Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1384
Peer-review started: October 16, 2023
First decision: December 19, 2023
Revised: December 24, 2023
Accepted: February 4, 2024
Article in press: February 4, 2024
Published online: April 15, 2024
Processing time: 177 Days and 11.7 Hours
Duodenal cancer is one of the most common subtypes of small intestinal cancer, and distant metastasis (DM) in this type of cancer still leads to poor prognosis. Although nomograms have recently been used in tumor areas, no studies have focused on the diagnostic and prognostic evaluation of DM in patients with primary duodenal cancer.
To develop and evaluate nomograms for predicting the risk of DM and personalized prognosis in patients with duodenal cancer.
Data on duodenal cancer patients diagnosed between 2010 and 2019 were extracted from the Surveillance, Epidemiology, and End Results database. Univariate and multivariate logistic regression analyses were used to identify independent risk factors for DM in patients with duodenal cancer, and univariate and multivariate Cox proportional hazards regression analyses were used to determine independent prognostic factors in duodenal cancer patients with DM. Two novel nomograms were established, and the results were evaluated by receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA).
A total of 2603 patients with duodenal cancer were included, of whom 457 cases (17.56%) had DM at the time of diagnosis. Logistic analysis revealed independent risk factors for DM in duodenal cancer patients, including gender, grade, tumor size, T stage, and N stage (P < 0.05). Univariate and multivariate COX analyses further identified independent prognostic factors for duodenal cancer patients with DM, including age, histological type, T stage, tumor grade, tumor size, bone metastasis, chemotherapy, and surgery (P < 0.05). The accuracy of the nomograms was validated in the training set, validation set, and expanded testing set using ROC curves, calibration curves, and DCA curves. The results of Kaplan-Meier survival curves (P < 0.001) indicated that both nomograms accurately predicted the occurrence and prognosis of DM in patients with duodenal cancer.
The two nomograms are expected as effective tools for predicting DM risk in duodenal cancer patients and offering personalized prognosis predictions for those with DM, potentially enhancing clinical decision-making.
Core Tip: Developed and evaluated were two new nomograms for predicting the risk of distant metastasis (DM) and providing personalized prognosis for patients with primary duodenal cancer. The study involved a total of 2603 duodenal cancer patients, among whom 457 (17.56%) had DM at the time of diagnosis. Independent risk factors for DM in duodenal cancer patients were identified. Additionally, independent prognostic factors for duodenal cancer patients with DM were determined. The results indicated that the nomograms accurately predicted the occurrence and prognosis of DM in duodenal cancer patients.
- Citation: Shang JR, Xu CY, Zhai XX, Xu Z, Qian J. Risk factors, prognostic factors, and nomograms for distant metastasis in patients with diagnosed duodenal cancer: A population-based study. World J Gastrointest Oncol 2024; 16(4): 1384-1420
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1384.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1384
Duodenal cancer, classified as a rare malignancy within gastrointestinal tumors, is recognized as a distinct clinicopathological subtype of small intestinal cancer[1]. The incidence rate of duodenal cancer is low, with a rate lower than 0.5 per 100000, it accounting for approximately 0.3% to 1.0% of all gastrointestinal malignancies[2]. However, among malignant tumors of the small intestine, duodenal cancer represents a significant portion, accounting for 25% to 35% of cases, making it one of the high-incidence malignancies of the digestive tract[3]. The incidence of malignant tumors in the small intestine has been steadily increasing, as reported by Yao et al[4]. From 1976 to 2016, the incidence has risen by 130%, accompanied by a relative increase in the mortality rate by 26%[5]. The duodenum, the most proximal portion of the small intestine, is frequently involved in duodenal cancer cases[6]. The number of patients with duodenal cancer has been progressively rising[7-9], highlighting the importance of research in this area. Most patients with duodenal cancer remain asymptomatic until the disease reaches an advanced stage. Additionally, the difficulty in identifying duodenal cancer through imaging examinations often leads to delays in diagnosis and subsequent poor prognosis[10,11]. Thus, there is a need for an enhanced understanding and improved diagnostic methods for this disease.
In patients with duodenal cancer who develop metastasis, The most common site of metastasis is the liver, occurring in 37.5% of cases. This was followed by lymph node metastasis at a rate of 12.5%) and lung metastasis occurring in 9.4% of cases. Overall, the metastasis rate in patients with duodenal cancer is 48.5%[12]. Notably, patients with duodenal cancer who experience distant metastasis (DM) often present with multiple lesions[13]. Hence, it is crucial to develop precise models for assessing the risk of DM in patients with duodenal cancer and evaluating their prognosis. Currently, there is a scarcity of studies offering dependable data regarding the association between clinicopathological characteristics and metastatic patterns in duodenal cancer. Furthermore, there is a lack of established predictive models for determining the likelihood of DM in duodenal cancer or for predicting the prognosis of patients with duodenal cancer and DM[14].
Recently, nomograms have gained widespread use for evaluating the prognosis of cancer patients because of their convenience and precision, making them an ideal choice for our purpose[15,16]. Considering this, we conducted a study using a representative cohort from the Surveillance, Epidemiology, and End Results (SEER) database to assess the incidence, risk factors, and prognosis of DM in patients diagnosed with duodenal cancer and developed two nomograms: One for predicting the likelihood of DM in patients with duodenal cancer and another for predicting the overall survival (OS) of duodenal cancer patients with DM.
The current data for this study on duodenal cancer were extracted from three SEER registry systems, SEER 8, SEER 12, and SEER 17, covering the period from 2010 to 2019. Only data from the period after 2010 were retrieved because the SEER database did not provide information on the site of DM. The inclusion criteria were as follows: (1) Cancer patients with a primary site in the duodenum retrieved using the topographical codes from the International Classification of Diseases for Oncology (ICD-O-3: C17.0); and (2) clinical pathological information, including primary tumor site, grade, histological type, tumor-node-metastasis, and tumor size. In addition, all patients without microscopic confirmation of duodenal cancer diagnosis were excluded. A total of 2603 patients diagnosed with duodenal cancer were included in the present study, of which 457 had DM. Finally, 2603 patients diagnosed with duodenal cancer, including 457 with DM, were included in the present study. All patients were used to form a diagnostic cohort to explore the risk factors for DM and develop a predictive nomogram. Additionally, out of 457 duodenal cancer patients with DM, 412 patients with a survival time of one month or more and available specific treatment information such as surgery, chemotherapy, and radiotherapy were used to form a prognostic cohort to study the prognostic factors for patients with DM and develop a novel prognostic nomogram.
In the diagnostic cohort, the patients were randomly divided into two sets: A training set consisting of 70% of the patients, and a validation set consisting of the remaining 30% of patients. Similarly, for the prognostic cohort, the training and validation sets were derived from corresponding patients with DM from the diagnostic cohort. For each cohort, patients in the training set were used to construct the nomogram and the corresponding patients in the validation set were used to validate the nomogram.
In this study, several variables were selected to identify risk factors for DM in patients with duodenal cancer. These variables included age, sex, race, marital status, grade, income, histological type, T stage, N stage, metastasis information, and tumor size. Survival analyses were conducted to investigate prognostic factors in patients with duodenal cancer and DM. Based on these factors, three treatment variables were included: surgery, radiotherapy, and chemotherapy. OS was the primary outcome and was defined as the time interval between the day of diagnosis and the day of death for any reason.
In the present study, all statistical analysis was performed with SPSS 25.0 and R software (version 4.2.3), and at P < 0.05 (two side) was considered as statistical significance. To assess the distribution of variables between the training and validation sets, all patients with duodenal cancer were randomly divided into these sets using the R software. Chi-square test or Fisher's exact test was used to compare the distribution of variables between the two sets.
In the diagnostic cohort, the univariate logistic analysis was to identify DM-related risk factors. Variables with P < 0.05 in the univariate analysis were incorporated into the multivariate logistic analysis with “Forward LR” in SPSS 25.0, to determine independent risk factors for DM in patients with duodenal cancer. Additionally, a novel diagnostic nomogram was built using the “rms” package based on independent risk factors. The receiver operating characteristic (ROC) curves of the nomogram and all independent variables were generated, and the corresponding area under the curve (AUC) was calculated to assess discrimination. Calibration curves and decision curve analysis (DCA) were used to evaluate the performance of the nomogram.
For prognostic factors, univariate Cox regression analysis was applied to determine the OS-related factors in duodenal cancer patients with DM. Significant variables (P < 0.05) were incorporated into the multivariate Cox analysis with “Forward LR” in SPSS 25.0, to further determine independent prognostic factors. A nomogram based on independent prognostic predictors was developed to predict the OS of duodenal cancer patients with DM and the individual risk score were calculated using the nomogram formula. In addition, time-dependent ROC curves of the nomogram and all independent prognostic variables at 12, 36, and 60 months were generated, and the corresponding time-dependent AUCs were applied to show discrimination. Calibration curves and DCA values at 12, 36, and 60 months were plotted to evaluate the nomogram. According to the median risk score, all patients with duodenal cancer with DM were divided into high- and low-risk groups. Kaplan-Meier (K-M) survival curves with the log-rank test were performed to show the difference in OS status between the two groups.
We performed a retrospective review of patients with duodenal cancer, based on the publicly available SEER program. A total of 2603 patients diagnosed with duodenal cancer were included in this study, with 1822 patients allocated to the training set and 781 patients to the validation set. Demographic and clinical characteristics of the duodenal cancer patients are summarized in Table 1. In the training set, the mean age was 63.73 years (range: 16-97 years), and in the validation set, it was 64.11 years (range: 25-96 years). As shown in Table 1, the most common tumor grade of differentiation was grade I (43.3 % in the training set and 45.3% in the validation set). The most common T and N stages were T1-T2 (51.8% in the training set and 52.5% in the validation set) and N0 (65.3% in the training set and 64.4% in the validation set). Regarding the histological type of duodenal cancer, adenocarcinoma (not otherwise specified), accounted for 35.8% in the training set and 36.0% in the validation set. Meanwhile, the Chi-squared test indicated that the distribution was completely random (Table 1).
| Training (%) (n = 1822) | Validation (n = 781) | Overall (n = 2603) | χ2 | P value | |
| Age, yr | 0.125 | 0.939 | |||
| < 60 | 625 (34.3) | 266 (34.1) | 891 (34.2) | ||
| > 70 | 645 (35.4) | 282 (36.1) | 927 (35.6) | ||
| 60-70 | 552 (30.3) | 233 (29.8) | 785 (30.2) | ||
| Race | 2.1543 | 0.3406 | |||
| Black | 358 (19.6) | 142 (18.2) | 500 (19.2) | ||
| Other | 166 (9.1) | 84 (10.8) | 250 (9.6) | ||
| White | 1298 (71.2) | 555 (71.1) | 1853 (71.2) | ||
| Sex | 1.795 | 0.180 | |||
| Female | 863 (47.4) | 393 (50.3) | 1256 (48.3) | ||
| Male | 959 (52.6) | 388 (49.7) | 1347 (51.7) | ||
| Marital status | 0.003 | 0.955 | |||
| Married | 1095 (60.1) | 471 (60.3) | 1566 (60.2) | ||
| Single | 727 (39.9) | 310 (39.7) | 1037 (39.8) | ||
| Income | 3.034 | 0.219 | |||
| $50000- $70000 | 844 (46.3) | 377 (48.3) | 1221 (46.9) | ||
| < $50000 | 294 (16.1) | 138 (17.7) | 432 (16.6) | ||
| > $70000 | 684 (37.5) | 266 (34.1) | 950 (36.5) | ||
| PRCDA | 0.635 | 0.426 | |||
| No | 1338 (73.4) | 561 (71.8) | 1899 (73.0) | ||
| Yes | 484 (26.6) | 220 (28.2) | 704 (27.0) | ||
| T | 0.092 | 0.761 | |||
| T1-T2 | 943 (51.8) | 410 (52.5) | 1353 (52.0) | ||
| T3-T4 | 879 (48.2) | 371 (47.5) | 1250 (48.0) | ||
| N | 0.160 | 0.689 | |||
| N0 | 1190 (65.3) | 503 (64.4) | 1693 (65.0) | ||
| N1-N2 | 632 (34.7) | 278 (35.6) | 910 (35.0) | ||
| Grade | 1.132 | 0.568 | |||
| I | 790 (43.3) | 354 (45.3) | 1144 (43.9) | ||
| II | 528 (29.0) | 212 (27.1) | 740 (28.4) | ||
| III-IV | 504 (27.7) | 215 (27.5) | 719 (27.6) | ||
| Tumor size, mm | 0.018 | 0.894 | |||
| < 25 | 868 (47.6) | 375 (48.0) | 1243 (47.8) | ||
| ≥ 25 | 954 (52.4) | 406 (52.0) | 1360 (52.2) | ||
| Histological type | 0.010 | 0.995 | |||
| Adenocarcinoma | 652 (35.8) | 281 (36.0) | 933 (35.8) | ||
| Carcinoid tumor | 661 (36.3) | 282 (36.1) | 943 (36.2) | ||
| Other | 509 (27.9) | 218 (27.9) | 727 (27.9 |
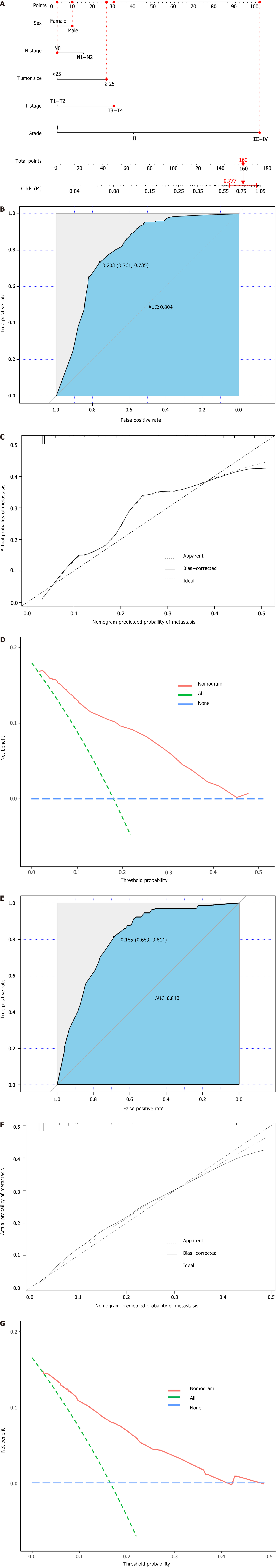
Among the total of 2603 cases analyzed, 457 cases (17.56%) were confirmed to have DM at the time of initial diagnosis, while 2146 cases (82.44%) did not have DM. Univariate logistic analysis of 11 potential factors revealed that five variables, namely sex, grade, T stage, N stage, and tumor size, were significantly associated with DM (Table 2). Subsequently, multivariate logistic regression analysis confirmed that male sex, higher T stage, higher N stage, higher grade, and larger tumor size were independent risk factors for the development of DM in patients with primary duodenal cancer (Table 2).
| Univariate analysis | Multivariate analysis | |||||
| OR | 95%CI | P value | OR | 95%CI | P value | |
| Age, yr | ||||||
| < 60 | Reference | |||||
| 60-70 | 1.164 | 0.939-1.445 | 0.246 | |||
| > 70 | 1.266 | 1.032-1.554 | 0.058 | |||
| Race | ||||||
| Black | Reference | |||||
| White | 1.013 | 0.817-1.265 | 0.92 | |||
| Other | 1.014 | 0.722-1.413 | 0.946 | |||
| Sex | ||||||
| Female | Reference | Reference | ||||
| Male | 1.245 | 1.050-1.477 | 0.035 | 1.165 | 0.965-1.408 | 0.183 |
| Marital status | ||||||
| Married | Reference | |||||
| Single | 1.056 | 0.888-1.254 | 0.603 | |||
| Income | ||||||
| $50000-$70000 | Reference | |||||
| < $50000 | 0.789 | 0.603-1.021 | 0.137 | |||
| > $70000 | 1.279 | 1.065-1.536 | 0.027 | |||
| PRCDA | ||||||
| No | Reference | |||||
| Yes | 1.118 | 0.925-1.347 | 0.330 | |||
| T | ||||||
| T1-T2 | Reference | Reference | ||||
| T3-T4 | 5.926 | 4.832-7.316 | < 0.001 | 1.779 | 1.360-2.336 | < 0.001 |
| N | ||||||
| N0 | Reference | Reference | ||||
| N1-N2 | 2.719 | 2.289-3.233 | < 0.001 | 1.306 | 1.070-1.595 | 0.027 |
| Grade | ||||||
| I | Reference | Reference | ||||
| II | 4.119 | 3.072-5.579 | < 0.001 | 2.174 | 1.560-3.053 | < 0.001 |
| III-IV | 16.616 | 12.710-22.039 | < 0.001 | 7.819 | 5.689-10.870 | < 0.001 |
| Tumor size, mm | ||||||
| < 25 | Reference | Reference | ||||
| ≥ 25 | 5.976 | 4.822-7.469 | < 0.001 | 1.649 | 1.249-2.186 | 0.003 |
| Histological type | ||||||
| Adenocarcinoma | Reference | |||||
| Carcinoid tumor | 0.106 | 0.078-0.142 | < 0.001 | |||
| Other | 0.740 | 0.612-0.894 | 0.009 | |||
A novel nomogram for predicting the risk of DM in patients with duodenal cancer was established based on five independent predictors (Figure 1A). The predictive accuracy of the nomogram was assessed using ROC curves in the training set, and the AUC of the nomogram was 0.804 (Figure 1B), indicating a strong predictive capability. To further validate the calibration of the nomogram, we generated calibration curves and DCA curves. The calibration curves demonstrated excellent consistency between the observed and predicted probabilities (Figure 1C). DCA curves (Figure 1D) further confirmed the reliability of the nomogram in providing valuable information for DM assessment. In the validation set, similar analyses were performed, verifying the predictive accuracy of the nomogram (Figure 1E-G). Additionally, ROC curves were generated for each individual predictor, revealing that the nomogram exhibited superior discriminative ability compared to individual factors in both the training and validation sets (Figure 2). Additionally, an expanded testing set was obtained from the SEER database to validate the applicability of the nomogram when external data were lacking. The AUC of the nomogram in the expanded testing set was 0.806 (Figure 3A). Additionally, the calibration, DCA, and ROC curves of all independent factors in the expanded testing set further demonstrated the good performance of the diagnostic nomogram (Figure 3B-D).
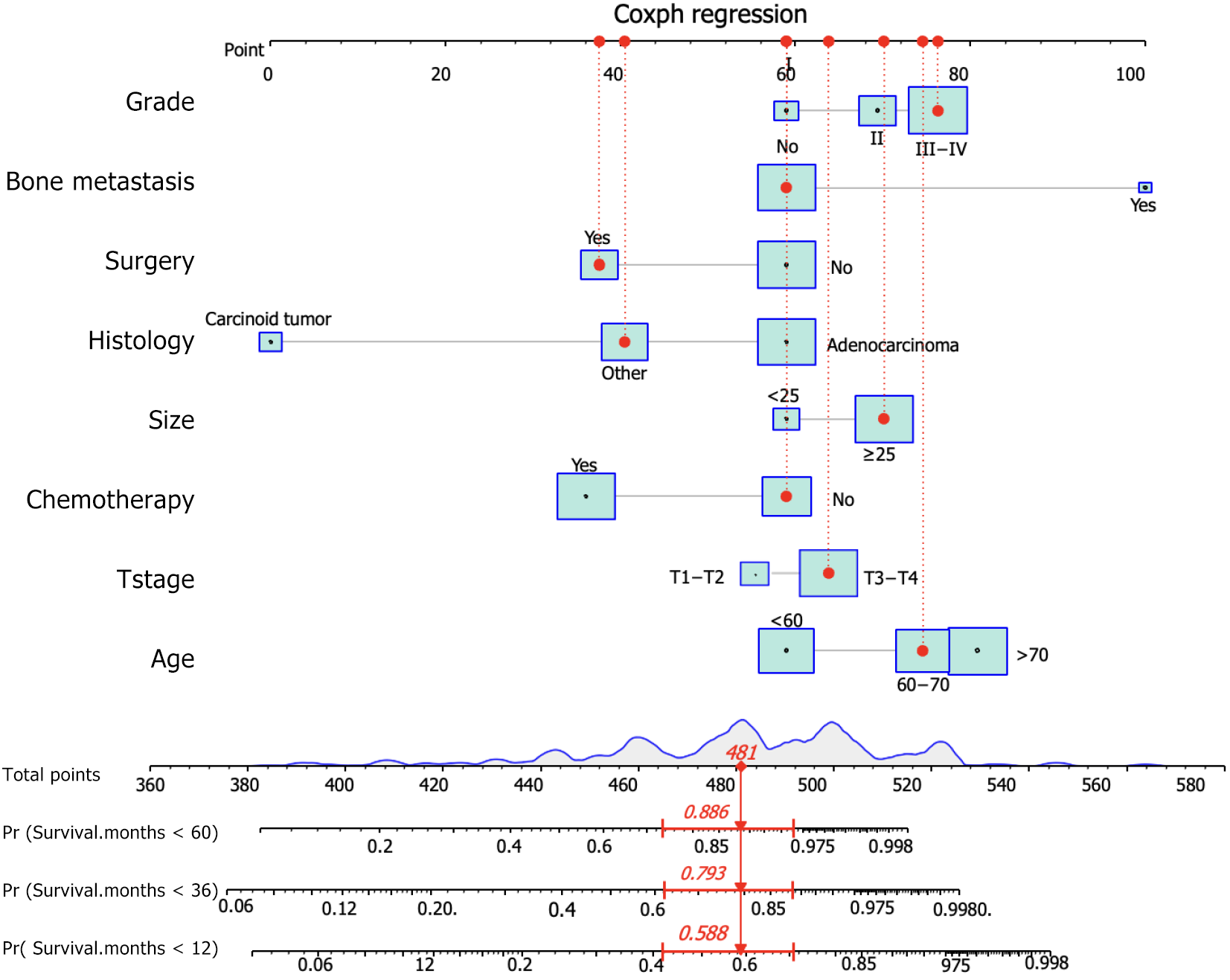
In this study, we examined 457 eligible patients diagnosed with duodenal cancer and DM to investigate potential prognostic factors. Among these patients, 121 (26.5%) underwent surgery, 56 (12.3%) received radiotherapy, and 246 (53.8%) underwent chemotherapy, as outlined in Table 3. Statistical analysis, including the Chi-square test and Fisher's exact test, revealed no significant differences in all variables between the training and validation sets. Undergoing both univariate and multivariate Cox regression analyses, robust prognostic factors were identified. The results revealed that older age (P < 0.001), higher T stage (P = 0.018), higher grade (P < 0.001), bone metastasis (P < 0.001), absence of surgery
| Training (%) (n = 319) | Validation (n = 138) | Overall (n = 457) | χ2 | P value | |
| Age, yr | 0.839 | 0.657 | |||
| < 60 | 94 (29.5) | 46 (33.3) | 140 (30.6) | ||
| > 70 | 124 (38.9) | 53 (38.4) | 177 (38.7) | ||
| 60-70 | 101 (31.7) | 39 (28.3) | 140 (30.6) | ||
| Race | 0.351 | 0.839 | |||
| Black | 61 (19.1) | 26 (18.8) | 87 (19.0) | ||
| Other | 29 (9.1) | 15 (10.9) | 44 (9.6) | ||
| White | 229 (71.8) | 97 (70.3) | 326 (71.3) | ||
| Sex | 1.010 | 0.315 | |||
| Female | 145 (45.5) | 55 (39.9) | 200 (43.8) | ||
| Male | 174 (54.5) | 83 (60.1) | 257 (56.2) | ||
| Marital status | 0.046 | 0.831 | |||
| Married | 190 (59.6) | 80 (58.0) | 270 (59.1) | ||
| Single | 129 (40.4) | 58 (42.0) | 187 (40.9) | ||
| Income | 1.765 | 0.414 | |||
| $50000-$70000 | 147 (46.1) | 57 (41.3) | 204 (44.6) | ||
| < $50000 | 43 (13.5) | 16 (11.6) | 59 (12.9) | ||
| > $70000 | 129 (40.4) | 65 (47.1) | 194 (42.5) | ||
| PRCDA | 2.04420 | 0.153 | |||
| No | 220 (69.0) | 105 (76.1) | 325 (71.1) | ||
| Yes | 99 (31.0) | 33 (23.9) | 132 (28.9) | ||
| T | 0.4560 | 0.500 | |||
| T1-T2 | 59 (18.5) | 30 (21.7) | 89 (19.5) | ||
| T3-T4 | 260 (81.5) | 108 (78.3) | 368 (80.5) | ||
| N | 0.043 | 0.837 | |||
| N0 | 146 (45.8) | 61 (44.2) | 207 (45.3) | ||
| N1-N2 | 173 (54.2) | 77 (55.8) | 250 (54.7) | ||
| Surgery | 0.468 | 0.494 | |||
| No | 238 (74.6) | 98 (71.0) | 336 (73.5) | ||
| Yes | 81 (25.4) | 40 (29.0) | 121 (26.5) | ||
| Chemotherapy | 0.324 | 0.569 | |||
| No | 144 (45.1) | 67 (48.6) | 211 (46.2) | ||
| Yes | 175 (54.9) | 71 (51.4) | 246 (53.8) | ||
| Radiation | 0.192 | 0.661 | |||
| No | 278 (87.1) | 123 (89.1) | 401 (87.7) | ||
| Yes | 41 (12.9) | 15 (10.9) | 56 (12.3) | ||
| Grade | 0.613 | 0.736 | |||
| I | 35 (11.0) | 12 (8.7) | 47 (10.3) | ||
| II | 78 (24.5) | 33 (23.9) | 111 (24.3) | ||
| III-IV | 206 (64.6) | 93 (67.4) | 299 (65.4) | ||
| Tumor size, mm | 0.157 | 0.692 | |||
| < 25 | 55 (17.2) | 21 (15.2) | 76 (16.6) | ||
| 25 | 264 (82.8) | 117 (84.8) | 381 (83.4) | ||
| Histology | 0.506 | 0.776 | |||
| Adenocarcinoma | 181 (56.7) | 78 (56.5) | 259 (56.7) | ||
| Carcinoid tumor | 24 (7.5) | 13 (9.4) | 37 (8.1) | ||
| Other | 114 (35.7) | 47 (34.1) | 161 (35.2) | ||
| Lung | 3.109 | 0.0779 | |||
| No | 290 (90.9) | 117 (84.8) | 407 (89.1) | ||
| Yes | 29 (9.1) | 21 (15.2) | 50 (10.9) | ||
| Bone | 0.072 | 0.788 | |||
| No | 304 (95.3) | 133 (96.4) | 437 (95.6) | ||
| Yes | 15 (4.7) | 5 (3.6) | 20 (4.4) | ||
| Brain | 0.588 | 2.326 | |||
| No | 317 (99.4) | 136 (98.6) | 453 (99.1) | ||
| Yes | 2 (0.6) | 2 (1.4) | 4 (0.9) | ||
| Liver | 0.315 | 0.575 | |||
| No | 56 (17.6) | 28 (20.3) | 84 (18.4) | ||
| Yes | 263 (82.4) | 110 (79.7) | 373 (81.6) | ||
| Univariate analysis | Multivariate analysis | |||||
| HR | 95%CI | P value | HR | 95%CI | P value | |
| Age, yr | ||||||
| < 60 | Reference | Reference | ||||
| 60-70 | 1.768 | 1.379-2.265 | < 0.001 | 1.648 | 1.280-2.123 | < 0.001 |
| > 70 | 2.276 | 1.793-2.891 | < 0.001 | 1.848 | 1.450-2.356 | 0.002 |
| Race | ||||||
| Black | Reference | |||||
| White | 1.042 | 0.825-1.316 | 0.771 | |||
| Other | 0.702 | 0.467-1.054 | 0.152 | |||
| Sex | ||||||
| Female | Reference | |||||
| Male | 1.216 | 1.001-1.478 | 0.098 | |||
| Marital status | ||||||
| Married | Reference | |||||
| Single | 1.267 | 1.045-1.536- | 0.044 | |||
| Income | ||||||
| $50000-$70000 | Reference | |||||
| < $50000 | 1.127 | 0.851-1.493 | 0.484 | |||
| > $70000 | 0.841 | 0.684-1.034 | 0.169 | |||
| PRCDA | ||||||
| No | Reference | |||||
| Yes | 0.981 | 0.796-1.210 | 0.882 | |||
| T | ||||||
| T1-T2 | Reference | Reference | ||||
| T3-T4 | 1.814 | 1.384-2.376 | < 0.001 | 1.481 | 1.126-1.947 | 0.018 |
| N | ||||||
| N0 | Reference | |||||
| N1-N2 | 1.078 | 0.890-1.307 | 0.519 | |||
| Grade | ||||||
| I | Reference | |||||
| II | 2.805 | 1.840-4.276 | < 0.001 | 2.833 | 1.827-4.394 | < 0.001 |
| III-IV | 3.642 | 2.442-5.432 | < 0.001 | 3.083 | 2.030-4.682 | < 0.001 |
| Tumor size, mm | ||||||
| < 25 | Reference | |||||
| ≥ 25 | 1.870 | 1.415-2.470 | < 0.001 | 1.410 | 1.055- 1.884 | 0.051 |
| Histological type | ||||||
| Adenocarcinoma | Reference | |||||
| Carcinoid tumor | 0.146 | 0.083-0.257 | < 0.001 | 0.163 | 0.088-0.300 | < 0.001 |
| Other | 0.471 | 0.379-0.586 | < 0.001 | 0.567 | 0.450-0.713 | < 0.001 |
| Surgery | ||||||
| No | ||||||
| Yes | 0.361 | 0.285-0.456 | < 0.001 | 0.455 | 0.357 -0.581 | < 0.001 |
| Chemotherapy | ||||||
| No | ||||||
| Yes | 0.767 | 0.631- 0.931 | 0.024 | 0.6004 | 0.491-0.734 | < 0.001 |
| Radiation | ||||||
| No | ||||||
| Yes | 1.232 | 0.944-1.608 | 0.197 | |||
| Bone | ||||||
| No | ||||||
| Yes | 3.094 | 2.065-4.635 | < 0.001 | 3.239 | 2.138- 4.908 | < 0.001 |
| Liver | ||||||
| No | ||||||
| Yes | 1.067 | 0.844- 1.347 | 0.650 | |||
| Brain | ||||||
| No | ||||||
| Yes | 1.958 | 0.854-4.486 | 0.183 | |||
| Lung | ||||||
| no | ||||||
| Yes | 1.332 | 0.987-1.797 | 0.116 | |||
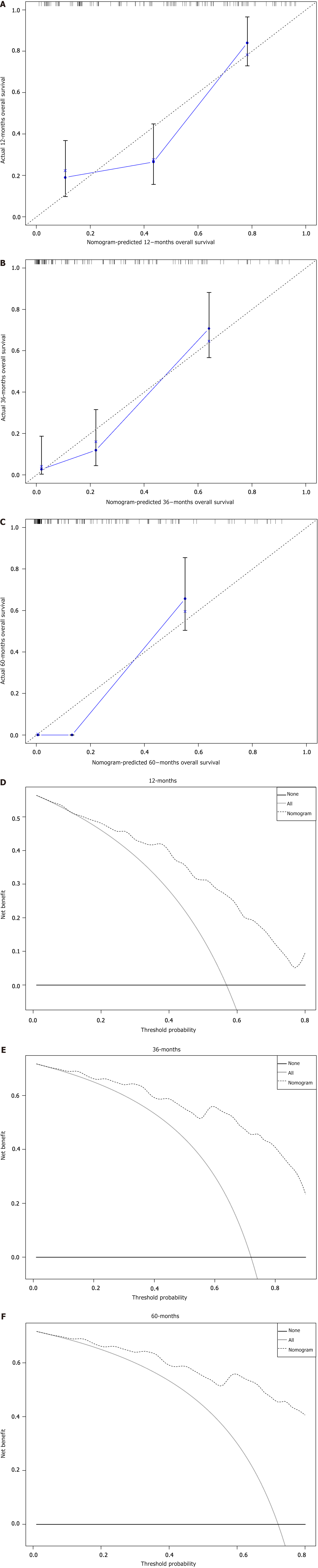
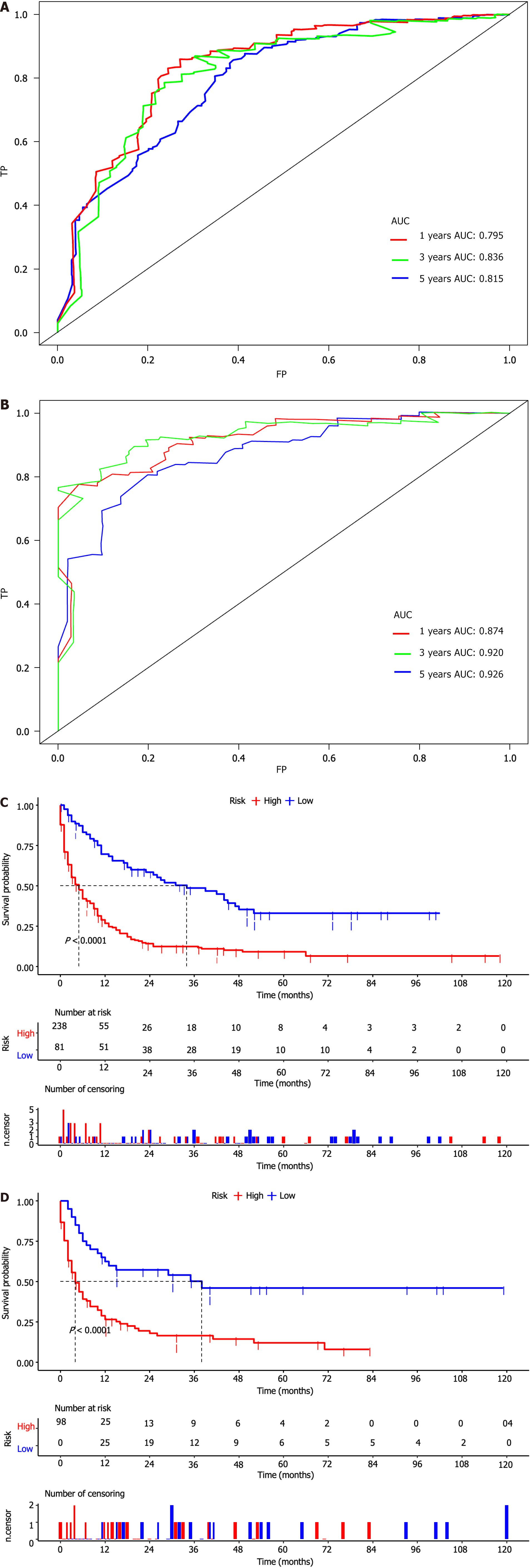
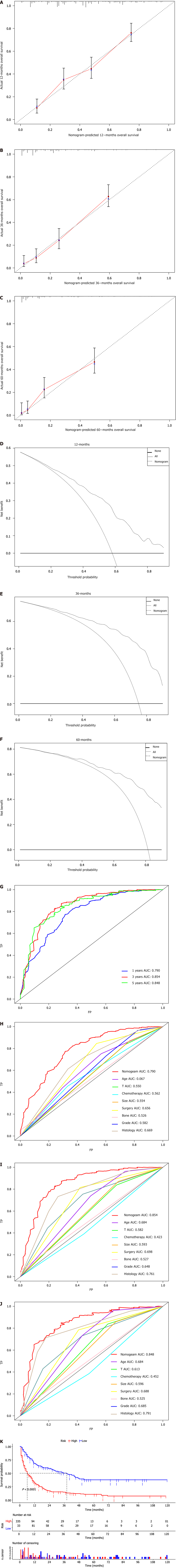
Based on these prognostic factors, we developed a nomogram to predict OS in patients with duodenal cancer and DM (Figure 4). The calibration curves for OS probability at 12, 36, and 60 months indicate strong consistency between the predicted OS and actual outcomes in the training set (Figure 5A-C). Furthermore, DCA curves confirm the favorable clinical utility of the nomogram (Figure 5D-F). In the validation set, both calibration curves and DCA curves also demonstrate good accuracy (Figure 6). ROC analysis revealed the nomogram's strong discrimination ability for predicting OS in duodenal cancer patients with DM. The area under the AUC values for the nomogram were 0.795, 0.836, and 0.815 at 12, 36, and 60 months in the training set (Figure 7A), and 0.874, 0.920, and 0.926 in the validation set (Figure 7B), respectively, demonstrating excellent predictive accuracy. K-M curves further highlighted the significant difference in OS between the high-risk and low-risk groups (Figure 7C and D). Moreover, we compared the discriminatory power of the nomogram with each independent prognostic factor and found that the nomogram consistently outperformed all individual factors at 12, 36, and 60 months (Figure 8).
In the expanded testing set, consisting of 468 patients with DM and comprehensive data on age, chemotherapy, and surgery from the SEER database, we observed the performance of the prognostic nomogram. The results demonstrated excellent calibration, as illustrated by the calibration plots (Figure 9A-C), signifying a strong concordance between the predicted OS and the actual outcomes for patients with DM. Additionally, DCA demonstrated that the prognostic nomogram served as an effective clinical tool (Figure 9D-F). Furthermore, the nomogram showed better discrimination than the three independent predictors at 12, 36, and 60 months (Figures 9G-I). The AUC for OS prediction at 12, 36, and 60 months was 0.804, 0.793, and 0.782, respectively (Figure 9J). Moreover, K-M survival analysis revealed distinct survival patterns between the high- and low-risk groups of patients (Figure 9K).
Distant organ metastasis is a common occurrence in duodenal cancer, a rare and aggressive malignant tumor. Approximately 26.7% of patients are diagnosed with metastasis at the time of diagnosis, with liver metastasis being the most prevalent (15.4%), followed by lung metastasis (8.1%), bone metastasis (4.6%), and brain metastases (1.2%)[17,18]. The grim prognosis associated with advanced-stage duodenal cancer is attributed to the challenges faced by patients with distant organ metastasis, who often cannot benefit from surgery, chemotherapy, and novel treatments, and are prone to experiencing various complications[19-22]. To address this, it is crucial to identify effective risk and prognostic factors for duodenal cancer patients with diabetes mellitus. In this research, we formulated a diagnostic nomogram to predict the presence of DM in recently diagnosed individuals with duodenal cancer. Additionally, we created a prognostic nomogram specifically tailored for patients already diagnosed with DM. By leveraging accessible variables, these nomograms provide diagnosis-related and prognosis-related scores, offering valuable guidance for clinical evaluation and intervention.
Recently, the focus of research on DM in duodenal cancer has increased, with many studies primarily consisting of case reports and a limited emphasis on clinical and pathological characteristics. In our study, we utilized a substantial dataset with meticulous clinical information sourced from the SEER database. Our findings reveal that the likelihood of DM in duodenal cancer patients is 17.6%. We identified five significant predictors of DM: Sex, T stage, N stage, tumor size, and grade. A study by Jiang et al[2] emphasized the varying rates and patterns of metastasis in patients with duodenal adenocarcinoma, particularly noting significant distinctions between males and females. Their research strongly suggests that males may be more susceptible to developing metastatic lesions in duodenal cancer, indicating a potentially more prominent role in the metastatic behavior of this malignancy. Our study aligns closely with these findings, further supporting the notion that male patients are at a greater risk of encountering duodenal cancer metastasis than their female counterparts. It's important to note that T stage encodes the depth and extent of tumor invasion, while N stage encodes the degree of lymph node involvement. In our study, we observed a positive correlation between higher T stage and the incidence of DM, as well as a positive correlation between higher N stage and the incidence of DM. These findings are consistent with previous research, indicating that cellular migration, invasion, and lymph node metastasis are crucial factors contributing to tumor progression and metastasis[23-25]. The correlation between tumor size and the occurrence of metastasis is undeniable, and our study confirms this relationship[26,27].
Given the notably unfavorable prognosis in duodenal cancer patients with DM, early detection of DM is of paramount importance, enabling timely initiation of appropriate measures such as surgical resection and chemotherapy[28]. Until now, many studies have only examined individual risk factors in isolation, and we have taken a step forward by developing an innovative diagnostic nomogram to predict the risk of DM in patients with duodenal cancer. This nomogram incorporated five independent predictive factors, allowing for a comprehensive assessment of DM risk. Through rigorous evaluation using calibration curves, ROC curves, and DCA, we demonstrated the exceptional performance of our nomogram. It holds the promise of significantly enhancing the current landscape of risk assessment, offering a more accurate and personalized approach to clinical decision making.
We further examined the prognostic factors of patients with duodenal cancer and DM. Age, histological type, T stage, tumor grade, tumor size, whether surgery or chemotherapy was performed, and the presence of bone or lung metastasis were identified as prognostic factors. We developed a corresponding prognostic nomogram, which suggests that patients with bone metastasis may require more aggressive treatments due to significantly lower OS than those with liver metastasis and lung metastasis[29]. As the incidence of duodenal cancer continues to increase, there is an urgent need for new treatment strategies. However, current adjuvant chemotherapy continues to play a crucial role in prolonging patients’ lives, and some clinical trials are still ongoing[30-32]. Surgery remains the primary choice for the treatment of early stage duodenal cancer, and it still plays a role in the management of patients with advanced-stage duodenal cancer[33,34]. Remarkably, our findings demonstrated that the lack of surgical intervention and chemotherapy exerted a substantial detrimental effect on OS, consistent with the aforementioned outcomes. Furthermore, our study revealed that radiotherapy did not significantly affect the prognosis, which is consistent with a previous study[35]. Patients who underwent surgical and chemotherapy interventions achieved superior outcomes compared to those who did not receive such treatments, underscoring the pivotal role of surgery and chemotherapy in the treatment of duodenal cancer. These compelling results provide clinicians with evidence to effectively persuade hesitant patients about the substantial benefits of surgery and chemotherapy. It is widely believed that older age in duodenal cancer patients with DM is associated with a poorer OS prognosis than in younger patients[14]. Our study affirmed that older patients indeed had a higher likelihood of experiencing a poorer OS. Importantly, we introduced a novel prognostic nomogram for predicting the prognosis of duodenal cancer patients with DM, and its discriminative ability was demonstrated to surpass that of any individual predictor. This suggests that the nomogram may offer a new avenue for personalized assessment in clinical decision-making.
Currently, there are no nomograms available for predicting the prognosis of duodenal cancer with DM. Compared with the available prognostic models, our study offers several advantages. First, our study focused on a different population than previous studies. For instance, Wang et al[36] only examined patients with small intestinal adenocarcinoma, whereas Modlin et al[37] included patients with small intestinal carcinoid tumors. In contrast, we specifically investigated the common duodenal cancer subtype with a poor prognosis and limited effective treatments. This clinical specificity has not been explored previously. Second, our study incorporated a smaller number of clinical variables, while achieving equivalent or enhanced AUC values. Third, in the absence of external data, our study conducted extensive validation using the SEER database to further validate the performance of the nomogram.
Nonetheless, there are certain limitations to this study. First, the relatively small sample size of duodenal cancer patients with DM (n = 457) may have introduced potential errors. Second, while we constructed a prediction model in the training set and validated it in the validation set, the nomograms lacked sufficient external data for complete validation, potentially leading to internal bias. Third, the information collected in the SEER database was about the disease at the time of initial diagnosis, which meant that the DM that occurred in the latter stage could not be included. Fourth, potential confounding factors, such as specific surgical approaches, chemotherapy, radiotherapy, and reasons for treatment selection, were unmeasured and therefore unreported in the SEER database, which may have impacted the results. Additionally, the predictors in this study encompassed only common clinical variables such as several critical variables such as carcinoembryonic antigen and CA-199 were not recorded in the SEER database. Finally, as this was a retrospective study, we need to confirm the nomograms designed in this study with relevant prospective studies in the future.
In conclusion, our study contributes novel insights into the diagnosis, prognosis, and treatment of duodenal cancer, particularly in the context of DM and the challenging subgroup of patients with DM. The innovative nomograms developed offer valuable tools for clinicians, providing a more accurate and personalized approach to risk assessment and clinical decision-making. While our study has shed light on critical factors influencing DM and prognosis, it is not without limitations. The relatively limited number of duodenal cancer patients with DM may introduce potential errors. Additionally, the nomograms lack external data for complete validation, potentially leading to internal bias. The retrospective nature of the study and the unavailability of certain critical variables in the SEER database further impact the generalizability of our findings. Despite these limitations, our study presents a foundation for future research. Prospective studies are warranted to confirm and further validate the nomograms designed in this study. This comprehensive approach to understanding and managing duodenal cancer, especially in high-risk subgroups, holds promise for improving patient outcomes and guiding clinical practice.
Duodenal cancer is a prevalent subtype of small intestinal cancer, and the prognosis for patients with distant metastasis (DM) in this type of cancer remains poor. However, there is a lack of studies focusing on the diagnostic and prognostic evaluation of DM in patients with primary duodenal cancer.
In this study, we aimed to utilize data from the Surveillance, Epidemiology, and End Results (SEER) database to investigate the risk factors for DM and identify prognostic factors in patients with duodenal cancer.
To develop nomogram predicting the risk of DM in patients with duodenal cancer and providing personalized prognosis predictions for those with DM, aiming to enhance clinical decision-making.
Data from duodenal cancer patients (2010-2019) were extracted from the SEER database. Univariate and multivariate logistic regression identified independent DM risk factors, while Cox proportional hazards regression determined prognostic factors in duodenal cancer patients with DM. Novel nomograms were created and evaluated using receiver operating characteristic (ROC) curves, calibration curves, and decision curve analysis (DCA).
Among 2603 duodenal cancer patients, 17.56% had DM at diagnosis. Logistic analysis identified risk factors (gender, grade, tumor size, T stage, N stage, P < 0.05). Cox analyses revealed prognostic factors (age, histological type, T stage, tumor grade, tumor size, bone metastasis, chemotherapy, surgery, P < 0.05). Nomogram accuracy was confirmed in training, validation, and testing sets (ROC, calibration, DCA curves). Kaplan-Meier curves (P < 0.001) indicated precise prediction of DM occurrence and prognosis.
This study on duodenal cancer highlights the poor prognosis linked to DM. Developed and evaluated using SEER database data, two nomograms predict DM risk and personalized prognosis. Validated for accuracy, these nomograms offer clinicians a valuable tool to enhance decision-making on DM risk and prognosis in duodenal cancer patients.
Future research should prospectively validate the nomograms, integrating additional factors for enhanced predictive accuracy. External validation across diverse datasets and assessing the nomograms' impact on treatment decisions are crucial. Evaluating feasibility for routine clinical use, conducting long-term follow-up studies, and considering patient-reported outcomes aim to improve applicability and enhance decision-making for duodenal cancer patients with DM.
The authors sincerely thank the Surveillance, Epidemiology, and End Results (SEER) program for their efforts in establishing the SEER database.
| 1. | Nakagawa K, Sho M, Fujishiro M, Kakushima N, Horimatsu T, Okada KI, Iguchi M, Uraoka T, Kato M, Yamamoto Y, Aoyama T, Akahori T, Eguchi H, Kanaji S, Kanetaka K, Kuroda S, Nagakawa Y, Nunobe S, Higuchi R, Fujii T, Yamashita H, Yamada S, Narita Y, Honma Y, Muro K, Ushiku T, Ejima Y, Yamaue H, Kodera Y. Clinical practice guidelines for duodenal cancer 2021. J Gastroenterol. 2022;57:927-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 51] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 2. | Jiang QL, Huang XH, Chen YT, Zhang JW, Wang CF. Prognostic Factors and Clinical Characteristics of Patients with Primary Duodenal Adenocarcinoma: A Single-Center Experience from China. Biomed Res Int. 2016;2016:6491049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, Kramer J, Siegel RL. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 1743] [Article Influence: 435.8] [Reference Citation Analysis (0)] |
| 4. | Yao H, Sokas C, Welch HG. Rising Incidence of Cancer of the Small Intestine: Overdiagnosis and Better Diagnosis of Low-lethality Disease. Gastroenterology. 2022;162:1749-1751.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Anzidei M, Napoli A, Zini C, Kirchin MA, Catalano C, Passariello R. Malignant tumours of the small intestine: a review of histopathology, multidetector CT and MRI aspects. Br J Radiol. 2011;84:677-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Aparicio T, Zaanan A, Svrcek M, Laurent-Puig P, Carrere N, Manfredi S, Locher C, Afchain P. Small bowel adenocarcinoma: epidemiology, risk factors, diagnosis and treatment. Dig Liver Dis. 2014;46:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 225] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 7. | Legué LM, Bernards N, Gerritse SL, van Oudheusden TR, de Hingh IH, Creemers GM, Ten Tije AJ, Lemmens VE. Trends in incidence, treatment and survival of small bowel adenocarcinomas between 1999 and 2013: a population-based study in The Netherlands. Acta Oncol. 2016;55:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Lu Y, Fröbom R, Lagergren J. Incidence patterns of small bowel cancer in a population-based study in Sweden: increase in duodenal adenocarcinoma. Cancer Epidemiol. 2012;36:e158-e163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Bilimoria KY, Bentrem DJ, Wayne JD, Ko CY, Bennett CL, Talamonti MS. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg. 2009;249:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 440] [Cited by in RCA: 482] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 10. | Bojesen RD, Andersson M, Riis LB, Nielsen OH, Jess T. Incidence of, phenotypes of and survival from small bowel cancer in Denmark, 1994-2010: a population-based study. J Gastroenterol. 2016;51:891-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Qubaiah O, Devesa SS, Platz CE, Huycke MM, Dores GM. Small intestinal cancer: a population-based study of incidence and survival patterns in the United States, 1992 to 2006. Cancer Epidemiol Biomarkers Prev. 2010;19:1908-1918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Nappo G, Funel N, Laurenti V, Stenner E, Carrara S, Bozzarelli S, Spaggiari P, Zerbi A. Ampullary Cancer: Histological Subtypes, Markers, and Clinical Behaviour-State of the Art and Perspectives. Curr Oncol. 2023;30:6996-7006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Ohara T, Iwai N, Okuda T, Tsuji T, Hara T, Okishio S, Kubota M, Oka K, Sakai H, Komaki T, Sakagami J, Kagawa K. [A Case of Duodenal Tumor Accompanied by Liver and Lymph Node Metastases after Ten Years of Observation]. Gan To Kagaku Ryoho. 2023;50:913-915. [PubMed] |
| 14. | Xu J, Yao Z, Liao G, OuYang X, Mao S, Cao J, Lai B. Prediction of distant metastasis and specific survival prediction of small intestine cancer patients with metastasis: A population-based study. Cancer Med. 2023;12:15037-15053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 15. | Balachandran VP, Gonen M, Smith JJ, DeMatteo RP. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16:e173-e180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 2577] [Article Influence: 234.3] [Reference Citation Analysis (0)] |
| 16. | Song C, Yu D, Wang Y, Wang Q, Guo Z, Huang J, Li S, Hu W. Dual Primary Cancer Patients With Lung Cancer as a Second Primary Malignancy: A Population-Based Study. Front Oncol. 2020;10:515606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Overman MJ, Pozadzides J, Kopetz S, Wen S, Abbruzzese JL, Wolff RA, Wang H. Immunophenotype and molecular characterisation of adenocarcinoma of the small intestine. Br J Cancer. 2010;102:144-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Todoroki T, Koike N, Morishita Y, Kawamoto T, Ohkohchi N, Shoda J, Fukuda Y, Takahashi H. Patterns and predictors of failure after curative resections of carcinoma of the ampulla of Vater. Ann Surg Oncol. 2003;10:1176-1183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Lee SY, Lee JH, Hwang DW, Kim SC, Park KM, Lee YJ. Long-term outcomes in patients with duodenal adenocarcinoma. ANZ J Surg. 2014;84:970-975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Gedaly R, Shoukouh-Amiri H, Shimizu A, Osama Gaber A, Sylvestre PB, Vera S. Liver transplantation for the treatment of nonresectable metastatic duodenal paraganglioma. Transpl Int. 2006;19:848-850. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Liu T, Sun Q, Chen J, Wang F, Li B, Qin W, Ye Z, Hu F. A comparison of neoadjuvant chemotherapy with gemcitabine versus docetaxel plus cisplatin in locoregionally advanced nasopharyngeal carcinoma: a propensity score matching analysis. Cancer Manag Res. 2018;10:6237-6245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Ziogas IA, Rallis KS, Tasoudis PT, Moris D, Schulick RD, Del Chiaro M. Management and outcomes of mixed adenoneuroendocrine carcinoma of the ampulla of Vater: A systematic review and pooled analysis of 56 patients. Eur J Surg Oncol. 2023;49:682-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Reference Citation Analysis (0)] |
| 23. | Lee KF, Lok HT, Fung AKY, Kung JWC, Lo EYJ, Chong CCN, Wong J, Ng KKC, Lai PBS. The Impact of Involved Resection Margin on Recurrence and Survival After Pancreaticoduodenectomy for Periampullary Carcinoma, with Emphasis on Pancreatic Head Carcinoma. World J Surg. 2023;47:717-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 24. | Ecker BL, McMillan MT, Datta J, Dempsey DT, Karakousis GC, Fraker DL, Drebin JA, Mamtani R, Giantonio BJ, Roses RE. Lymph node evaluation and survival after curative-intent resection of duodenal adenocarcinoma: A matched cohort study. Eur J Cancer. 2016;69:135-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Zhou YJ, Wang QW, Zhang QW, Chen JN, Wang XY, Gao YJ, Li XB. Patterns of Lymph Node Metastasis in Patients With T1/T2 Gastroduodenal Neuroendocrine Neoplasms: Implications for Endoscopic Treatment. Front Endocrinol (Lausanne). 2021;12:658392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Shi J, Liu S, Cao J, Shan S, Zhang J, Wang Y. Development and validation of lymph node ratio-based nomograms for primary duodenal adenocarcinoma after surgery. Front Oncol. 2022;12:962381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 27. | Wang SJ, Li YF, Liao S, Wei YZ, Zhou YM. Proposal of a new T-stage classification system for ampullary carcinoma based on Surveillance, Epidemiology and End Result (SEER) database. Hepatobiliary Pancreat Dis Int. 2021;20:568-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 28. | Patel PH, Likos-Corbett M, Doyle J, Kumar S, Bhogal RH. Node negative duodenal adenocarcinoma is associated with long-term patient survival following pancreaticoduodenal resection. ANZ J Surg. 2022;92:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Guo T, Ng KK, Chiang HW, Ma MF, Lin Y, Qian JM. Duodenal Neuroendocrine Carcinoma Presenting with Disseminated Liver and Bone Metastases as the Primary Manifestation: Case Report and Literature Review. Cell Biochem Biophys. 2015;72:305-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Seo D, Park BG, Jung D, Hwang HK, Kim SH, Hong SS, Kang CM. Pancreatoduodenectomy following neoadjuvant chemotherapy in duodenal adenocarcinoma. Ann Hepatobiliary Pancreat Surg. 2023;27:114-119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 31. | Zhang Z, Lei Y, Wang D, Yang L, Lou C. Case Report: A case of advanced duodenal adenocarcinoma in complete remission after chemotherapy combined with targeted therapy and radiotherapy. Front Oncol. 2022;12:968110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 32. | Han L, Ding N, Li L, Wei X, Hu J, Liu B, Qian X. Hepatoid adenocarcinoma of the duodenal papilla with hepatic metastases: A case report and literature review. Front Oncol. 2022;12:948892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 33. | Hwang JS, So H, Oh D, Song TJ, Park DH, Seo DW, Lee SK, Kim MH, Hong SM, Yang J, Lee SS. Long-term outcomes of endoscopic papillectomy for early-stage cancer in duodenal ampullary adenoma: Comparison to surgical treatment. J Gastroenterol Hepatol. 2021;36:2315-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Chadalavada P, Shah TU. Updates in endoscopic management of ampullary and duodenal adenomas. Curr Opin Gastroenterol. 2023;39:496-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 35. | Guo X, Bian SB, Peng Z, Wang N, Wei B, Cui JX, Wang XX, Xie TY, Xi HQ, Chen L. [Surgical selection and metastatic warning of splenic lymph node dissection in advanced gastric cancer radical surgery: a prospective, single-center, randomized controlled trial]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23:144-151. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Wang D, Li C, Li Y, Liu W, Zhao L, Güngör C, Tan F, Zhou Y. Specific survival nomograms based on SEER database for small intestine adenocarcinoma. Ann Palliat Med. 2021;10:7440-7457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Modlin IM, Gustafsson BI, Pavel M, Svejda B, Lawrence B, Kidd M. A nomogram to assess small-intestinal neuroendocrine tumor ('carcinoid') survival. Neuroendocrinology. 2010;92:143-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Njemanze P, Nigeria S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD