Published online Apr 15, 2024. doi: 10.4251/wjgo.v16.i4.1204
Peer-review started: January 13, 2024
First decision: January 30, 2024
Revised: February 2, 2024
Accepted: March 6, 2024
Article in press: March 6, 2024
Published online: April 15, 2024
Processing time: 88 Days and 10.1 Hours
Multiple primary malignant tumors (MPMTs) was first described by Billroth as early as 1889, with the first report published by Warren and Gates in 1932. Since then, numerous cases have been reported. A literature review of 1104269 patients with cancer revealed that the incidence of MPMTs ranged from 0.73 to 11.7%. In recent years, however, there has been a significant upward trend in the incidence of this phenomenon, which may be associated with many different factors, including the advancement of modern diagnostic procedures facilitating the examination and diagnosis of more MPMTs, increased exposure to chemotherapy and radiotherapy that exacerbate the risk of new malignant tumors in patients with cancer, and prolonged survival of patients with cancer allowing sufficient time for the development of new primary cancers.
To analyze the incidence, clinical features, treatment factors, prevalence, and prognosis of patients with MPMTs in the gastrointestinal tract treated in a single center. Additionally, we analyzed the different tumor combinations, time interval between the occurrence of tumors, and staging.
This retrospective cohort study analyzed 8059 patients with pathologically confirmed gastrointestinal malignant tumors treated at the Gansu Province Hospital in Lanzhou, Gansu, China between June 2011 and June 2020. Of these, 85 patients had MPMTs. The clinical features, treatment factors, prevalence, and prognosis of this latter cohort were analyzed.
The incidence of MPMTs in patients with gastrointestinal malignant tumors was 1.05% (85/8059), including 83 double primary malignant tumors and two triple primary malignant tumors of which 57 (67.06%) were synchronous MPMTs (SMPMTs) and 28 (32.94%) were metachronous MPMTs (MMPMTs). The most frequent associations were found between the rectum colon cancers within the SMPMT category and the gastric-colon cancers within the MMPMT category. For the MMPMTs, the median interval was 53 months. The overall 1-, 3- and 5-year survival rates from diagnosis of the first primary cancer were 91.36%, 65.41%, and 45.97%, respectively; those from diagnosis of the second primary cancer were 67.90%, 29.90%, and 17.37%, respectively.
MPMTs in the gastrointestinal tract have a high incidence and poor prognosis. Thus, it is necessary to perform both gastroscopy and colonoscopy in patients with gastrointestinal tumors. Multidisciplinary comprehensive diagnosis and treatment may improve the diagnosis rate and treatment efficiency of MPMTs.
Core Tip: Despite improvement in understanding of multiple primary malignant tumors (MPMTs), their pathogenesis remains unclear. Herein, we analyzed the incidence of MPMTs in the gastrointestinal tract, the different tumor combinations, time intervals between the occurrence of tumors, staging, clinical course, and prognostic features. Our aim was to determine whether the gastrointestinal tract is particularly susceptible to second or third primary cancers, and to promote early diagnosis. Our results suggested that MPMTs in the gastrointestinal tract have a high incidence and poor prognosis, and both gastroscopy and colonoscopy are necessary in patients with gastrointestinal tumors. Multidisciplinary comprehensive diagnosis and treatment may improve MPMT diagnosis and treatment.
- Citation: Zhu CL, Peng LZ. Clinical analysis of multiple primary gastrointestinal malignant tumors: A 10-year case review of a single-center. World J Gastrointest Oncol 2024; 16(4): 1204-1212
- URL: https://www.wjgnet.com/1948-5204/full/v16/i4/1204.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i4.1204
Multiple primary malignant tumors (MPMTs) are defined as the coexistence of two or more independent primary malignancies of different histologies in a single patient, either simultaneously or consecutively. MPMT was first described by Billroth as early as 1889, with the first report published by Warren and Gates in 1932[1]. Since then, numerous cases have been reported. A literature review of 1104269 patients with cancer revealed that the incidence of MPMTs ranged from 0.73% to 11.7%[2]. In recent years, however, there has been a significant upward trend in the incidence of this phenomenon, which may be associated with many different factors, including the advancement of modern diagnostic procedures facilitating the examination and diagnosis of more MPMTs[3], increased exposure to chemotherapy and radiotherapy that exacerbate the risk of new malignant tumors in patients with cancer[4,5], and prolonged survival of patients with cancer allowing sufficient time for the development of new primary cancers[6]. By analyzing the clinicopathologic data of 15321 patients with malignancies, Zhai et al[7] found that the most common MPMTs were digestive system malignancies, and the most frequent associations of MPMTs sites in the same system were digestive-digestive system malignancies. The major site of MPMTs within the digestive system was the large intestine (colon, rectum), followed by the stomach[8].
This study aimed to analyze the incidence, tumor combinations, time interval between the occurrence of tumors, staging, clinical course, and prognostic features of MPMTs in the gastrointestinal tract. The overarching aim of this investigation was to thereby determine whether the gastrointestinal tract is particularly susceptible to second or third primary cancers, and to aid in the early diagnosis of these lesions by clarifying any such tendency.
For this retrospective cohort study, data from a total of 8059 patients with pathologically-proven gastrointestinal malignant tumors treated at our hospital (The Gansu Province Hospital, Lanzhou, Gansu, China) between June 2011 and June 2020 were analyzed. Of these, 85 patients were diagnosed with MPMTs.
We adopted the criteria proposed by Warren and Gates in 1932 for the diagnosis of MPMTs: (1) Each tumor must be a pathologically proven as malignant; (2) Each tumor must be histologically distinct; and (3) the possibility of the tumor resulting from the metastasis of another must be excluded[1]. MPMTs can be sub-classified into either synchronous MPMTs (SMPMTs) and metachronous MPMTs (MMPMTs), according to their time interval, defined as the time between the date of diagnosis of the first primary cancer and the date of diagnosis of the second primary cancer. Patients are considered to have SMPMTs if the interval time is within 6 months; if the interval time is > 6 months, they are considered to have MMPMTs[9]. All patient details have been de-identified. The reporting of this study conforms to the strengthening the reporting of observational studies in epidemiology statement guidelines[10].
Data were analyzed for patient characteristics, tumor pathology, and treatments received. Kaplan–Meier survival analysis was performed to estimate overall survival using Graphpad Prism version 8.0.2 (GraphPad Software, San Diego, California). Descriptive data are presented as numbers and percentages, and differences between groups were evaluated using chi-square tests. A P-value of < 0.05 was considered statistically significant.
Among 8059 patients with pathologically-proven gastrointestinal malignant tumors, 85 had MPMTs, representing an incidence of 1.05%. Among these 85 patients, 83 cases were double primary malignant tumors and two were triple primary malignant tumors. Of these, 57 were males and 28 were females, with a male-to-female ratio of 2.04:1. The median ages at diagnosis for the first and second primary cancers were 58 years (range, 26–89 years) and 61 years (range, 35–89 years), respectively. Of these patients, 59 (69.41%) and 67 (78.82%) were aged > 50 years at diagnosis of the first and second primary cancers, respectively.
Among the 85 patients with MPMTs, 28 (32.94%) had SMPMTs, and 57 (67.06%) had MMPMTs. Of the 28 SMPMTs, 27 had double primary malignant tumors and one had a triple primary malignant tumor. Of the 57 MMPMTs, 56 cases were double primary malignant tumors and one case was triple primary malignant tumor. The median interval between diagnoses of the first primary and second primary cancers was 24 months (range, 0–318 months). Among the MMPMTs, the median interval was 53 months (range, 6–318 months; Table 1).
| Variable | First primary tumor | Second primary tumor | Third primary tumor |
| No. of patients, n | 85 | 85 | 2 |
| Median age, yr (range) | 58 (26-89) | 61 (35-89) | |
| Sex, n | |||
| Male | 57 | 57 | 0 |
| Female | 28 | 28 | 2 |
| P value | > 0.1 | ||
| Age, n | |||
| ≤ 50 yr | 26 | 18 | 0 |
| > 50 yr | 59 | 67 | 2 |
| P value | > 0.1 | ||
| Tumor distribution | |||
| Stomach | 41.46 (34/85) | 12.94 (11/85) | 0 |
| Small bowel | 1.18 (1/85) | 3.53 (3/85) | 0 |
| Colon | 32.94 (28/85) | 43.53 (37/85) | 50 (1/2) |
| Rectum | 25.88 (22/85) | 40.00 (34/85) | 50 (1/2) |
| P value | < 0.05 | ||
| Pathological type | |||
| Adenocarcinoma | 100 (85/85) | 97.65 (83/85) | 100 (2/2) |
| Neuroendocrine carcinoma | 0 | 1.18 (1/85) | 0 |
| GIST | 0 | 1.18 (1/85) | 0 |
| P value | > 0.1 | ||
| Clinical stage | |||
| Tis | 11.32 (6/53) | 8.22 (6/73) | |
| I | 13.20 (7/53) | 12.33 (9/73) | 50 (1/2) |
| II | 30.19 (16/53) | 28.76 (21/73) | 50 (1/2) |
| III | 37.74 (20/53) | 27.40 (20/73) | |
| IV | 7.55 (4/53) | 23.29 (17/73) | |
| P value | > 0.1 | ||
| Treatment | |||
| Surgery | 17.65 (15/85) | 14.12 (12/85) | 50 (1/2) |
| Surgery + chemotherapy | 76.47 (65/85) | 48.23 (41/85) | 50 (1/2) |
| Chemotherapy ± radiotherapy | 3.53 (3/85) | 21.18 (18/85) | |
| Best supportive care | 2.35 (2/85) | 16.47 (14/85) | |
| P value | < 0.001 |
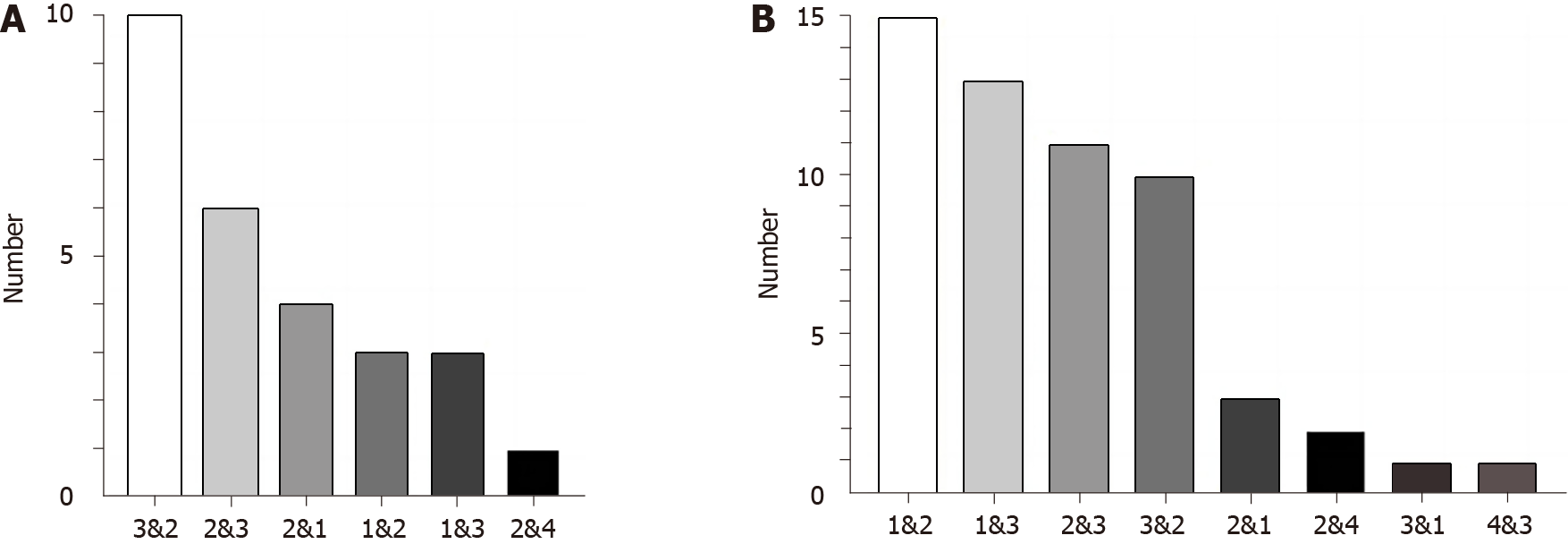
The major site for MPMTs in the gastrointestinal tract was the colon (38.37%), followed by the rectum (33.14%) and stomach (26.16%; Table 2). Common tumor associations in double primary malignancies mainly included rectum-colon cancers (n = 20), gastric-colon cancers (n = 18), and colon-rectum cancers (n = 17). In the SMPMT group, the most common associations were found between the rectum-colon cancers (n = 10) and colon-rectum cancers (n = 6), followed by colon-gastric cancers (n = 3; Figure 1A). In the MMPMT group, the most common associations were found between the gastric-colon cancers (n = 15) and gastric-rectum cancers (n = 13), followed by colon-rectum cancers (n = 11; Figure 1B). Overall, we found that nearly all tumors were adenocarcinomas, with the exception of one neuroendocrine carcinoma and one gastrointestinal stromal tumor (GIST) (Table 1).
| Sites | Number of MPMTs | Total number | Incidence (%) | Incidence (%) in gastrointestinal MPMTs |
| Stomach | 45 | 3618 | 1.24 | 26.16 |
| Small bowel | 4 | 126 | 3.17 | 2.33 |
| Colon | 66 | 1450 | 4.55 | 38.37 |
| Left colon | 41 | 23.84 | ||
| Right colon | 25 | 14.53 | ||
| Rectum | 57 | 2865 | 1.99 | 33.14 |
According to the American Joint Committee on Cancer 8th edition, malignancy clinical staging was possible in 62.35% (53/85), 85.88% (73/85), and 100% (2/2) of the first, second, and third primary cancers, respectively. The most common stages of the first, second, and third primary cancers were III, II, and II, respectively (Table 1).
To ascertain the treatment modalities applied in these patients, complete clinical information regarding the cancer therapy received was collected for further study. Among the first primary cancers, 17.65% (15/85) underwent only surgery, 76.47% (65/85) underwent surgery and chemotherapy, 3.53% (3/85) underwent only chemotherapy and/or radiotherapy, and 2.35% (2/85) received the best supportive care. The total resection rate was 94.12% (80/85). Among the second primary cancer, 14.12% (12/85) underwent only surgery, 48.23% (41/85) underwent surgery and chemotherapy, 21.18% (18/85) underwent only chemotherapy and/or radiotherapy, and 16.47% (14/85) received the best supportive care. The total resection rate was 62.35% (53/85). Compared with the first primary cancer, the resection rate of the second primary cancer was lower (P < 0.05). In MMPMTs, 14.04% (8/57) of cases underwent only surgery and 85.96% (49/57) underwent chemotherapy or radiotherapy before the diagnosis of the second primary cancer (Table 1).
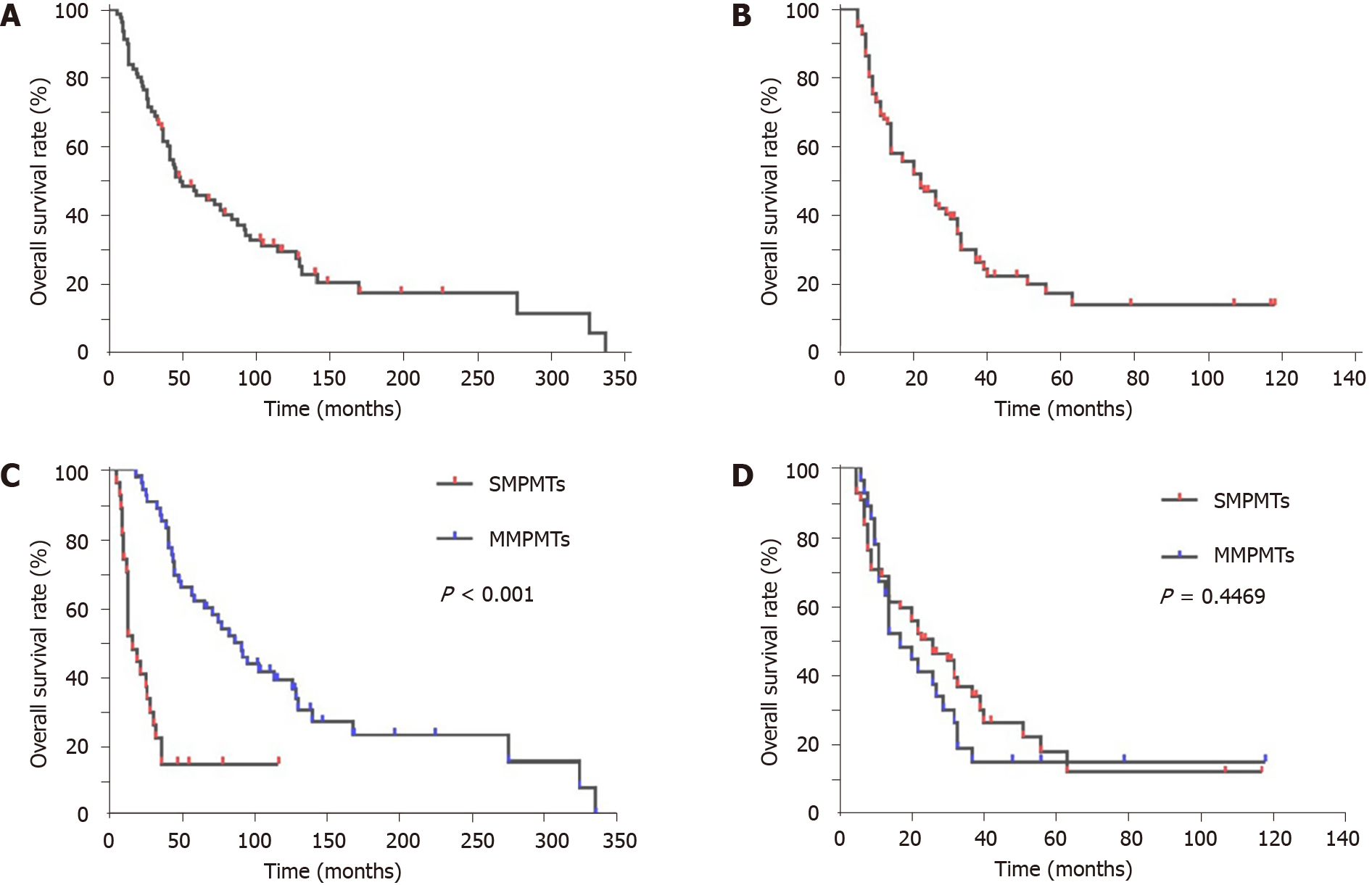
Until April 30, 2021, 81 (95.29%) patients with MPMTs were effectively monitored, while four were lost to follow-up, resulting in a missing rate of 4.71%. Among the patients who attended follow-up, 62 died, while 19 remained alive. The overall 1-, 3-, and 5-year survival rates from diagnosis of the first primary cancer of the 81 patients were 91.36%, 65.41%, and 45.97%, respectively (Figure 2A), while those from diagnosis of the second primary cancer were 67.90%, 29.90%, and 17.37%, respectively (Figure 2B). The MMPMT group showed a longer survival time than the SMPMT group (P < 10-3) after diagnosis of the first primary cancer (Figure 2C). Following diagnosis of the second primary cancer, no difference was observed in the survival rate among the two groups; however, within the first year, the MMPMT group had a longer survival time than the SMPMT group, although this situation was reversed after another year (Figure 2D).
With the advancement of anti-cancer therapies and modern diagnostic procedures, along with the expansion of the aging population, an increasing number of MPMTs have been diagnosed. However, the incidence of MPMTs varies from region to region. A literature review of 1104269 patients with cancer reported that the incidence of MPMTs was 0.73%–11.7%[2]. Further, the incidence was estimated at 8% by the National Cancer Institute’s Surveillance, Epidemiology, and End Results data[11]. A review of from 2919023 malignant cancers in 69 European cancer registries revealed 183683 cases of MPMTs, representing an overall incidence of 6.3%, ranging geographically from 0.4% (Italy) to 12.9% (Iceland)[12]. In China, different studies have reported incidence rates of MPMTs varying between 0.4% and 3.66%[13]. Zhai et al[7] found that the ratio of MPMTs sites in the same system was 27.54%, 93.48% of which were in the digestive system. The most common tumor pairs were digestive-digestive tumors (25.75%). The major site for MPMTs of the digestive system was the large intestine (colon, 23.17%; rectum, 25.82%), followed by the stomach (23.17%)[8]. Analysis of 8059 gastrointestinal cancer cases revealed an incidence of 1.05% for MPMTs in the gastrointestinal tract. In order to improve the diagnosis rate of MPMTs, we performed colonoscopy for patients with gastric cancer and gastroscopy for patients with colorectal cancer. The most common tumor pairs were rectum-colon cancers, followed by gastric-colon cancers and colon-rectum cancers. In the MMPMT group, 28 of 56 patients (50%) with double primary malignant tumors had gastric cancer as the first primary lesion. Of these, 15 (53.57%) had a second primary cancer in the colon and 13 (46.43%) had one in the rectum. However, among 16 patients who had colon cancer as the first primary lesion, 11 (68.75%) had second primary lesions in the rectum and only three (18.75%) had them in the stomach. Among 11 patients with rectum cancer as the first primary lesion, 10 (90.91%) had second primary lesions in the colon and only one (9.09%) had them in the stomach. In summary, the first primary gastric cancer was more likely to develop into second primary colorectal cancer, while the first primary colorectal cancer was more likely to develop into the second primary colorectal cancer, and rarely developed into the first second gastric cancer. In this study, men were more likely to suffer from MPMTs than women, with a sex ratio of 2.04:1. This result was higher than the male-to-female incidence ratio (1.18:1[12]) of MPMTs in total cancer, but similar to the male-to-female incidence ratio of gastrointestinal cancer found in past studies, which revealed male:female ratios of 2.4:1[14] in gastric cancer and 1.28:1[15] in colorectal cancer. In our study, 78.82% of the patients were over 50 years old when the second primary cancer was diagnosed, which is generally consistent with earlier studies[16]. Furthermore, the second primary cancer most frequently occurs 5 to 10 years after the first primary cancer[17]. Our results showed that the median interval was 53 months (range, 6–318 months) in the MMPMTs. These results indicate that clinicians should be more concerned about patients who have survived for more than 5 years from diagnosis of the first primary cancer, as well as those who are > 50 years of age. In addition, we also found that the proportion of patients with stage IV tumors in the second primary cancer was higher than that in the first primary cancer. This may be related to the fact that many patients fail to follow up promptly after the first primary cancer is diagnosed. Clinicians should therefore encourage cancer patients to adhere to timely follow-up appointments.
Due to a large number of studies reported in recent years, MPMTs are now better understood. However, their pathogenesis remains unclear. Many papers have reported that intense exposure to carcinogens (such as tobacco, alcohol, and environmental toxins), unhealthy lifestyle, hormonal and nutritional factors[6], genetic susceptibilities[17], and antineoplastic therapies (radio/chemotherapy and hormonal treatment)[18] could be significant factors contributing to the development of MPMTs. Indeed, many studies have confirmed that both chemotherapy and radiotherapy exert carcinogenic effects. Radiotherapy elevates the risk of various tumors including gastrointestinal tumors[19], while chemotherapy agents such as arsenic trioxide, alkylating agents, topoisomerase II inhibitors, and anthracyclines can cause acute myeloid leukemia[4]. Alkylating agents can also induced the occurrence of sarcomas, as well as bone and lung cancers[20,21]. The use of cyclophosphamide and tamoxifen have also been associated with bladder[22] and endometrial cancer[23], respectively. Of the MMPMT patients enrolled in this study, 85.96% (49/57) had undergone chemotherapy before the second primary cancer was diagnosed. This suggests that chemotherapy may play an important role in the initiation of MPMTs.
Close relationships between many genes, such as BRCA1/BRCA2, TP53, ATM, POLD1, PABL2, SMAD4, MMR, and EGFR, and the occurrence of MPMTs have been previously established[17]. Some of these genes are involved in genetic syndromes including Lynch, Li-Fraumeni, Cowden, Hereditary breast-ovarian cancer, PTEN hamartoma tumor, and Peutz-Jeghers. These genetic syndromes are also associated with MPMTs[24]. In addition to gene mutation and genetic syndromes, the microsatellite stability (MSI) phenotype can also cause MPMTs[25]. Therefore, clinical workers should pay more attention to the important role of genetic instability in the occurrence and development of MPMTs. Testing for MSI in the first primary cancer may help to identify patients at high risk of developing MPMTs. Furthermore, some tumors have a high risk for developing MPMTs; for example, 6%–8% of patients with soft tissue sarcoma (STS) and 10%–20% of patients with GIST developed MPMTs[17]. Thus, patients with GIST and STS should be monitored closely due to their increased susceptibility. However, in this study, we found that all of the tumors were adenocarcinomas, with the exception of one neuroendocrine carcinoma and one GIST. Therefore, the relationship between STS, GIST, and gastrointestinal MPMTs needs further study.
The therapeutic principle for MPMTs depends on the location and stage of each tumor, pathological tumor types, and physical condition of the patient. Treatment options for patients with MPMTs should be determined by multi-disciplinary teams of experts from oncology surgery, oncology medicine, radiotherapy, pathology, endoscopy, and other departments. The economic capability and willingness of patients may also influence both treatment plans and outcomes. Our study found that surgery combined with chemotherapy was the dominant treatment strategy for both the first and second primary cancers. This result was also consistent with the treatment strategies for gastrointestinal malignant tumors. The resection rate of the second primary cancer was lower than that of the first primary cancer. A possible reason for this is the high proportion of stage IV second primary cancers caused by failed timely reviews following diagnosis of the first primary cancer. In addition, some patients were unwilling to undergo surgical treatment.
In this study, the MMPMT group showed a longer survival time from diagnosis of the first primary cancer than the SMPMT group. However, the survival rate from diagnosis of the second primary cancer among the two groups showed no difference. This suggests that the second primary cancer may be the main factor affecting the survival time of patients with MMPMTs. Concurrently, we found that the short-term survival rate of patients with SMPMT was lower than that of patients with MMPMT, while the long-term survival rate was higher from the diagnosis of the second primary cancer. The reason for this may be that patients with SMPMTs have a higher short-term mortality due to higher tumor load, while patients with MMPMTs are more likely to develop resistance to chemotherapy caused by previous chemotherapy for the first primary cancer, and thus have poorer long-term survival rates. It is also worth mentioning that one patient in our study who was 84 years of age, with four primary tumors, had survived for more than 50 years. If MPMTs can be detected early, the prognosis is better than that for a single recurrence or metastasis of the primary tumor.
Our study has some limitations. Firstly, we collected and analyzed only 85 MPMTs in patients with gastrointestinal malignant tumors from a single hospital. Additionally, the sample size selected for this study was not scientifically calculated. Furthermore, we did not analyze the etiology of MPMTs in gastrointestinal malignant tumors, as this was a retrospective study with non-standardized data and records, and genetic testing was rarely performed on this cohort.
Because of the high incidence and poor prognosis of MPMTs in the gastrointestinal tract, colonoscopy should be performed among for patients with gastric cancer, while gastroscopy should be performed for patients with colorectal cancer. Despite this investigation, the etiology of MPMTs in gastrointestinal malignant tumors remains unknown and further studies with large sample sizes are needed. Increasing the awareness of MPMTs among clinicians and patients with cancer contributes to early diagnoses and treatment, as well as better prognoses. Surgery combined with chemotherapy remains the primary treatment method for MPMTs in the gastrointestinal tract. Multidisciplinary comprehensive diagnosis and treatment may improve the diagnosis rate and treatment efficiency of MPMTs.
A literature review of 1104269 patients with cancer revealed that the incidence of multiple primary malignant tumors (MPMTs) ranged from 0.73% to 11.7%. In recent years, however, there has been a significant upward trend in the incidence of this phenomenon.
The overarching motivation of this investigation was to thereby determine whether the gastrointestinal tract is particularly susceptible to second or third primary cancers, and to aid in the early diagnosis of these lesions by clarifying any such tendency.
The aim of this study was to analyze the incidence, clinical features, treatment factors, prevalence, and prognosis of patients with MPMTs in the gastrointestinal tract treated in a single center.
The study analyzed 8059 patients with pathologically confirmed gastrointestinal malignant tumors between June 2011 and June 2020. Of these, 85 patients had MPMTs. The clinical features, treatment factors, prevalence, and prognosis of this latter cohort were analyzed.
The incidence of MPMTs in patients with gastrointestinal malignant tumors was 1.05%, including 83 double primary malignant tumors and two triple primary malignant tumors of which 67.06% were synchronous MPMTs (SMPMTs) and 32.94% were metachronous MPMTs (MMPMTs). The most frequent associations were found between the rectum colon cancers within the SMPMT category and the gastric-colon cancers within the MMPMT category.
MPMTs in the gastrointestinal tract have a high incidence and poor prognosis.
It is necessary to perform both gastroscopy and colonoscopy in patients with gastrointestinal tumors. Multidisciplinary comprehensive diagnosis and treatment may improve the diagnosis rate and treatment efficiency of MPMTs.
| 1. | Yang XB, Zhang LH, Xue JN, Wang YC, Yang X, Zhang N, Liu D, Wang YY, Xun ZY, Li YR, Sun HS, Zhao LJ, Zhao HT. High incidence combination of multiple primary malignant tumors of the digestive system. World J Gastroenterol. 2022;28:5982-5992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 2. | Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol. 2003;26:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 168] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Jiang Y, Miao Z, Wang J, Chen J, Lv Y, Xing D, Wang X, Wang Y, Cao Z, Zhao Z. Clinical characteristics and prognosis associated with multiple primary malignant tumors in non-Hodgkin lymphoma patients. Tumori. 2019;105:474-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Babacan NA, Aksoy S, Cetin B, Ozdemir NY, Benekli M, Uyeturk U, Ali Kaplan M, Kos T, Karaca H, Oksuzoglu B, Zengin N, Buyukberber S. Multiple primary malignant neoplasms: multi-center results from Turkey. J BUON. 2012;17:770-775. [PubMed] |
| 5. | Travis LB, Hill DA, Dores GM, Gospodarowicz M, van Leeuwen FE, Holowaty E, Glimelius B, Andersson M, Wiklund T, Lynch CF, Van't Veer MB, Glimelius I, Storm H, Pukkala E, Stovall M, Curtis R, Boice JD Jr, Gilbert E. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 476] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 6. | Frödin JE, Ericsson J, Barlow L. Multiple primary malignant tumors in a national cancer registry--reliability of reporting. Acta Oncol. 1997;36:465-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Zhai C, Cai Y, Lou F, Liu Z, Xie J, Zhou X, Wang Z, Fang Y, Pan H, Han W. Multiple Primary Malignant Tumors - A Clinical Analysis of 15,321 Patients with Malignancies at a Single Center in China. J Cancer. 2018;9:2795-2801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 8. | Cheng HY, Chu CH, Chang WH, Hsu TC, Lin SC, Liu CC, Yang AM, Shih SC. Clinical analysis of multiple primary malignancies in the digestive system: a hospital-based study. World J Gastroenterol. 2005;11:4215-4219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | KAPSINOW R. Multiple primary cancer. A classification with report of cases. J La State Med Soc. 1962;114:194-200. [PubMed] |
| 10. | Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M; STROBE initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163-W194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 849] [Cited by in RCA: 1452] [Article Influence: 76.4] [Reference Citation Analysis (0)] |
| 11. | Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the Surveillance, Epidemiology, and End Results (SEER) Program. Oncologist. 2007;12:20-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 680] [Cited by in RCA: 745] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 12. | Rosso S, De Angelis R, Ciccolallo L, Carrani E, Soerjomataram I, Grande E, Zigon G, Brenner H; EUROCARE Working Group. Multiple tumours in survival estimates. Eur J Cancer. 2009;45:1080-1094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Zheng Y, Sun Y, Kuai Y, Fu G, An H, Chen J, Zhu J, Wo Y, Wu Y, Song K, Xu Q, Wu D, Huang D, Wang Q, Pan H. Gene expression profiling for the diagnosis of multiple primary malignant tumors. Cancer Cell Int. 2021;21:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Yang L, Zheng R, Wang N, Yuan Y, Liu S, Li H, Zhang S, Zeng H, Chen W. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 154] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 15. | Du LB, Li HZ, Wang YQ, Zhu C, Zheng RS, Zhang SW, Chen WQ, He J. [Report of colorectal cancer incidence and mortality in China, 2013]. Zhonghua Zhong Liu Za Zhi. 2017;39:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 16. | Seegobin K, Staggs E, Khawaja R, Maharaj S, Gautam S, Smotherman C, Rana F. Pilot study on the occurrence of multiple cancers following cancer-related therapy at the University of Florida, Jacksonville (2011-2016). J Investig Med. 2018;66:1050-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Lv M, Zhang X, Shen Y, Wang F, Yang J, Wang B, Chen Z, Li P, Li S. Clinical analysis and prognosis of synchronous and metachronous multiple primary malignant tumors. Medicine (Baltimore). 2017;96:e6799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 18. | Liu Z, Liu C, Guo W, Li S, Bai O. Clinical analysis of 152 cases of multiple primary malignant tumors in 15,398 patients with malignant tumors. PLoS One. 2015;10:e0125754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Travis LB, Fosså SD, Schonfeld SJ, McMaster ML, Lynch CF, Storm H, Hall P, Holowaty E, Andersen A, Pukkala E, Andersson M, Kaijser M, Gospodarowicz M, Joensuu T, Cohen RJ, Boice JD Jr, Dores GM, Gilbert ES. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97:1354-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 626] [Cited by in RCA: 597] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 20. | Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, Joensuu T, Lynch CF, van Leeuwen FE, Holowaty E, Storm H, Glimelius I, Pukkala E, Stovall M, Fraumeni JF Jr, Boice JD Jr, Gilbert E. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. J Natl Cancer Inst. 2002;94:182-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 408] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 21. | Storm HH, Lynge E, Osterlind A, Jensen OM. Multiple primary cancers in Denmark 1943-80; influence of possible underreporting and suggested risk factors. Yale J Biol Med. 1986;59:547-559. [PubMed] |
| 22. | Bruni C, Furst DE. The burning question: To use or not to use cyclophosphamide in systemic sclerosis. Eur J Rheumatol. 2020;7:S237-S241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Emons G, Mustea A, Tempfer C. Tamoxifen and Endometrial Cancer: A Janus-Headed Drug. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 24. | Da M, Peng L, Zhang Y, Yao J, Duan Y, Wen Y. Synchronous double primary gastric and endometrial cancer: a case report and literature review. Int J Clin Exp Pathol. 2015;8:8573-8578. [PubMed] |
| 25. | Yun HR, Yi LJ, Cho YK, Park JH, Cho YB, Yun SH, Kim HC, Chun HK, Lee WY. Double primary malignancy in colorectal cancer patients--MSI is the useful marker for predicting double primary tumors. Int J Colorectal Dis. 2009;24:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ghannam WM, Egypt S-Editor: Li L L-Editor: A P-Editor: Yu HG