Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.773
Peer-review started: November 9, 2023
First decision: December 6, 2023
Revised: December 16, 2023
Accepted: January 15, 2024
Article in press: January 15, 2024
Published online: March 15, 2024
Processing time: 123 Days and 23 Hours
Laparoscopic rectal cancer radical surgery is a complex procedure affected by various factors. However, the existing literature lacks standardized parameters for the pelvic region and soft tissues, which hampers the establishment of consistent conclusions.
To comprehensively assess 16 pelvic and 7 soft tissue parameters through computerized tomography (CT)-based three-dimensional (3D) reconstruction, providing a strong theoretical basis to address challenges in laparoscopic rectal cancer radical surgery.
We analyzed data from 218 patients who underwent radical laparoscopic surgery for rectal cancer, and utilized CT data for 3D pelvic reconstruction. Specific anatomical points were carefully marked and measured using advanced 3D modeling software. To analyze the pelvic and soft tissue parameters, we emp
The investigation highlighted significant sex disparities in 14 pelvic bone parameters and 3 soft tissue parameters. Males demonstrated larger measurements in pelvic depth and overall curvature, smaller measurements in pelvic width, a larger mesorectal fat area, and a larger anterior-posterior abdominal diameter. By contrast, females exhibited wider pelvises, shallower depth, smaller overall curvature, and an increased amount of subcutaneous fat tissue. However, there were no significant sex differences observed in certain parameters such as sacral curvature height, superior pubococcygeal diameter, rectal area, visceral fat area, waist circumference, and transverse abdominal diameter.
The reconstruction of 3D CT data enabled accurate pelvic measurements, revealing significant sex differences in both pelvic and soft tissue parameters. This study design offer potential in predicting surgical difficulties and creating personalized surgical plans for male rectal cancer patients with a potentially “difficult pelvis”, ultimately improving surgical outcomes. Further research and utilization of these parameters could lead to enhanced surgical methods and patient care in laparoscopic rectal cancer radical surgery.
Core Tip: This study utilized three-dimensional computerized tomography reconstruction to comprehensively evaluate 16 pelvic and 7 soft tissue parameters in 218 rectal cancer patients undergoing laparoscopic surgery. Significant sex differences were found in 14 pelvic and 3 soft tissue parameters. Females exhibited wider, shallower pelvises with smaller overall curvature compared to males. Males had narrower, deeper pelvises with greater curvature and increased mesorectal fat. These findings can help predict surgical difficulties in males with a potentially “difficult pelvis” to guide operative planning and improve outcomes.
- Citation: Zhou XC, Ke FY, Dhamija G, Chen H, Wang Q. Study on sex differences and potential clinical value of three-dimensional computerized tomography pelvimetry in rectal cancer patients. World J Gastrointest Oncol 2024; 16(3): 773-786
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/773.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.773
Rectal cancer is currently one of the most common malignant tumors. Compared to Western countries, China has a higher incidence of rectal cancer compared to colon cancer, with 60% to 70% of cases located in the middle and lower rectum[1]. Due to its deep location in the pelvic cavity and close anatomical relationship with adjacent tissues and organs, surgical treatment of middle and lower rectal cancer is relatively more challenging particularly for some low rectal cancer patients with obese and narrow male pelvises. Since the initial report proposed by British scholar Heald in 1982, total mesorectal excision (TME) has been recognized as a fundamental principle in the curative resection of rectal cancer[2]. However, the specific difficulty of rectal cancer radical surgery is affected by many factors, such as the patient’s own situation including the patient’s sex, body mass index (BMI), visceral fat area (VFA), mesorectal fat area (MFA), and the specific condition of the tumor (e.g., size, location, distance from the anal edge, stage, adhesion with surrounding tissues and organs), the spatial structure of the patient’s pelvis, and the surgeon’s experience. Among these factors, the spatial structure of the patient’s pelvis has a significant impact on the surgical procedure. Some studies have found that the size and shape of the pelvis are also one of the most important factors affecting the surgery of rectal cancer[3,4].
There are also related studies that have shown that VFA is closely related to the operative time and intraoperative blood loss of laparoscopic TME for rectal cancer. Compared with BMI, it can better reflect the impact of obesity on the difficulty of surgery[5,6]. Some scholars believe that MFA can be used as a predictor of the technical difficulty of TME for rectal cancer, because the larger fat area of mesorectum causes the space between pelvic fascia and visceral fascia wrapping around the mesorectum to become narrower[7]. In this case, it will take more time to obtain a suitable surgical field during the pelvic surgery of rectal cancer. Therefore, it is very necessary for colorectal surgeons to understand thoroughly the overall structure of the pelvis before operation, and predict the difficulty of surgery in advance through the measurement of the pelvic anatomical diameters, angles, ratios, and soft tissue parameters such as VFA and MFA, and formulate appropriate and accurate surgical treatment plans.
Currently, the pelvic skeletal and soft tissue parameters measured in most literature are relatively limited[8-12], Shimada et al[8] evaluated pelvic shape only using the anteroposterior and transverse diameters of the pelvic inlet and outlet and pelvic depth (sacral promontory to tip of coccyx) on three-dimensional (3D) volume-rendered images, and the anteroposterior diameter/transverse diameter ratio. Hausen et al[9] used the transverse diameter of the pelvic inlet, interspinous distance, intertuberous distance, the diameters of obstetric conjugate, pelvic height (promontory to intertuberous connecting line), pelvic depth (superior aspect of the symphysis to intertuberous connecting line), sagittal outlet, and sagittal midpelvic. Bertani et al[10] only used the anteroposterior and transverse diameters of the pelvic inlet and outlet and pelvic depth (sacral promontory to tip of coccyx). Curtis et al[12] only used the anteroposterior diameter of the pelvic inlet and outlet, pelvic depth (sacral promontory to tip of coccyx), interspinous distance, and mesorectal area. The measurement indicators were not completely unified, thus preventing the derivation of consistent conclusions.
Based on the aforementioned controversial issues, the present study retrospectively analyzed clinical, radiological, and pathological data from 218 patients who underwent laparoscopic radical surgery for rectal cancer. Computerized tomography (CT) scan data were collected for each patient and used to perform 3D reconstruction and measurement of 16 defined pelvic bone parameters and 7 soft tissue parameters. These parameters were statistically compared between male and female patients. This study provides a theoretical basis for addressing the abovementioned problems by measuring these parameters and drawing conclusions.
Complete clinical, pathological, and radiographic data were collected from 218 patients who underwent laparoscopic rectal cancer radical surgery at Wenzhou Central Hospital (Wenzhou, Zhejiang, China) from February 2013 to June 2022.
CT scanning data collection: The process of collecting CT scan data involves several steps and preparations. Starting from approximately 2 d before the CT examination, the patient is advised to follow a semi-liquid diet. However, for the 12 h leading up to the examination, fasting is required, and it is important for the patient to have empty bowels. Within 2 h prior to the examination, the patient needs to orally consume 1500-2000 mL of water to fill the intestines, while also ensuring the bladder is moderately distended by holding urine.
During the examination, the patient should be in a supine position, aligning the spinal axis with the midline and ensuring that the line connecting the anterior superior iliac spines on both sides is horizontal. A 64-slice multi-detector CT scanner, specifically the SIEMENS SOMATOM Definition AS+, is used to scan from the diaphragmatic dome to the anal area.
The scan consists of multiple phases, including a non-enhanced phase and three dynamic contrast-enhanced phases. The arterial phase is scanned at 25-28 s, or via intelligent monitoring-triggered scanning within the upper segment of the abdominal aorta. The portal venous phase is scanned at 60-70 s, and the delayed phase is scanned at 180 s. The tube voltage is set at 120 kV, and the tube current is automatically determined by the scanner based on the positioning image. The rotation time is 0.4 s, the matrix is set at 512 × 512, and the pitch is 0.6. The single-phase scan time typically lasts around 8-10 s.
For the contrast-enhanced scan, the Ulrich Medical high-pressure injector (Ulrich Medical, Ulm, Germany) is used to administer the contrast agent. The specific contrast agents used are Omnipaque (iohexol injection) 350 mgI/mL (GE Healthcare, Shanghai Co., Ltd) or Iopamiro (iopamidol injection) 370 mgI/mL (Shanghai Bolaike Xinyi Pharmaceutical Co., Ltd). The contrast agent is administered at a dose of 1.5-2.0 mL/kg, at a rate of 3-4 mL/s. Additionally, 60 mL normal saline is administered (20 mL for pressure testing before contrast agent injection and 40 mL for flushing) at an injection rate of 3.0-4.0 mL/s. The acquired CT scan data are reconstructed using a soft tissue standard algorithm with a slice thickness and spacing of 1.0 mm, producing axial, coronal, sagittal, and multiplanar reconstruction images of the lesions. The CT scan data are transferred to a workstation and copied to a portable hard drive In DICOM format for future reference and repeat measurements.
3D reconstruction of the pelvis: Based on thin-slice CT scanning, the Digital Imaging and Communications in Medicine (DICOM) dataset of CT is imported into E3D Digital Medical 3D Modeling and Design Software (Master Edition V19.12, Nanjing Huiqing Information Technology Co., Ltd., Nanjing, China). This software can directly read CT’s original continuous axial images in DICOM format. The 3D reconstruction area is cropped after selecting the appropriate editing range through optional editing tools. After complete cropping, the bone tissue reconstruction threshold is set to 100-1200 using the threshold segmentation tool in the 3D reconstruction module. After creating a mask using threshold segmentation, irrelevant parts are removed using the simple seed point and cluster separation tools. Finally, selecting the “Rebuild from Smooth Mask” option in the solid modeling module, a 3D pelvis model, including parts of the lumbar vertebrae and upper femur, is reconstructed.
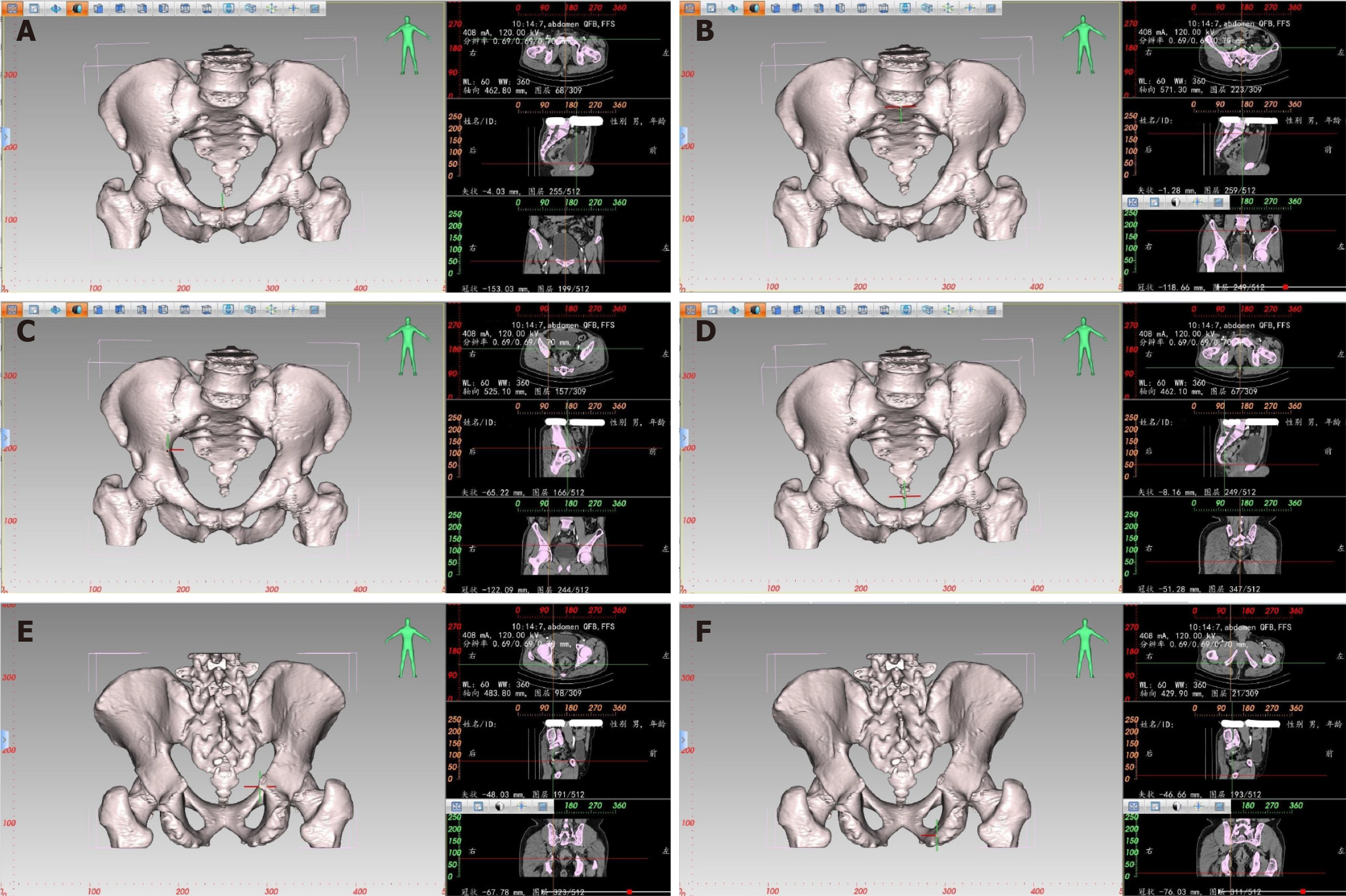
Pelvic measurement: The E3D Digital Medical 3D Modeling and Design Software can create a 3D digital model of the pelvis. It combines the characteristics of the bones in different planes, such as transverse, coronal, and sagittal, to locate and measure the corresponding distance and angle. For example, to locate the highest point of the pubic symphysis (Figure 1), the pubic symphysis is first identified in the sagittal plane by following the changes in CT values. Each click of the mouse on the plane generates two vertical lines. When the highest point of the pubic symphysis is located, the X-axis precisely passes through the upper edge of the pubis in the coronal plane, while the Y-axis and Z-axis are positioned at the center of the coronal and transverse planes, respectively. The same method is used to locate other anatomical landmarks. The software calculates the absolute distance between two points with an accuracy of up to 0.01 mm. Window width and window level can be adjusted to enhance the visibility of the bones for precise positioning. Similarly, for 3D pelvic measurements, the points of interest include the midpoint of the sacral promontory, the maximum distance between the left and right iliac crests, the coccyx tip, the ischial spine, and the ischial tuberosity.
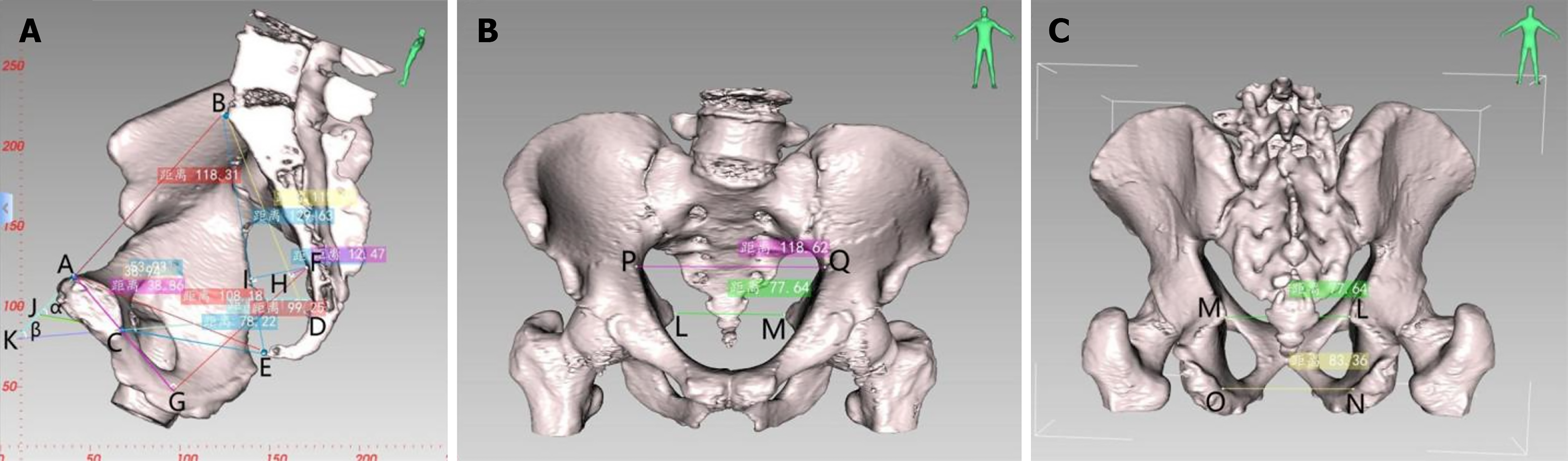
CT-based pelvic bone measurements involve a series of pelvic dimensions and angles, including anterior-posterior diameter of pelvic inlet (AB), transverse diameter of pelvic inlet (PQ), anterior-posterior diameter of mid-pelvis (CD), anterior-posterior diameter of pelvic outlet (CE), interischial spine diameter (LM), interischial tuberosity diameter (NO), superior-inferior diameter of the pubic symphysis (AC), sacrococcygeal distance (BE), superior-inferior diameter of the sacrum (BD), sacrococcygeal curvature height (FI), sacral curvature height (FH), superior pubococcygeal diameter (AE), anterior-posterior sacropubic distance (FG), sacrococcygeal angle (α), and sacropubic angle (β) (Table 1 and Figure 2).
| Parameter name | Definition |
| Anterior-posterior diameter of pelvic inlet (AB) | Distance between the midpoint of the superior pubic symphysis and the midpoint of the anterior margin of the sacral promontory |
| Transverse diameter of pelvic inlet (PQ) | Maximum distance between the left and right iliopectineal line |
| Anterior-posterior diameter of mid-pelvis (CD) | Distance between the midpoint of the inferior pubic symphysis and the midpoint of the sacrococcygeal junction |
| Anterior-posterior diameter of pelvic outlet (CE) | Distance between the midpoint of the inferior pubic symphysis and the tip of the coccyx |
| Interischial spine diameter (LM) | Shortest distance between the ischial spines |
| Interischial tuberosity diameter (NO) | Shortest distance between the ischial tuberosities |
| Superior-inferior diameter of the pubic symphysis (AC) | Distance between the superior and inferior borders of the pubic symphysis |
| Sacrococcygeal distance (BE) | Distance between the midpoint of the anterior margin of the sacral promontory and the tip of the coccyx |
| Superior-inferior diameter of sacrum (BD) | Distance between the midpoint of the anterior margin of the sacral promontory and the midpoint of the sacrococcygeal junction |
| Sacrococcygeal angle (α) | The angle between the extended lines of the anterior-posterior diameter of pelvic inlet and the anterior-posterior diameter of pelvic outlet |
| Sacropubic angle (β) | Angle between the extended lines of the anteroposterior diameter of pelvic inlet and the anterior-posterior diameter of mid-pelvis |
| Sacrococcygeal curvature height (FI) | Vertical distance from the most convex point of the sacrococcygeal curve to the sacrococcygeal distance |
| Sacral curvature height (FH) | Vertical distance from the most convex point of the sacral curve to the superior-inferior diameter of sacrum |
| Superior pubococcygeal diameter (AE) | Distance between the midpoint of the superior pubic symphysis and the tip of the coccyx |
| Anterior-posterior sacropubic distance (FG) | Vertical distance from the most convex point of the sacrococcygeal curve to the superior-inferior diameter of pubic symphysis or its extended line |
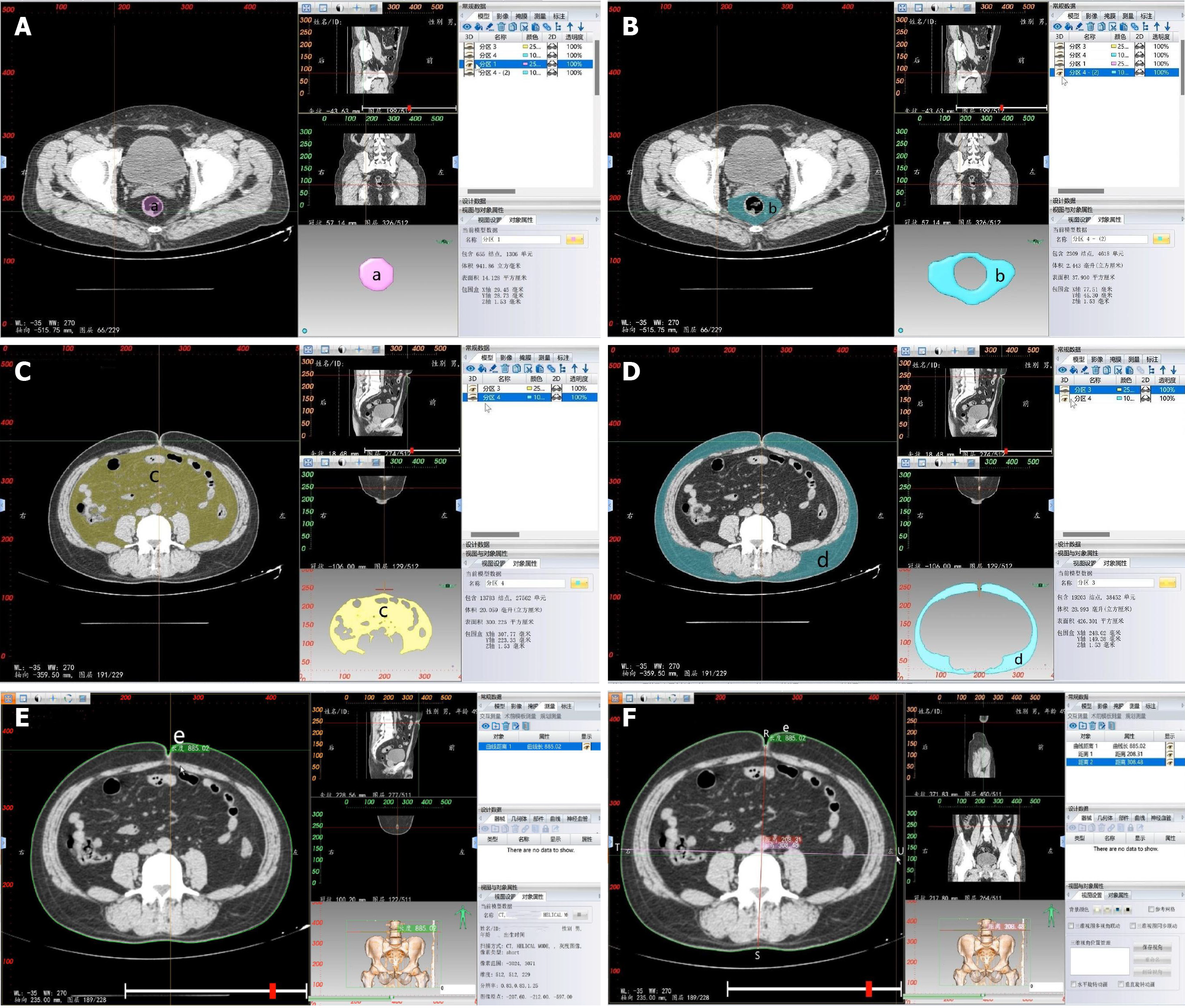
Soft tissue measurement: CT-based 3D reconstruction can also provide measurements of soft tissue parameters, including rectal area (a), MFA (b), VFA (c), subcutaneous fat area (SFA) (d), waist circumference (WC) (e), anterior-posterior abdominal diameter (APAD) (RS) and transverse abdominal diameter (TAD) (TU) (Table 2 and Figure 3).
| Parameter name | Definition |
| Rectal area (a) | Area of the rectum at the level of the ischial spine |
| Mesorectal fat area (b) | Area of the mesorectal fat at the level of the ischial spine |
| Visceral fat area (c) | Area of visceral fat at the level of the umbilicus |
| Subcutaneous fat area (d) | Area of subcutaneous fat at the level of the umbilicus |
| Waist circumference (e) | Circumference of the waist at the level of the umbilicus |
| Anterior-posterior abdominal diameter (RS) | It refers to the distance measured from the line connecting the umbilicus to the spinous process at the level of the umbilicus, between the abdominal wall section and the lumbar section |
| Transverse abdominal diameter (TU) | It refers to the distance measured from the level of the umbilicus to the horizontal line at the anterior edge of the lumbar vertebrae |
Based on thin-slice CT scanning, the DICOM dataset of the CT is imported into the E3D Digital Medical 3D Modeling and Design Software (Master Edition v19.12; Nanjing Huiqing Information Technology Co., Ltd.). The software directly reads the original continuous CT images in the DICOM format. The 3D reconstruction area can be cropped by selecting the appropriate editing options.
In the transverse section of the CT 2D image, the level of the ischial spine is selected, and in the 2D editing menu of the 3D reconstruction, the range of the mesorectal region is outlined and confirmed. The 2D painting brush tool is selected in the cluster separation menu, and the corresponding color is chosen to fill the rectal region. The remaining area, representing the mesorectal fat, is differentiated using a different color. The rectum and mesorectal fat are separated by clicking the separate and confirm buttons. In the mask panel, the solid modeling of both entities is reconstructed using a smooth mask, resulting in 3D images of both areas. The areas of interest can then be measured in the model section (Figure 3A and B).
Based on CT thin-slice scanning, the CT DICOM dataset was imported into E3D Digital Medical 3D Modeling and Design Software (Master Edition v19.12; Nanjing Huiqing Information Technology Co., Ltd.). The E3D software can directly read the original CT continuous slice images in DICOM format. By selecting the appropriate editing options, the 3D reconstruction area was cropped. After complete cropping, the fat tissue reconstruction threshold was set from -190 to -30 using the threshold segmentation tool in the 3D reconstruction.
In the sagittal and transverse sections of the CT 2D images, the most concave point of the navel was selected. The simplified brush tool was chosen in the 3D reconstruction menu, and two layers above and below the selected horizontal position in the transverse section were removed. The process was completed by double-clicking the left mouse button. Then the brush tool was selected in the mass separation menu, and different colors were assigned to distinguish between visceral fat and subcutaneous fat. Separation and confirmation buttons were clicked for each. This produced masks for both. In the mask panel, the solid modeling of both structures was reconstructed using the smooth mask reconstruction tool, resulting in 3D images of both structures. The areas of both structures can be automatically measured in the model section (Figure 3C and D).
Based on CT thin-slice scanning, the CT DICOM dataset was imported into E3D Digital Medical 3D Modeling and Design Software (Master Edition v19.12; Nanjing Huiqing Information Technology Co., Ltd.). The E3D software can directly read the original CT continuous slice images in DICOM format. By selecting the appropriate editing options, the 3D reconstruction area was cropped.
In the sagittal and transverse sections of the CT 2D images, the most concave point of the navel was selected. The image window level was adjusted to the fat tissue level for measurement. The caliper tool was chosen to outline the abdominal wall along its periphery in the measurement and analysis menu. The process was completed by right-clicking. The resulting measurement value represents the WC. In the measurement and analysis menu, the ruler tool was selected. The distance was measured from the most concave point of the navel to the line connecting the sacral prominence and the abdominal wall in the transverse section, yielding the APAD value. At the level of the anterior margin of the lumbar vertebrae, the distance between two points on the outer side of the abdominal wall was measured to obtain the TAD (Figure 3E and F).
The experimental data were analyzed using R software (version 4.2.1). The significance level was 0.05, and P < 0.05 indicated statistical significance. For single-factor analysis, the normality of the quantitative data was assessed using the Shapiro-Wilk test. Normally distributed data are presented as mean ± standard deviation (SD). Group comparisons were conducted using the paired sample t-test (for normally distributed, repeated measurements) or the Wilcoxon rank-sum test (for non-normally distributed data). For comparing means between two samples, if the data satisfied the assumptions of normality and homogeneity of variance, the independent sample t-test or analysis of variance was used; otherwise, the non-parametric Mann-Whitney U test was employed. If it did not conform to the normal distribution, it was expressed as the median (Q1, Q3), and the non-parametric Mann-Whitney U test or Kruskal-Wallis test were used for comparison between groups. Non-parametric Mann-Whitney U test or Kruskal-Wallis test were used for unidirectional ordered data of count data and rank data. For two-way unordered data, non-parametric tests such as the χ2 test, continuity corrected χ2 test, or Fisher’s exact test were used. Correlation analysis was performed using Pearson’s product-moment correlation coefficient for normally distributed bivariate data and Spearman’s rank correlation coefficient for the non-normally distributed quantitative, count, and ordered categorical data.
First, the distribution of variables was analyzed. According to the Shapiro-Wilk test, the following variables were normally distributed: Anterior-posterior diameter of pelvic inlet, transverse diameter of pelvic inlet, superior-inferior diameter of the pubic symphysis, sacrococcygeal distance, superior-inferior diameter of sacrum, sacrococcygeal angle, sacral curvature height, superior pubococcygeal diameter, WC, and TAD. However, the following variables did not follow a normal distribution: Anterior-posterior diameter of mid-pelvis, anterior-posterior diameter of pelvic outlet, interischial spine diameter, interischial tuberosity diameter, sacropubic angle, sacrococcygeal curvature height, anterior-posterior sacropubic distance, anterior-posterior diameter of pelvic inlet/sacrococcygeal distance, VFA, SFA, APAD, rectal area, and MFA. The statistical results are shown in Table 3.
| Variable name | W value | P value |
| Anterior-posterior diameter of pelvic inlet | 0.9905098 | 0.1642 |
| Transverse diameter of pelvic inlet | 0.9914321 | 0.2294 |
| Anterior-posterior diameter of mid-pelvis | 0.9758847 | 0.0009 |
| Anterior-posterior diameter of pelvic outlet | 0.9716010 | 0.0002 |
| Interischial spine diameter | 0.9840155 | 0.0146 |
| Interischial tuberosity diameter | 0.9848102 | 0.0195 |
| Superior-inferior diameter of the pubic symphysis | 0.9878909 | 0.0617 |
| Sacrococcygeal distance | 0.9947691 | 0.6552 |
| Superior-inferior diameter of sacrum | 0.9883327 | 0.0729 |
| Sacrococcygeal angle | 0.9931951 | 0.4174 |
| Sacropubic angle | 0.9720830 | 0.0003 |
| Sacrococcygeal curvature height | 0.8480501 | 0.0000 |
| Sacral curvature height | 0.9947091 | 0.6454 |
| Superior pubococcygeal diameter | 0.9918030 | 0.2615 |
| Anterior-posterior sacropubic distance | 0.9114720 | 0.0000 |
| Anterior-posterior diameter of pelvic inlet/sacrococcygeal distance | 0.9723249 | 0.0003 |
| Visceral fat area | 0.9564061 | 0.0000 |
| Subcutaneous fat area | 0.9635344 | 0.0000 |
| Waist circumference | 0.9939451 | 0.5244 |
| Anterior-posterior abdominal diameter | 0.9863249 | 0.0343 |
| Transverse abdominal diameter | 0.9939991 | 0.5327 |
| Rectal area | 0.9221744 | 0.0000 |
| Mesorectal fat area | 0.9777443 | 0.0016 |
Among the 218 patients included in the study, there were 139 males and 79 females. The age range was 32 years to 89 years, and the BMI ranged from 14.42 to 31.25 kg/m². In the American Society of Anesthesiologists (ASA) grading, there were 22 cases classified as ASA grade I, 185 cases as ASA grade II, and 11 cases as ASA grade III. Among the 218 patients, 19 had a history of abdominal surgery and 8 received neoadjuvant chemotherapy. In terms of pTNM/ypTNM staging, there were 9 cases classified as stage 0, 53 cases as stage I, 63 cases as stage II, and 93 cases as stage III. The general data are shown in Table 4.
| Range | mean ± SD or median (Q1, Q3) | No. of cases (percentage), n (%) | |
| Age (yr) | 32-89 | 66 (59, 74) | |
| Sex | |||
| Male | 139 (63.76) | ||
| Female | 79 (36.24) | ||
| BMI (kg/m2) | 14.42-31.25 | 22.57 ± 3.17 | |
| ASA grading | |||
| I | 22 (10.09) | ||
| II | 185 (84.86) | ||
| III | 11 (5.05) | ||
| History of previous abdominal surgery | 19 (8.72) | ||
| Neoadjuvant therapy | 8 (3.67) | ||
| pTNM/ypTNM staging | |||
| 0 | 9 (4.13) | ||
| I | 53 (24.31) | ||
| II | 63 (28.9) | ||
| III | 93 (42.66) |
A single experienced senior radiologist performed pelvic measurements. To evaluate differences within the measurement group, the pelvic skeletal and soft tissue parameters of 20 patients were measured twice by the same observer at a 4-wk interval, and the initial results were not visible during the repeat measurements. The data were analyzed using paired sample t-tests or Wilcoxon signed-rank tests, and inter-observer differences were calculated using Pearson’s product-moment correlation coefficient or Spearman’s rank correlation coefficient. The two measurements were highly correlated (P < 0.05), indicating reliable and accurate measurements.
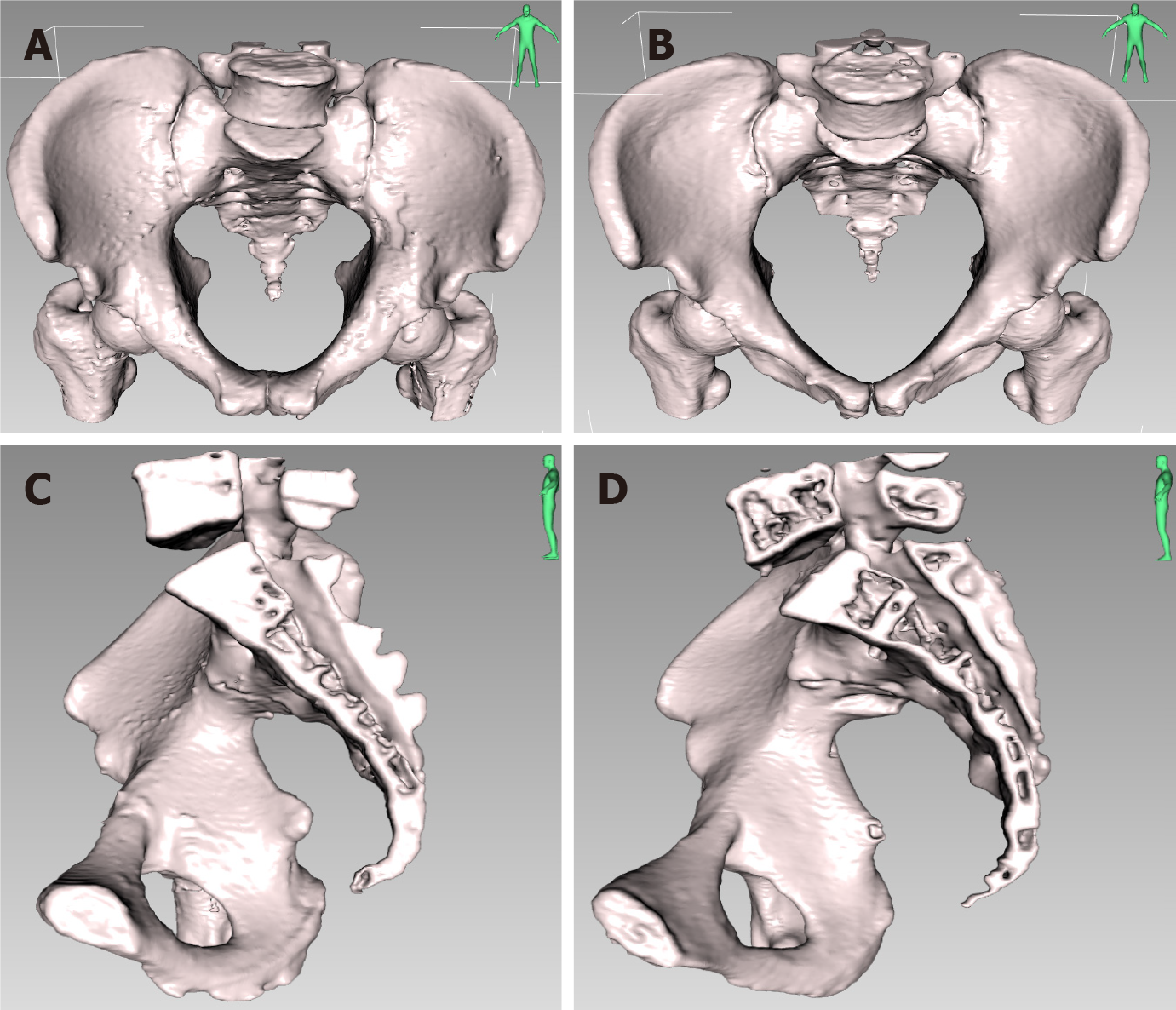
The measured normality of the 16 pelvic and 7 soft tissue parameters was assessed using the Shapiro-Wilk test. The results showed that some parameters followed a normal distribution while others did not. Normally distributed data are presented as the mean ± SD, and group comparisons between sexes were performed using either the independent sample t-test (for normally distributed data with equal variances) or the non-parametric Mann-Whitney U test (for non-normally distributed data). Non-normally distributed data are presented as median (Q1, Q3), and group comparisons were performed using the non-parametric Mann-Whitney U test. The statistical results are shown in Table 5. The 3D comparison of pelvic parameters between males and females is shown in Figure 4.
| Variable name | Total cases (n = 218), mean ± SD or median (Q1, Q3) | Male (n = 139), mean ± SD or median (Q1, Q3) | Female (n = 79), mean ± SD or median (Q1, Q3) | Mean comparison between sexes, P value |
| Anterior-posterior diameter of pelvic inlet in mm | 114.36 ± 11.99 | 110.57 ± 10.84 | 121.03 ± 11.01 | 0.0000a |
| Transverse diameter of pelvic inlet in mm | 123.3 ± 8.14 | 119.81 ± 6.57 | 129.44 ± 6.93 | 0.0000a |
| Anterior-posterior diameter of mid pelvis in mm | 111.59 (105.76, 117.26) | 109.06 (103.5, 114.76) | 113.94 (109.81, 121.44) | 0.0000a |
| Anterior-posterior diameter of pelvic outlet in mm | 88.34 (82.35, 95.73) | 86.45 (80.03, 91.33) | 93.12 (85.32, 99.8) | 0.0000a |
| Interischial spine diameter in mm | 95.92 (88.9, 104.09) | 90.77 (84.88, 96.05) | 106.73 (102.53, 112.28) | 0.0000a |
| Interischial tuberosity diameter | 104.88 (96.78, 116.95) | 99.58 (93.26, 108.12) | 117.37 (109.52, 126.2) | 0.0000a |
| Superior-inferior diameter of pubic symphysis in mm | 41.8 ± 4.92 | 43.11 ± 4.99 | 39.49 ± 3.82 | 0.0000a |
| Sacrococcygeal distance in mm | 122.92 ± 12.02 | 125.23 ± 11.53 | 118.84 ± 11.83 | 0.0002a |
| Superior-inferior diameter of sacrum in mm | 105.36 ± 10.35 | 106.76 ± 9.79 | 102.9 ± 10.89 | 0.0101a |
| Sacrococcygeal curvature height in mm | 39.15 (35.81, 42.96) | 39.7 (36.42, 44.05) | 38.3 (34.67, 41.45) | 0.0204a |
| Sacral curvature height in mm | 20.87 ± 5.66 | 21.14 ± 5.74 | 20.38 ± 5.53 | 0.3385 |
| Superior pubococcygeal diameter in mm | 119.39 ± 9.27 | 119.41 ± 8.68 | 119.34 ± 10.29 | 0.9596 |
| Anterior-posterior sacropubic distance in mm | 119.6 (113.39, 126.28) | 117.03 (112.54, 123.58) | 123.86 (116.48, 130.88) | 0.0000a |
| Sacrococcygeal angle (°) | 48.37 ± 7.28 | 50.7 ± 6.67 | 44.26 ± 6.47 | 0.0000a |
| Sacropubic angle (°) | 33.01 (29.28, 36.75) | 34.24 (30.42, 37.42) | 31.48 (28.09, 35.24) | 0.0028a |
| Anterior-posterior diameter of pelvic inlet/sacrococcygeal distance | 0.93 (0.85, 0.99) | 0.88 (0.83, 0.94) | 0.99 (0.96, 1.08) | 0.0000a |
| Rectal area in cm2 | 16.67 (12.12, 21.69) | 16.69 (11.48, 21.99) | 16.57 (12.44, 21.39) | 0.8389 |
| Mesorectal fat area in cm2 | 38.57 (26.71, 51.24) | 44.17 (32.87, 53.19) | 30.95 (21.2, 43.35) | 0.0000a |
| Visceral fat area in cm2 | 202.65 (122.4, 259.89) | 219.86 (125.28, 277.74) | 174.12 (120.58, 242) | 0.1184 |
| Subcutaneous fat area in cm2 | 255.35 (184.82, 338.59) | 235.63 (138.73, 294.54) | 319.87 (226.14, 403.15) | 0.0000a |
| Waist circumference in cm | 81.69 ± 9.26 | 82.02 ± 9.45 | 81.1 ± 8.95 | 0.4744 |
| Anterior-posterior abdominal diameter in cm | 18.22 (16.14, 19.93) | 18.26 (16.52, 20.35) | 17.72 (15.29, 19.33) | 0.0447a |
| Transverse abdominal diameter in cm | 28.69 ± 2.78 | 28.72 ± 2.67 | 28.65 ± 2.99 | 0.8653 |
Traditional X-ray pelvic measurements have been widely used in obstetrics to predict cephalopelvic disproportion and assess the need for cesarean section surgery[13]. However, X-ray measurements have lower sensitivity and specificity, which limits their clinical application. CT and magnetic resonance imaging (MRI) examinations are commonly used imaging methods for preoperative staging of rectal cancer in clinical practice, and they also provide a reliable technique for pelvic measurement in terms of diameter. However, most domestic and international literature focuses on 2D measurements using CT and MRI, with limited reports on 3D measurements. Some scholars have evaluated pelvic shape using several pelvic diameters, the ratio of diameters and angles on CT 3D volume-rendered images in rectal cancer patients, and rare scholars determined pelvic dimensions at term pregnancy with 3D MRI pelvimetry[8-11,14,15]. Compared to CT 3D reconstruction imaging, conventional preoperative MRI has a thicker slice (5 mm) and longer imaging time, resulting in blurry and unclear images after 3D reconstruction. Therefore, it is challenging to routinely implement it in clinical practice. On the other hand, CT thin-layer scanning can achieve a slice thickness of 1 mm or below. Based on this type of CT dataset, 3D reconstruction of CT images provides a clearer, more 3D, and visually realistic structure of the pelvic space. Unlike 2D CT images, which can be affected by improper patient positioning and misalignment with the imaging device, the structure of the reconstructed image from thin-layer CT scanning is not affected by such factors. Therefore, it allows for accurate positioning and measurement in 3D space[8,16].
As widely known, the female pelvis is generally broader and shallower compared to the male pelvis, which facilitates the delivery of the fetus during childbirth. Colorectal surgeons have realized that in performing surgery for mid-to-low rectal cancer, it is usually easier to operate in the female pelvis compared to the male pelvis[3]. However, even within the same sex, there are individual differences in the difficulty of pelvic surgical procedures. Some studies have confirmed the significant differences in pelvic measurement diameters between males and females[16,17]. Nevertheless, the two sexes vary considerably and overlap in pelvic measurement diameters[18]. Currently, most domestic and international literature provides relatively limited pelvic measurement parameters, and the measurement indicators are not entirely standardized, making it difficult to draw consistent conclusions.
This study demonstrates notable differences between males and females in 14 pelvic bone parameters and 3 soft tissue parameters. The parameters showing significant disparities are as follows: Anterior-posterior diameter of pelvic inlet, transverse diameter of pelvic inlet, anterior-posterior diameter of mid-pelvis, anterior-posterior diameter of pelvic outlet, interischial spine diameter, interischial tuberosity diameter, superior-inferior diameter of the pubic symphysis, sacrococcygeal distance, superior-inferior diameter of sacrum, sacrococcygeal curvature height, anterior-posterior sacropubic distance, sacrococcygeal angle, sacropubic angle, anterior-posterior diameter of pelvic inlet/sacrococcygeal distance, MFA, SFA, and APAD (all P < 0.05). Among these 14 bony parameters, all except for superior-inferior diameter of the pubic symphysis, sacrococcygeal distance, superior-inferior diameter of sacrum, sacrococcygeal curvature height, sacrococcygeal angle, and sacropubic angle reflect wider pelvic width in the female pelvis. Conversely, superior-inferior diameter of the pubic symphysis, sacrococcygeal distance, and superior-inferior diameter of sacrum reflect pelvic depth, which is more significant in the male pelvis. The larger the sacrococcygeal angle and sacropubic angle, the longer the sacrococcygeal bone and the straighter the sacral bone. The results of this study are consistent with literature reports[8-11,15], but there are also reports showing no difference in sacrococcygeal distance between sexes[12], which is related to the small sample size (71 cases in total, male/female ratio: 38:33). This study revealed a significant difference in the sacrococcygeal arch height between males and females, with more extensive measurements found in the male pelvic region.
CT scanning is also a convenient and practical method for assessing abdominal morphology and fat distribution. Among the three soft tissue parameters that exhibit sex differences, MFA and APAD are more prominent in males, whereas SFA is more significant in females. MFA is typically defined as the area of mesorectal fat at the interischial spine level, corresponding to the upper part of the rectum, located 8-10 cm from the anal margin. It is considered an accurate representation of the total volume of mesorectal fat[19]. A larger MFA leads to a narrower space between the pelvic fascia and mesorectum, which requires more time to achieve an appropriate surgical field[7]. The results of this study demonstrate that males have larger MFA and APAD than females (P < 0.05), indicating a relatively thicker mesorectal adipose tissue in males compared to females. This suggests that the bone space of male pelvic surgery and the space of internal soft tissue retraction of mesorectal are smaller. In comparison, a larger APAD suggests a deeper operative space for abdominal surgery, potentially increasing the difficulty of surgery in male patients. The measurement results of MFA in both sexes in this study are similar to those reported by Curtis et al[12], which showed a larger cross-sectional area of mesorectum in males than in females. Conversely, male SFA is smaller than female SFA, consistent with literature reports[20], likely due to the higher estrogen secretion in females, resulting in a thicker subcutaneous fat layer than in males.
The other two pelvic bony and four soft tissue parameters show no significant differences between males and females. These include sacral curvature height, superior pubococcygeal diameter, rectal area, VFA, WC, and TAD (all P > 0.05). sacral curvature height reflects the degree of sacral curvature, while superior pubococcygeal diameter reflects pelvic width. The high overlap of these parameters between males and females suggests that the measurement parameters themselves may be a more helpful factor for predicting the difficulty of surgery than sex[21]. The results of this study showed no significant difference in rectal area, VFA, WC, or TAD between males and females (all P > 0.05). VFA reflects the total area of the greater omentum, retroperitoneum, and mesentery[7]. The results of this study showed that the VFA of male was not significantly greater than that of female, contrary to the findings of Clark et al[22]. This may be attributed to the relatively leaner Asian population in our study cohort, with a lower average BMI.
Anterior-posterior diameter of pelvic inlet, anterior-posterior diameter of mid-pelvis, anterior-posterior diameter of pelvic outlet, and anterior-posterior sacropubic distance reflect the anterior-posterior width of the pelvis. The transverse diameter of pelvic inlet, interischial spine diameter, and interischial tuberosity diameter reflect the lateral width of the pelvis. The superior-inferior diameter of the pubic symphysis, sacrococcygeal distance, and sacrococcygeal distance reflect the superior-inferior depth of the pelvis. Sacrococcygeal curvature height reflects the curvature of the sacrococcygeal bone, while MFA reflects the thickness of the mesorectal fascia. When the pelvis has a larger anterior-posterior and lateral width, shallower superior-inferior depth, and less sacrococcygeal bone curvature, along with smaller MFA, it suggests that the bone space of the operation and the space of the internal soft tissue retraction in the mesocentery are larger, potentially reducing the difficulty of rectal cancer surgery.
The results of this study indicate that the male pelvis is narrower, deeper, and has straighter sacrococcygeal bones with greater overall curvature. On the other hand, the female pelvis is wider, shallower, and has smaller overall curvature than the male pelvis. Additionally, the mesorectum is relatively thicker in males, and the APAD is significantly larger in males, while the SFA is significantly smaller in males compared to females. These findings suggest that comparing pelvic bony and soft tissue parameters between males and females can provide a more accurate assessment of surgical difficulty in male rectal cancer patients with potentially “difficult pelvis”. This information can guide the development of more specific surgical plans, improving surgical safety, quality, and patient prognosis. However, this study also had several limitations. First, the present study was a retrospective single-center analysis. Second, it is important to note that the participants in this study were solely from the eastern region of China. As a result, the findings of this study can only be generalized to the Asian population and may not accurately represent the variations in pelvic structure between males and females across different ethnicities. Third, the interobserver variability was not studied during the measurements. To ensure the validity of our conclusions, further research must be conducted with diverse cohorts from multiple centers worldwide.
The method of pelvic measurement based on 3D reconstruction using CT data is reliable and accurate. There are significant differences between males and females in pelvic bony and soft tissue parameters. Female pelvises are wider, shallower, and have smaller overall curvature than male pelvises, while male pelvises are narrower, deeper, and have straighter sacrococcygeal bones with greater overall curvature. The mesorectum is relatively thicker in males, and the APAD is significantly larger in males, while subcutaneous fat tissue is significantly less abundant in males than females.
This study focused on analyzing the pelvic bone parameters and soft tissue parameters in patients who underwent laparoscopic radical surgery for rectal cancer. The significance of this research lies in understanding the sex disparities in these parameters and their implications for surgical outcomes.
The main motivation behind this research was to address the key problems in accurately measuring the pelvic bone and soft tissue parameters in patients with rectal cancer. By understanding the precise measurements of the pelvic anatomical diameters, angles, ratios, and soft tissue parameters, clinicians can make informed decisions regarding surgical planning and treatment strategies. Solving these measurement challenges can significantly contribute to improving patient outcomes and advancing research in the field of rectal cancer treatment.
The main objectives of this study were to investigate the sex differences in pelvic bone and soft tissue parameters and to determine their potential impact on laparoscopic rectal cancer surgery. By achieving these objectives, this research contributes to the existing knowledge in the field and provides a theoretical basis for addressing related surgical challenges.
To achieve the research objectives, a retrospective analysis of clinical, radiological, and pathological data from 218 patients was conducted. Computerized tomography scan data was utilized for 3D pelvic reconstruction, and statistical methods such as paired sample t-tests, Wilcoxon rank-sum tests, and correlation analysis were employed to analyze the parameters.
The investigation revealed significant sex disparities in 14 pelvic bone parameters and 3 soft tissue parameters. Males exhibited larger measurements in pelvic depth and overall curvature, while females demonstrated wider pelvises and shallower depth. These findings contribute to a better understanding of the anatomical differences between sexes and their implications for laparoscopic rectal cancer surgery.
This study proposes new insights into the sex-specific anatomical variations in pelvic bone and soft tissue parameters. The findings highlight the importance of considering these differences during surgical planning and decision-making. By recognizing and addressing these disparities, surgeons can optimize surgical outcomes and improve patient care.
The direction of future research in this field should focus on further exploring the impact of sex disparities in pelvic bone and soft tissue parameters on surgical techniques and patient outcomes. Additionally, investigating the relationship between these parameters and postoperative complications or functional outcomes would provide valuable insights for improving surgical strategies and patient quality of life.
| 1. | Hospital Authority of National Health and Family Planning Commission of the People′s Republic of China; Chinese Society of Oncology. [Chinese Protocol of Diagnosis and Treatment of Colorectal Cancer]. Zhonghua Wai Ke Za Zhi. 2018;56:241-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Heald RJ, Husband EM, Ryall RD. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69:613-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1985] [Cited by in RCA: 1973] [Article Influence: 44.8] [Reference Citation Analysis (1)] |
| 3. | Veenhof AA, Engel AF, van der Peet DL, Sietses C, Meijerink WJ, de Lange-de Klerk ES, Cuesta MA. Technical difficulty grade score for the laparoscopic approach of rectal cancer: a single institution pilot study. Int J Colorectal Dis. 2008;23:469-475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 4. | Gu J, Bo XF, Xiong CY, Wu AW, Zhang XP, Li M, An Q, Fang J, Li J, Zhang X, Wang HY, Gao F, You WC. Defining pelvic factors in sphincter-preservation of low rectal cancer with a three-dimensional digital model of pelvis. Dis Colon Rectum. 2006;49:1517-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Chen B, Zhang Y, Zhao S, Yang T, Wu Q, Jin C, He Y, Wang Z. The impact of general/visceral obesity on completion of mesorectum and perioperative outcomes of laparoscopic TME for rectal cancer: A STARD-compliant article. Medicine (Baltimore). 2016;95:e4462. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Kang J, Baek SE, Kim T, Hur H, Min BS, Lim JS, Kim NK, Lee KY. Impact of fat obesity on laparoscopic total mesorectal excision: more reliable indicator than body mass index. Int J Colorectal Dis. 2012;27:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 7. | Yamaoka Y, Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Furutani A, Manabe S, Torii K, Koido K, Mori K. Mesorectal fat area as a useful predictor of the difficulty of robotic-assisted laparoscopic total mesorectal excision for rectal cancer. Surg Endosc. 2019;33:557-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 8. | Shimada T, Tsuruta M, Hasegawa H, Okabayashi K, Ishida T, Asada Y, Suzumura H, Kitagawa Y. Pelvic inlet shape measured by three-dimensional pelvimetry is a predictor of the operative time in the anterior resection of rectal cancer. Surg Today. 2018;48:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zur Hausen G, Gröne J, Kaufmann D, Niehues SM, Aschenbrenner K, Stroux A, Hamm B, Kreis ME, Lauscher JC. Influence of pelvic volume on surgical outcome after low anterior resection for rectal cancer. Int J Colorectal Dis. 2017;32:1125-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Bertani E, Chiappa A, Della Vigna P, Radice D, Papis D, Cossu L, Biffi R, Bianchi PP, Luca F, Andreoni B. The Impact of pelvimetry on anastomotic leakage in a consecutive series of open, laparoscopic and robotic low anterior resections with total mesorectal excision for rectal cancer. Hepatogastroenterology. 2014;61:1574-1581. [PubMed] |
| 11. | Kaufmann D, Lauscher JC, Gröne J, Zur Hausen G, Kreis ME, Hamm B, Niehues SM. CT-based measurement of the inner pelvic volume. Acta Radiol. 2017;58:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Curtis NJ, Thomas C, Dennison G, Ockrim JB, Conti JA, Dalton R, Allison AS, Francis NK. Factors Predicting Operative Difficulty of Laparoscopic Total Mesorectal Excision. Dis Colon Rectum. 2019;62:1467-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Abitbol MM, Taylor UB, Castillo I, Rochelson BL. The cephalopelvic disproportion index. Combined fetal sonography and x-ray pelvimetry for early detection of cephalopelvic disproportion. J Reprod Med. 1991;36:369-373. [PubMed] |
| 14. | Liao KD, Yu YH, Li YG, Chen L, Peng C, Liu P, Chen CL, Chen RY, Zhong M, Wang Y. Three-dimensional magnetic resonance pelvimetry: A new technique for evaluating the female pelvis in pregnancy. Eur J Radiol. 2018;102:208-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Lorenzon L, Bini F, Landolfi F, Quinzi S, Balducci G, Marinozzi F, Biondi A, Persiani R, D'Ugo D, Tirelli F, Iannicelli E. 3D pelvimetry and biometric measurements: a surgical perspective for colorectal resections. Int J Colorectal Dis. 2021;36:977-986. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Wang C, Xiao Y, Qiu H, Yao J, Pan W. Factors affecting operating time in laparoscopic anterior resection of rectal cancer. World J Surg Oncol. 2014;12:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Verschueren RC, Mulder NH, Van Loon AJ, De Ruiter AJ, Szabo BG. The anatomical substrate for a difference in surgical approach to rectal cancer in male and female patients. Anticancer Res. 1997;17:637-641. [PubMed] |
| 18. | Salerno G, Daniels IR, Brown G, Heald RJ, Moran BJ. Magnetic resonance imaging pelvimetry in 186 patients with rectal cancer confirms an overlap in pelvic size between males and females. Colorectal Dis. 2006;8:772-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Boyle KM, Chalmers AG, Finan PJ, Sagar PM, Burke D. Morphology of the mesorectum in patients with primary rectal cancer. Dis Colon Rectum. 2009;52:1122-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Lee KH, Kang BK, Ahn BK. Higher visceral fat area/subcutaneous fat area ratio measured by computed tomography is associated with recurrence and poor survival in patients with mid and low rectal cancers. Int J Colorectal Dis. 2018;33:1303-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Killeen T, Banerjee S, Vijay V, Al-Dabbagh Z, Francis D, Warren S. Magnetic resonance (MR) pelvimetry as a predictor of difficulty in laparoscopic operations for rectal cancer. Surg Endosc. 2010;24:2974-2979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Clark W, Siegel EM, Chen YA, Zhao X, Parsons CM, Hernandez JM, Weber J, Thareja S, Choi J, Shibata D. Quantitative measures of visceral adiposity and body mass index in predicting rectal cancer outcomes after neoadjuvant chemoradiation. J Am Coll Surg. 2013;216:1070-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aydin S, Turkey S-Editor: Wang JJ L-Editor: Filipodia P-Editor: Zhao S