorectal Dis. 2021;36:949-958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Fujino S, Miyoshi N, Kitakaze M, Yasui M, Ohue M, Osawa H, Ide Y, Sueda T, Tei M, Takeda T, Danno K, Suzuki Y, Noura S, Ohshima K, Morii E, Takahashi H, Uemura M, Yamamoto H, Murata K, Doki Y, Eguchi H; Clinical Study Group of Osaka University, Colorectal Cancer Treatment Group (CSGOCG). Lymph node metastasis in T1 colorectal cancer: Risk factors and prediction model. Oncol Lett. 2023;25:191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 26. | Kudo SE, Ichimasa K, Villard B, Mori Y, Misawa M, Saito S, Hotta K, Saito Y, Matsuda T, Yamada K, Mitani T, Ohtsuka K, Chino A, Ide D, Imai K, Kishida Y, Nakamura K, Saiki Y, Tanaka M, Hoteya S, Yamashita S, Kinugasa Y, Fukuda M, Kudo T, Miyachi H, Ishida F, Itoh H, Oda M, Mori K. Artificial Intelligence System to Determine Risk of T1 Colorectal Cancer Metastasis to Lymph Node. Gastroenterology. 2021;160:1075-1084.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 27. | Shahidi N, Vosko S, van Hattem WA, Sidhu M, Bourke MJ. Optical evaluation: the crux for effective management of colorectal neoplasia. Therap Adv Gastroenterol. 2020;13:1756284820922746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Kudo Se, Lambert R, Allen JI, Fujii H, Fujii T, Kashida H, Matsuda T, Mori M, Saito H, Shimoda T, Tanaka S, Watanabe H, Sung JJ, Feld AD, Inadomi JM, O'Brien MJ, Lieberman DA, Ransohoff DF, Soetikno RM, Triadafilopoulos G, Zauber A, Teixeira CR, Rey JF, Jaramillo E, Rubio CA, Van Gossum A, Jung M, Vieth M, Jass JR, Hurlstone PD. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68:S3-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 372] [Article Influence: 20.7] [Reference Citation Analysis (1)] |
| 29. | Burgess NG, Pellise M, Nanda KS, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, P'Ng H, McLeod D, Bourke MJ. Clinical and endoscopic predictors of cytological dysplasia or cancer in a prospective multicentre study of large sessile serrated adenomas/polyps. Gut. 2016;65:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 30. | Burgess NG, Hourigan LF, Zanati SA, Brown GJ, Singh R, Williams SJ, Raftopoulos SC, Ormonde D, Moss A, Byth K, Mahajan H, McLeod D, Bourke MJ. Risk Stratification for Covert Invasive Cancer Among Patients Referred for Colonic Endoscopic Mucosal Resection: A Large Multicenter Cohort. Gastroenterology. 2017;153:732-742.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 175] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 31. | Puig I, López-Cerón M, Arnau A, Rosiñol Ò, Cuatrecasas M, Herreros-de-Tejada A, Ferrández Á, Serra-Burriel M, Nogales Ó, Vida F, de Castro L, López-Vicente J, Vega P, Álvarez-González MA, González-Santiago J, Hernández-Conde M, Díez-Redondo P, Rivero-Sánchez L, Gimeno-García AZ, Burgos A, García-Alonso FJ, Bustamante-Balén M, Martínez-Bauer E, Peñas B, Pellise M; EndoCAR group, Spanish Gastroenterological Association and the Spanish Digestive Endoscopy Society. Accuracy of the Narrow-Band Imaging International Colorectal Endoscopic Classification System in Identification of Deep Invasion in Colorectal Polyps. Gastroenterology. 2019;156:75-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 32. | Sumimoto K, Tanaka S, Shigita K, Hirano D, Tamaru Y, Ninomiya Y, Asayama N, Hayashi N, Oka S, Arihiro K, Yoshihara M, Chayama K. Clinical impact and characteristics of the narrow-band imaging magnifying endoscopic classification of colorectal tumors proposed by the Japan NBI Expert Team. Gastrointest Endosc. 2017;85:816-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 114] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 33. | Moss A, Bourke MJ, Williams SJ, Hourigan LF, Brown G, Tam W, Singh R, Zanati S, Chen RY, Byth K. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology. 2011;140:1909-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 479] [Cited by in RCA: 449] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 34. | Kobayashi N, Saito Y, Sano Y, Uragami N, Michita T, Nasu J, Matsuda T, Fu KI, Fujii T, Fujimori T, Ishikawa T, Saito D. Determining the treatment strategy for colorectal neoplastic lesions: endoscopic assessment or the non-lifting sign for diagnosing invasion depth? Endoscopy. 2007;39:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 35. | Backes Y, Moss A, Reitsma JB, Siersema PD, Moons LM. Narrow Band Imaging, Magnifying Chromoendoscopy, and Gross Morphological Features for the Optical Diagnosis of T1 Colorectal Cancer and Deep Submucosal Invasion: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Backes Y, Schwartz MP, Ter Borg F, Wolfhagen FHJ, Groen JN, de Vos Tot Nederveen Cappel WH, van Bergeijk J, Geesing JMJ, Spanier BWM, Didden P, Vleggaar FP, Lacle MM, Elias SG, Moons LMG; Dutch T1 CRC Working Group. Multicentre prospective evaluation of real-time optical diagnosis of T1 colorectal cancer in large non-pedunculated colorectal polyps using narrow band imaging (the OPTICAL study). Gut. 2019;68:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 37. | Yasue C, Chino A, Takamatsu M, Namikawa K, Ide D, Saito S, Igarashi M, Fujisaki J. Pathological risk factors and predictive endoscopic factors for lymph node metastasis of T1 colorectal cancer: a single-center study of 846 lesions. J Gastroenterol. 2019;54:708-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 90] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 38. | Shahidi N, Bourke MJ. How to Manage the Large Nonpedunculated Colorectal Polyp. Gastroenterology. 2021;160:2239-2243.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Fujiya M, Tanaka K, Dokoshi T, Tominaga M, Ueno N, Inaba Y, Ito T, Moriichi K, Kohgo Y. Efficacy and adverse events of EMR and endoscopic submucosal dissection for the treatment of colon neoplasms: a meta-analysis of studies comparing EMR and endoscopic submucosal dissection. Gastrointest Endosc. 2015;81:583-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 279] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 40. | Lim XC, Nistala KRY, Ng CH, Lin SY, Tan DJH, Ho KY, Chong CS, Muthiah M. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal polyps: A meta-analysis and meta-regression with single arm analysis. World J Gastroenterol. 2021;27:3925-3939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 41. | De Ceglie A, Hassan C, Mangiavillano B, Matsuda T, Saito Y, Ridola L, Bhandari P, Boeri F, Conio M. Endoscopic mucosal resection and endoscopic submucosal dissection for colorectal lesions: A systematic review. Crit Rev Oncol Hematol. 2016;104:138-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 135] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 42. | Park JJ, Cheon JH, Kwon JE, Shin JK, Jeon SM, Bok HJ, Lee JH, Moon CM, Hong SP, Kim TI, Kim H, Kim WH. Clinical outcomes and factors related to resectability and curability of EMR for early colorectal cancer. Gastrointest Endosc. 2011;74:1337-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 43. | Watanabe D, Toyonaga T, Ooi M, Yoshizaki T, Ohara Y, Tanaka S, Kawara F, Ishida T, Morita Y, Umegaki E, Matsuda T, Sumi Y, Nishio M, Yokozaki H, Azuma T. Clinical outcomes of deep invasive submucosal colorectal cancer after ESD. Surg Endosc. 2018;32:2123-2130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Shahidi N, Vosko S, Gupta S, Whitfield A, Cronin O, O'Sullivan T, van Hattem WA, Sidhu M, Tate DJ, Lee EYT, Burgess N, Williams SJ, Bourke MJ. A Rectum-Specific Selective Resection Algorithm Optimizes Oncologic Outcomes for Large Nonpedunculated Rectal Polyps. Clin Gastroenterol Hepatol. 2023;21:72-80.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 45. | Schmidt A, Bauerfeind P, Gubler C, Damm M, Bauder M, Caca K. Endoscopic full-thickness resection in the colorectum with a novel over-the-scope device: first experience. Endoscopy. 2015;47:719-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 148] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 46. | Zwager LW, Bastiaansen BAJ, van der Spek BW, Heine DN, Schreuder RM, Perk LE, Weusten BLAM, Boonstra JJ, van der Sluis H, Wolters HJ, Bekkering FC, Rietdijk ST, Schwartz MP, Nagengast WB, Ten Hove WR, Terhaar Sive Droste JS, Rando Munoz FJ, Vlug MS, Beaumont H, Houben MHMG, Seerden TCJ, de Wijkerslooth TR, Gielisse EAR, Hazewinkel Y, de Ridder R, Straathof JA, van der Vlugt M, Koens L, Fockens P, Dekker E; Dutch eFTR Group. Endoscopic full-thickness resection of T1 colorectal cancers: a retrospective analysis from a multicenter Dutch eFTR registry. Endoscopy. 2022;54:475-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 47. | Kuellmer A, Mueller J, Caca K, Aepli P, Albers D, Schumacher B, Glitsch A, Schäfer C, Wallstabe I, Hofmann C, Erhardt A, Meier B, Bettinger D, Thimme R, Schmidt A; FTRD study group. Endoscopic full-thickness resection for early colorectal cancer. Gastrointest Endosc. 2019;89:1180-1189.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 48. | Dolan RD, Bazarbashi AN, McCarty TR, Thompson CC, Aihara H. Endoscopic full-thickness resection of colorectal lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2022;95:216-224.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 49. | Meier B, Caca K, Schmidt A. Hybrid endoscopic mucosal resection and full-thickness resection: a new approach for resection of large non-lifting colorectal adenomas (with video). Surg Endosc. 2017;31:4268-4274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 50. | Meier B, Stritzke B, Kuellmer A, Zervoulakos P, Huebner GH, Repp M, Walter B, Meining A, Gutberlet K, Wiedbrauck T, Glitsch A, Lorenz A, Caca K, Schmidt A. Efficacy and Safety of Endoscopic Full-Thickness Resection in the Colorectum: Results From the German Colonic FTRD Registry. Am J Gastroenterol. 2020;115:1998-2006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 51. | Brown CJ, Raval MJ. Advances in minimally invasive surgery in the treatment of colorectal cancer. Expert Rev Anticancer Ther. 2008;8:111-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 52. | Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24:2200-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 402] [Article Influence: 25.1] [Reference Citation Analysis (2)] |
| 53. | Lee L, Edwards K, Hunter IA, Hartley JE, Atallah SB, Albert MR, Hill J, Monson JR. Quality of Local Excision for Rectal Neoplasms Using Transanal Endoscopic Microsurgery Versus Transanal Minimally Invasive Surgery: A Multi-institutional Matched Analysis. Dis Colon Rectum. 2017;60:928-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 54. | Stipa F, Tierno SM, Russo G, Burza A. Trans-anal minimally invasive surgery (TAMIS) versus trans-anal endoscopic microsurgery (TEM): a comparative case-control matched-pairs analysis. Surg Endosc. 2022;36:2081-2086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 55. | McCarty TR, Bazarbashi AN, Hathorn KE, Thompson CC, Aihara H. Endoscopic submucosal dissection (ESD) versus transanal endoscopic microsurgery (TEM) for treatment of rectal tumors: a comparative systematic review and meta-analysis. Surg Endosc. 2020;34:1688-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (2)] |
| 56. | Arezzo A, Passera R, Saito Y, Sakamoto T, Kobayashi N, Sakamoto N, Yoshida N, Naito Y, Fujishiro M, Niimi K, Ohya T, Ohata K, Okamura S, Iizuka S, Takeuchi Y, Uedo N, Fusaroli P, Bonino MA, Verra M, Morino M. Systematic review and meta-analysis of endoscopic submucosal dissection versus transanal endoscopic microsurgery for large noninvasive rectal lesions. Surg Endosc. 2014;28:427-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 57. | Dekkers N, Dang H, van der Kraan J, le Cessie S, Oldenburg PP, Schoones JW, Langers AMJ, van Leerdam ME, van Hooft JE, Backes Y, Levic K, Meining A, Saracco GM, Holman FA, Peeters KCMJ, Moons LMG, Doornebosch PG, Hardwick JCH, Boonstra JJ. Risk of recurrence after local resection of T1 rectal cancer: a meta-analysis with meta-regression. Surg Endosc. 2022;36:9156-9168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 58. | Wang AY, Hwang JH, Bhatt A, Draganov PV. AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary. Gastroenterology. 2021;161:2030-2040.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 59. | Pioche M, Walter T. Endoscopic removal of colorectal T1 cancers: Why is a 1-year follow-up recommended by ESGE when resection is R0 and curative? Endosc Int Open. 2019;7:E816-E817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 60. | Patenotte A, Yzet C, Wallenhorst T, Subtil F, Leblanc S, Schaefer M, Walter T, Lambin T, Fenouil T, Lafeuille P, Chevaux JB, Legros R, Rostain F, Rivory J, Jacques J, Lépilliez V, Pioche M. Diagnostic endoscopic submucosal dissection for colorectal lesions with suspected deep invasion. Endoscopy. 2023;55:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 61. | Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T, Takao M, Shinohara T, Murakami Y, Fujimori T, Kaneko K, Saito Y. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551-9; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 207] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 62. | Huisman JF, Dang H, Moons LMG, Backes Y, Dik VK, Groen JN, Ter Borg F, van Bergeijk JD, Geesing JMJ, Spanier BWM, Terhaar Sive Droste JS, Overwater A, van Lelyveld N, Kessels K, Lacle MM, Offerhaus GJA, Brohet RM, Knijn N, Vleggaar FP, van Westreenen HL, de Vos Tot Nederveen Cappel WH, Boonstra JJ; Dutch T1 CRC working group. Diagnostic value of radiological staging and surveillance for T1 colorectal carcinomas: A multicenter cohort study. United European Gastroenterol J. 2023;11:551-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 63. | Moons LMG, Bastiaansen BAJ, Richir MC, Hazen WL, Tuynman J, Elias SG, Schrauwen RWM, Vleggaar FP, Dekker E, Bos P, Fariña Sarasqueta A, Lacle M, Hompes R, Didden P. Endoscopic intermuscular dissection for deep submucosal invasive cancer in the rectum: a new endoscopic approach. Endoscopy. 2022;54:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (1)] |
| 64. | Metter K, Aepli P, Dumoulin FL, Hayee B, Grund KE, Farin G, Frei R. Endoscopic submucosal resection: a technique using novel devices for incision and resection of neoplastic lesions. Endoscopy. 2022;54:1001-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (1)] |
| 65. | Schoeman S, Shahidi N, Bourke MJ. Snare-based full-thickness endoscopic resection for deeply invasive colorectal neoplasia. Gastrointest Endosc. 2020;92:731-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 66. | Noren ER, Wickham C, Lee SW. Current techniques for combined endoscopic and laparoscopic surgery (CELS). Ann Laparosc Endosc Surg. 2019;4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 67. | Hanevelt J, Moons LMG, Hentzen JEKR, Wemeijer TM, Huisman JF, de Vos Tot Nederveen Cappel WH, van Westreenen HL. Colonoscopy-Assisted Laparoscopic Wedge Resection for the Treatment of Suspected T1 Colon Cancer. Ann Surg Oncol. 2023;30:2058-2065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 68. | Leicher LW, Huisman JF, van Grevenstein WMU, Didden P, Backes Y, Offerhaus GJA, Laclé MM, Moll FCP, Geesing JMJ, Smakman N, Droste JSTS, Verdaasdonk EGG, Ter Borg F, Talsma AK, Erkelens GW, van der Zaag ES, Schrauwen RW, van Wely BJ, Schot I, Vermaas M, van Bergeijk JD, Sietses C, Hazen WL, Wasowicz DK, Ramsoekh D, Tuynman JB, Alderlieste YA, Renger RJ, Oort FA, Bilgen EJS, Vleggaar FP, Vasen HFA, de Vos Tot Nederveen Cappel WH, Moons LMG, van Westreenen HL. Colonoscopic-Assisted Laparoscopic Wedge Resection for Colonic Lesions: A Prospective Multicenter Cohort Study (LIMERIC-Study). Ann Surg. 2022;275:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 69. | Lezoche E, Baldarelli M, Lezoche G, Paganini AM, Gesuita R, Guerrieri M. Randomized clinical trial of endoluminal locoregional resection versus laparoscopic total mesorectal excision for T2 rectal cancer after neoadjuvant therapy. Br J Surg. 2012;99:1211-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 235] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 70. | Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, Faucheron JL, Jafari M, Portier G, Meunier B, Sileznieff I, Prudhomme M, Marchal F, Pocard M, Pezet D, Rullier A, Vendrely V, Denost Q, Asselineau J, Doussau A. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 268] [Article Influence: 29.8] [Reference Citation Analysis (1)] |
| 71. | Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens JJME, van Meerten E, Tanis PJ, de Hingh IHJT, van der Schelling GP, Acherman Y, Leijtens JWA, Bremers AJA, Beets GL, Hoff C, Verhoef C, Marijnen CAM, de Wilt JHW; CARTS Study Group. Long-term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 2019;154:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 72. | Garcia-Aguilar J, Shi Q, Thomas CR Jr, Chan E, Cataldo P, Marcet J, Medich D, Pigazzi A, Oommen S, Posner MC. A phase II trial of neoadjuvant chemoradiation and local excision for T2N0 rectal cancer: preliminary results of the ACOSOG Z6041 trial. Ann Surg Oncol. 2012;19:384-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 217] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 73. | Kennecke HF, O'Callaghan CJ, Loree JM, Moloo H, Auer R, Jonker DJ, Raval M, Musselman R, Ma G, Caycedo-Marulanda A, Simianu VV, Patel S, Pitre LD, Helewa R, Gordon VL, Neumann K, Nimeiri H, Sherry M, Tu D, Brown CJ. Neoadjuvant Chemotherapy, Excision, and Observation for Early Rectal Cancer: The Phase II NEO Trial (CCTG CO.28) Primary End Point Results. J Clin Oncol. 2023;41:233-242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 74. | Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 436] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 75. | van Oostendorp SE, Smits LJH, Vroom Y, Detering R, Heymans MW, Moons LMG, Tanis PJ, de Graaf EJR, Cunningham C, Denost Q, Kusters M, Tuynman JB. Local recurrence after local excision of early rectal cancer: a meta-analysis of completion TME, adjuvant (chemo)radiation, or no additional treatment. Br J Surg. 2020;107:1719-1730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 76. | Li J, Huang F, Cheng P, Zhang M, Lu Z, Zheng Z. Patient outcomes after non-curative endoscopic submucosal dissection for early colorectal cancer: a single-center, retrospective cohort study. Transl Cancer Res. 2021;10:5123-5132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 77. | Spadaccini M, Bourke MJ, Maselli R, Pioche M, Bhandari P, Jacques J, Haji A, Yang D, Albéniz E, Kaminski MF, Messmann H, Herreros de Tejada A, Sferrazza S, Pekarek B, Rivory J, Geyl S, Gulati S, Draganov P, Shahidi N, Hossain E, Fleischmann C, Vespa E, Iannone A, Alkandari A, Hassan C, Repici A; ESD Western Alliance (EWA). Clinical outcome of non-curative endoscopic submucosal dissection for early colorectal cancer. Gut. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 78. | Chen Y, Jing W, Chen M, Wang Z, Wu J, Yang J, Yang L, Deng K. Long-term outcomes of local resection versus surgical resection for high-risk T1 colorectal cancer: a systematic review and meta-analysis. Gastrointest Endosc. 2023;97:1016-1030.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country of origin: Canada
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade C
Scientific Significance: Grade C
P-Reviewer: Li SC, United States S-Editor: Lin C L-Editor: A P-Editor: Li X