Published online Mar 15, 2024. doi: 10.4251/wjgo.v16.i3.1029
Peer-review started: November 22, 2023
First decision: December 8, 2023
Revised: December 21, 2023
Accepted: January 4, 2024
Article in press: January 4, 2024
Published online: March 15, 2024
Processing time: 111 Days and 1.2 Hours
CALD1 has been discovered to be abnormally expressed in a variety of malignant tumors, including gastric cancer (GC), and is associated with tumor progression and immune infiltration; however, the roles and mechanisms of CALD1 in epithe
To investigate the role and mechanism of CALD1 in GC progression, invasion, and migration.
In this study, the relationship between CALD1 and GC, as well as the possible network regulatory mechanisms of CALD1, was investigated by bioinformatics and validated by experiments. CALD1-siRNA was synthesized and used to trans
Bioinformatics results showed that CALD1 was highly expressed in GC tissues, and CALD1 was significantly higher in EMT-type GC tissues than in tissues of other types of GC. The prognosis of patients with high expression of CALD1 was worse than that of patients with low expression, and a prognostic model was constructed and evaluated. The experimental results were consistent with the results of the bioinformatics analysis. The expression level of CALD1 in GC cell lines was all higher than that in gastric epithelial cell line GES-1, with the strongest expression found in AGS and MKN45 cells. Cell activity was significantly reduced after CALD1-siRNA trans
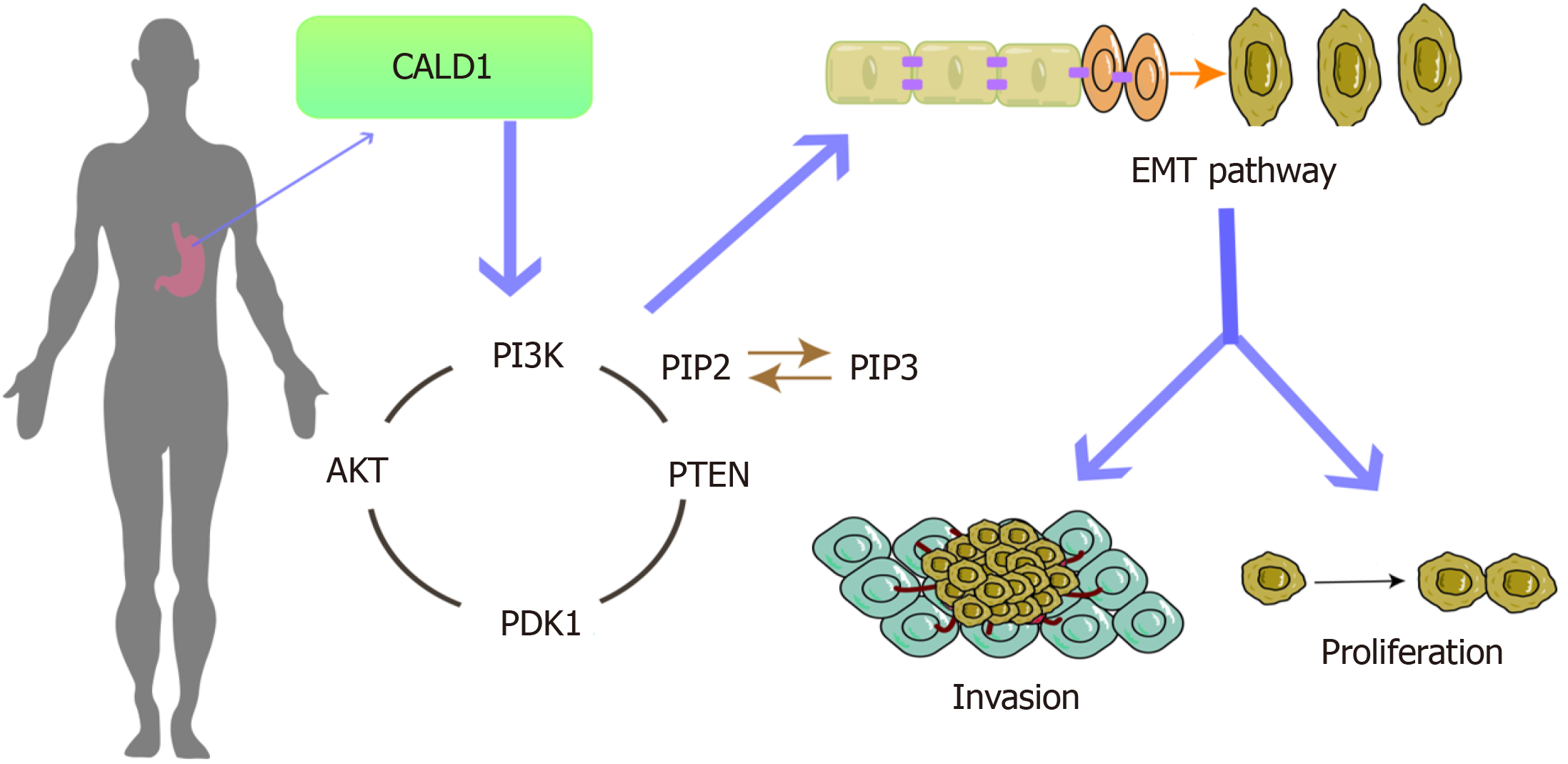
Increased expression of CALD1 is a key factor in the progression, invasion, and metastasis of GC, which may be associated with regulating the PI3K-Akt pathway to promote EMT.
Core Tip: In this study, the relationship between CALD1 and gastric cancer (GC) and the possible network regulatory mechanisms of CALD1 were explored by bioinformatics methods and validated by experiments. We conducted functional analysis and verification through tissue and cell experiments, delving into possible pathways and mechanisms involved. It was showed that CALD1 may participate in the proliferation, invasion, and migration, and epithelial-mesenchymal transition (EMT)-related gene and protein expression in GC cells. Our study suggested that CALD1, through PI3K-Akt signaling pathway activation, may regulate EMT in GC cells, representing a potentially novel target for GC treatment.
- Citation: Ma WQ, Miao MC, Ding PA, Tan BB, Liu WB, Guo S, Er LM, Zhang ZD, Zhao Q. CALD1 facilitates epithelial-mesenchymal transition progression in gastric cancer cells by modulating the PI3K-Akt pathway. World J Gastrointest Oncol 2024; 16(3): 1029-1045
- URL: https://www.wjgnet.com/1948-5204/full/v16/i3/1029.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i3.1029
Gastric cancer (GC) is a major malignancy of the digestive tract that ranks fifth in incidence and fourth in mortality, imposing a significant societal burden[1,2]. The rise in health consciousness, as well as advances in diagnostic technologies such as endoscopy and computed tomography, has improved the early detection and treatment of GC. None
The CALD1 gene encodes the Caldesmon protein, which has high and low molecular weight variants and functions as a cytoskeletal-associated protein. It is involved in cellular adhesion, cytoskeletal organization, and angiogenesis, as well as cell proliferation, apoptosis, motility, and adhesion. As a result, it may influence tumor proliferation, invasion, and metastasis[7-9]. CALD1 expression is abnormal in various cancers, including GC, and it correlates with tumor stage and prognosis[8-13]. In GC research, CALD1 expression has been linked with immune infiltration and prognosis, though its specific mechanisms remain unknown[8].
Tumor cells can acquire invasive mesenchymal characteristics via the epithelial-mesenchymal transition (EMT) process, resulting in increased motility and invasiveness, decreased adhesion, and altered cell polarity, all of which can lead to metastasis from the primary tumor site[14]. EMT is triggered by key transcription factors such as SNAIL, and involves changes in gene expression mediated by the TGF-β, PI3K-Akt, and ERK-MAPK pathways, among others[15,16]. However, the exact role and mechanism of CALD1 in GC-related EMT are unknown. Therefore, this study aimed to investigate the relationship between CALD1 and GC based on the TCGA and GEO databases. We assessed CALD1 mRNA and protein expression in GC tissues, explored the relationship between CALD1 expression and clinicopathological features, and investigated the role and mechanism of CALD1 in GC tissues and cell lines through a combination of experimental and bioinformatics approaches.
This study involved 80 GC patients undergoing radical surgery from January to December 2022 at the Department of Surgery, The Fourth Hospital of Hebei Medical University. Postoperative confirmation revealed that all patients had gastric adenocarcinoma, with no preoperative treatments or concurrent secondary tumors. Uniformly sized tumors and adjacent non-tumor tissues were excised, placed in cryopreservation tubes, and stored at -80 °C in liquid nitrogen. Additionally, tissues from a previous cohort of 60 patients were included in immunohistochemical staining and follow-up studies. The Medical Ethics Committee of The Fourth Hospital of Hebei Medical University granted ethical approval, and all patients provided informed consent.
The human GC cell lines HGC27, NCI-N87, AGS, and MKN45, and the gastric epithelial cell line GES-1, obtained from the National Cell Resource Center of the National Biomedical Resource, were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin, in a humidified 37 °C incubator with 5% CO2. Cell growth and sterility were monitored daily, with medium changes every 1-2 d. For cell detachment for passage or subsequent experiments, 0.25% trypsin was used.
Single-cell suspensions at a density of 1 × 106 cells/mL were prepared, seeded into 6-well plates, and cultured. siRNA and overexpression plasmids were used to transfect cells using Lipofectamine 2000 according to the manufacturer's in
Paraffin-embedded GC tumor and adjacent tissue specimens were sectioned at 4 μm, deparaffinized, and underwent antigen retrieval. Immunohistochemical staining was done according to the kit instructions, and the results were eva
Cells were harvested and lysed for protein extraction, and the bicinchoninic acid method was used to quantify protein concentrations. Proteins were denatured, separated using SDS-PAGE, and transferred to PVDF membranes. Membranes were blocked and then incubated with primary and secondary antibodies before being imaged with the Odyssey dual-color infrared fluorescence scanning system. Relative protein expression was assessed by comparing grayscale values, with β-actin serving as an internal reference. This procedure was repeated three times.
Total RNA was extracted using Trizol, quantified, and reverse-transcribed into cDNA. qPCR amplification was per
Cells were seeded in 96-well plates and incubated for 48 h. CCK-8 reagent was added at different time points. After incubation, the optical density at 450 nm was measured using a microplate reader.
After seeding single-cell suspensions in 24-well plates and allowing cells to reach confluence, cell transfection was performed. Once the cells were confluent, a scratch was made, followed by washing, serum-free medium addition, and microscopic examination at 0 and 48 h post-scratch.
Single-cell suspensions were seeded into Transwell chambers in a 24-well plate. After 24 h, cells on the inner surface were removed, fixed, stained, and examined under an inverted phase-contrast microscope.
Single-cell suspensions of lentivirus-infected cells were subcutaneously injected into BALB/c nude mice. Tumor growth was tracked, and tumors were dissected for analysis at the end of the experiment.
Various bioinformatics analyses were performed, including gene set enrichment analysis, protein-protein interaction (PPI) network analysis, differential gene expression enrichment analysis, Pearson correlation analysis, and Cox proportional hazards model analysis. The goal of these analyese was to determine the effect of CALD1 expression on survival and to create a predictive nomogram.
Statistical analyses were performed using IBM SPSS statistical software version 25.0. The t-test, one-way ANOVA, and Kaplan-Meier method were used for data comparisons and survival curve generation. The data are presented as the mean ± SD.
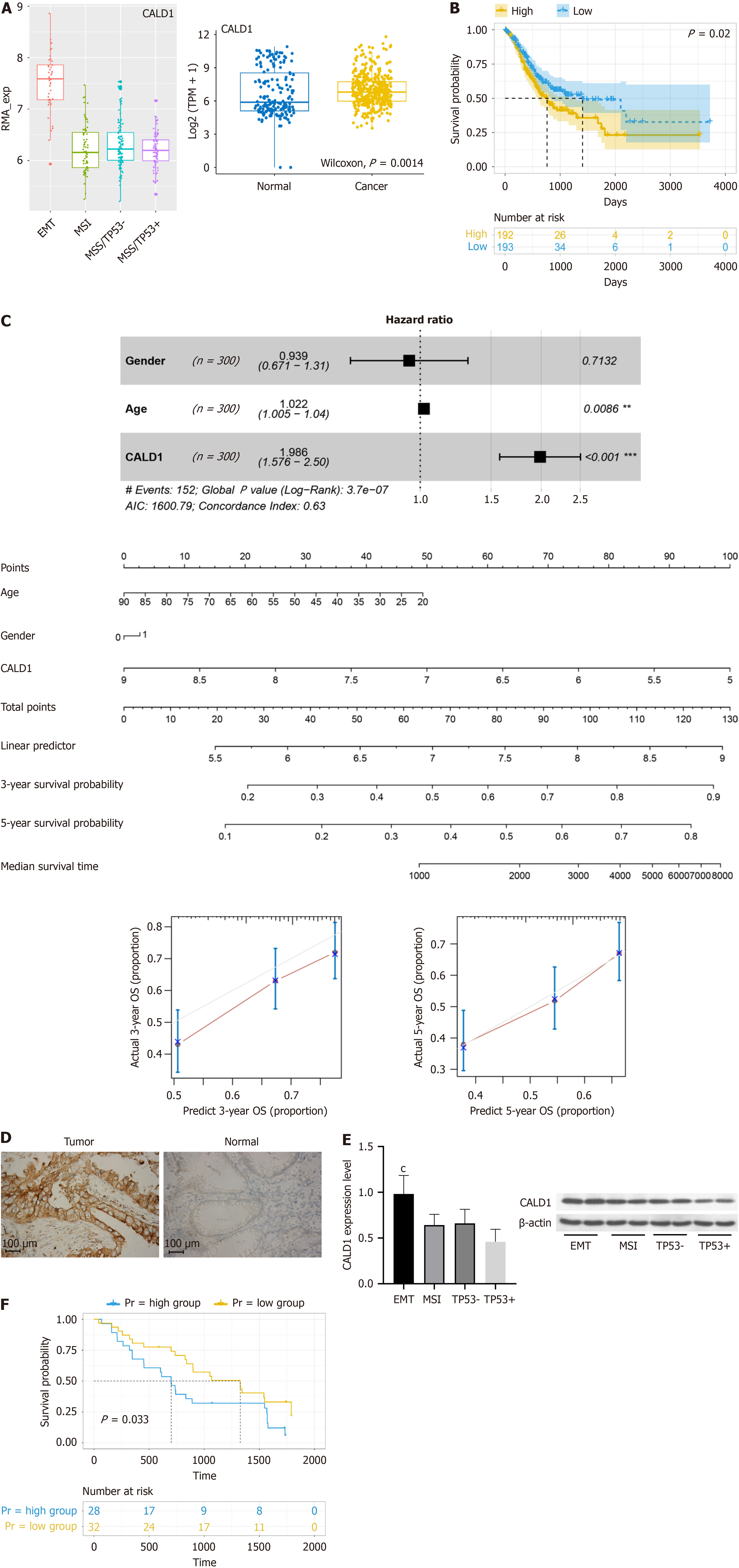
Analysis of CALD1 expression in GC revealed its higher levels in GC cases, particularly in the EMT subtype, according to the Asian Cancer Research Group (ACRG) classification (Figure 1A). Patients with higher levels of CALD1 expression had a worse prognosis than those with lower levels (Figure 1B). A prognostic model that included age, gender, and CALD1 expression, was developed and validated for accuracy using a calibration curve (Figure 1C). Immunohistochemical analysis revealed increased CALD1 protein expression in GC tissues compared to adjacent non-cancerous tissues in a subset of 60 cases (Figure 1D). Both CALD1 mRNA and protein levels were significantly higher in the EMT subtype of GC, as demonstrated by qPCR and Western blot in 20 cases (Figure 1E), and high expression was associated with a poor prognosis in another subset of 60 cases (Figure 1F).
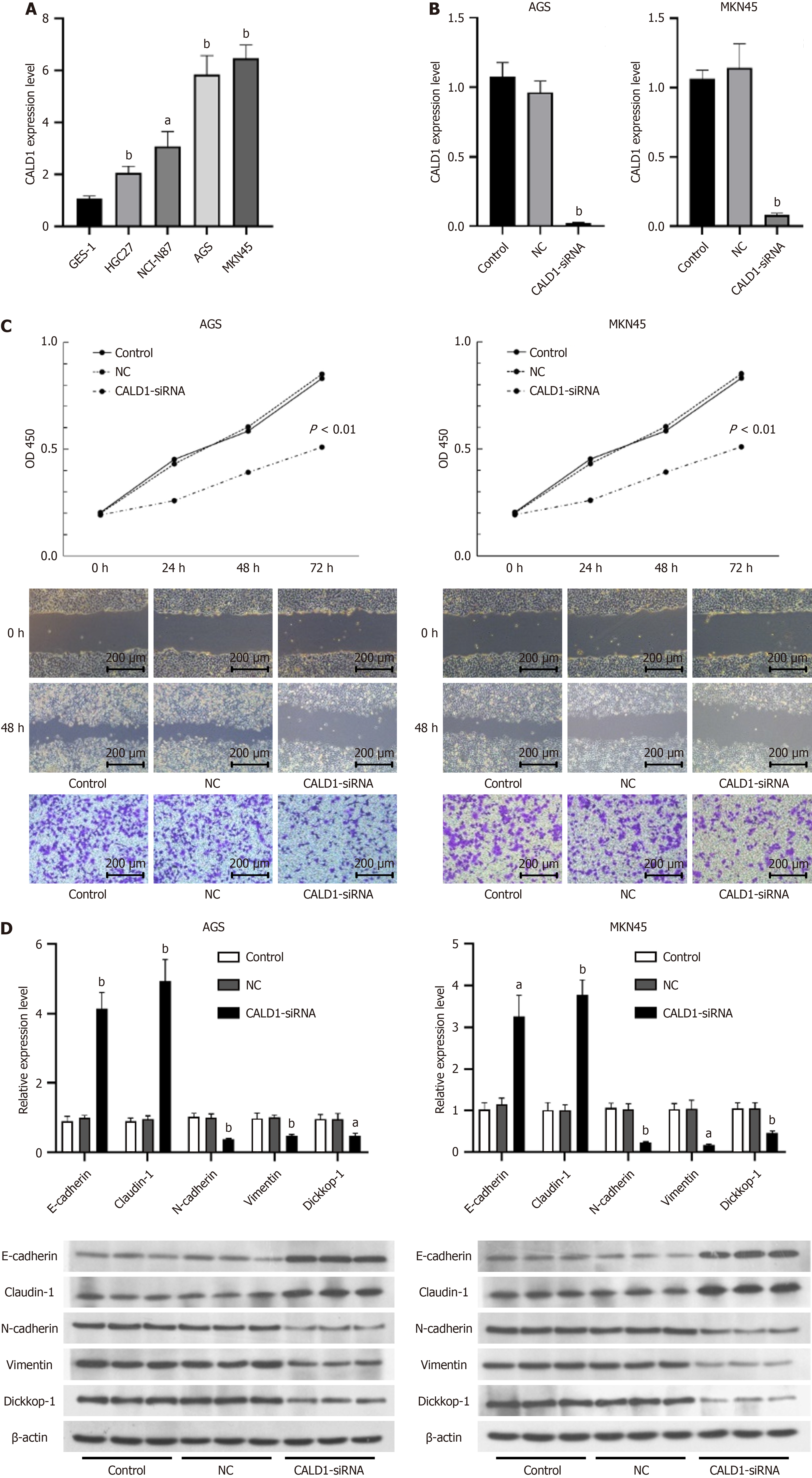
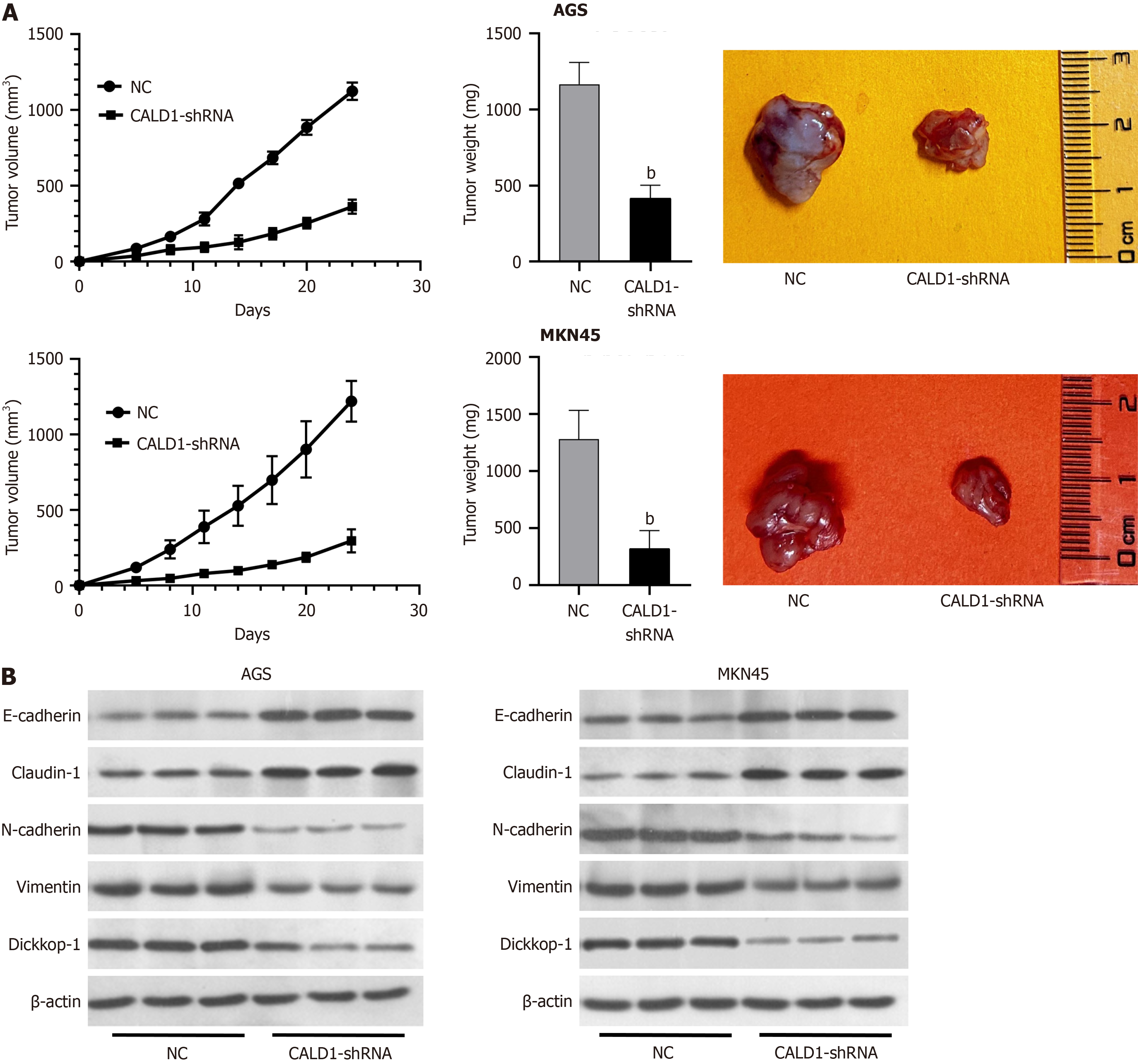
Inhibition of CALD1 reduced proliferation, invasion, and migration in GC cells. Among the various cell lines tested, AGS and MKN45 had significantly higher CALD1 expression and were chosen for further investigation (Figure 2A). CALD1-siRNA had a significant inhibitory effect in these cell lines (Figure 2B). Post-transfection, there was a significant decrease in cell activity, migration, and invasion (Figure 2C). CALD1 inhibition also increased E-cadherin and Claudin-1 expression while decreasing N-cadherin, Vimentin, and Dickkop-1 (DKK-1) mRNA and protein levels, as confirmed by RT-qPCR and Western blot (Figure 2D).
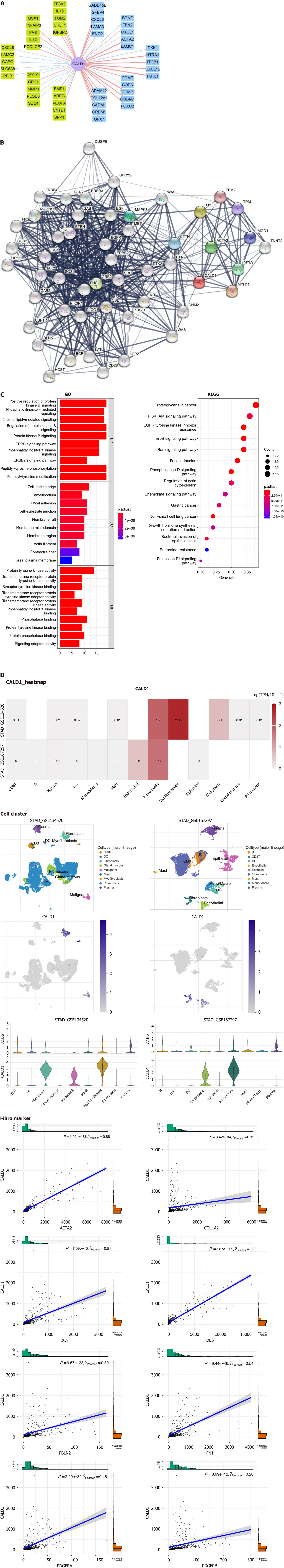
Bioinformatics analysis revealed significant associations of CALD1 with the PI3K-Akt-mTOR and EMT signaling pathways in GC samples (Figure 3A). PPI network analysis revealed multiple proteins that interact with CALD1 (Figure 3B). GO and KEGG enrichment analyses revealed that CALD1 was involved in a network of various biological processes that include protein kinase B signaling and protein tyrosine kinase activity (Figure 3C). Single-cell data showed high CALD1 expression in fibroblasts, as evidenced by bulk data analysis, which revealed a positive correlation between CALD1 and fibroblast surface molecule expression (Figure 3D).
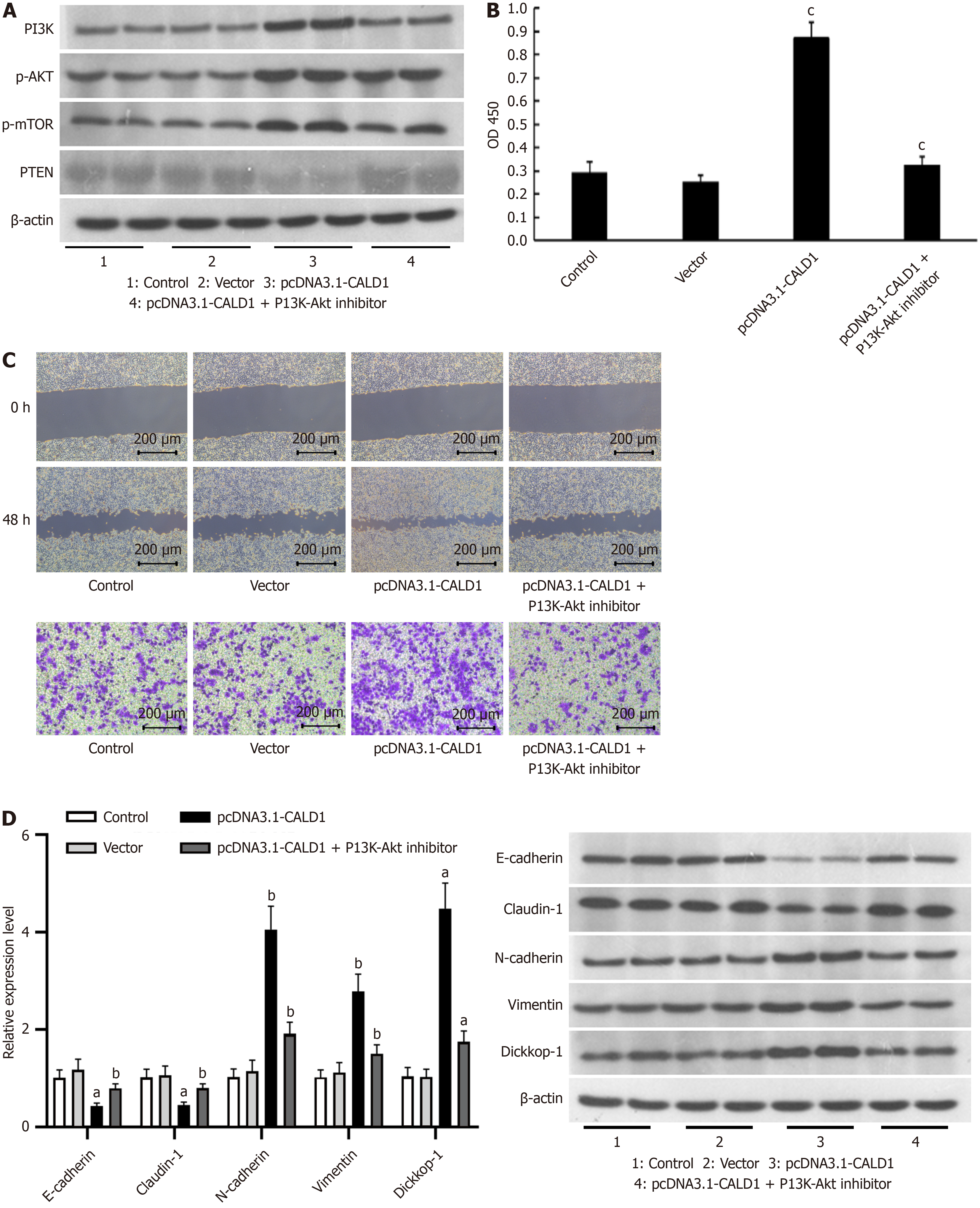
Additional studies confirmed the role of CALD1 in enhancing GC cell proliferation, invasion, and migration via the PI3K-Akt pathway. Western blot analysis revealed that CALD1 overexpression increased PI3K, p-AKT, and p-mTOR expression, while decreasing PTEN expression. However, integrating CALD1 overexpression with PI3K-Akt pathway inhibition moderated these effects (Figure 4A). The CCK-8 assay results showed that the effect of CALD1 on tumor cell activity was reduced after the addition of a PI3K-Akt inhibitor (Figure 4B). Scratch and Transwell assays revealed that CALD1 had a reduced impact on cell migration and invasion after inhibitor treatment (Figure 4C). In AGS and MKN45 cells, CALD1 overexpression altered the expression of EMT-related genes and proteins, an effect that was mitigated by the PI3K-Akt inhibitor (Figure 4D).
In an animal model, CALD1 inhibition led to reduced tumor growth in nude mice. Tumors derived from CALD1-shRNA-transfected cells were significantly lighter and smaller than those in the control group (P < 0.05), as shown by Western blot analysis, which also revealed changes in EMT-related protein expression (Figure 5A and B), thus de
GC is a complex, multifaceted disease that progresses through multiple steps and stages, with tumor invasion and metastasis playing important roles in the high mortality rates observed in advanced stages of GC[3-6]. It is critical to comprehend the underlying mechanisms of GC development. Identifying molecules integral to its initiation and pro
The ACRG divides GC into distinct molecular subtypes: MSS/EMT, MSS/TP53+, MSI/TP53+, and MSS/TP53-. Each subtype is associated with varying prognostic outcomes, with the MSS/EMT subtype being linked to the poorest prog
Our study employed bioinformatics analysis to establish that CALD1 is significantly overexpressed in GC compared to normal tissues. Further investigation, consistent with the ACRG molecular classification, revealed significantly higher expression of CALD1 in the EMT subtype of GC than in other subtypes. Futhermore, increased CALD1 expression was linked to a worse prognosis, implying a role for CALD1 in the progression of EMT-subtype GC. This suggests the role of CALD1 in the development of aggressive EMT-subtype GC. To substantiate these observations, we procured GC and adjacent normal tissue samples for bioinformatics analysis, which corroborated our initial findings. The correlation of high CALD1 expression with poorer patient outcomes implicates its significant role in GC progression.
Further validation using qPCR experiments showed increased CALD1 expression in GC cell lines compared to gastric epithelial cell lines. Functional assays, including CCK-8, scratch, and Transwell assays, revealed that siRNA-mediated CALD1 knockdown significantly reduced cell viability, migration, and invasion. This demonstrates a strong link between CALD1 and GC cell activity, invasion, and metastasis, emphasizing its critical role in the initiation and progression of GC.
It is well-established that the progression of GC is closely associated with the EMT process in GC cells. EMT, which is triggered by various genes and pathways, allows GC cells to switch from an epithelial to a more aggressive mesenchymal phenotype, thereby facilitating tumor progression[18]. This process involves the downregulation of epithelial cell markers and the upregulation of mesenchymal cell markers, both of which are important factors promoting tumor metastasis[19,20].
In our investigation, CALD1 expression inhibition in AGS and MKN45 cells, specifically, resulted in increased ex
CALD1, which is found at 7q33, encodes the Caldesmon protein, which exists in two isoforms with different molecular weights and cellular origins. Caldesmon has diverse roles in cellular processes such as migration, invasion, and proliferation by regulating actin cytoskeleton remodeling[8,9,21,22]. Previous research has linked CALD1 to tumor angio
Using bioinformatics analysis, the PPI network of CALD1 was established and GO and KEGG data analysis was performed, revealing CALD1 enrichment in the PI3K-Akt signaling pathway, which is known for its significant influence on GC development. Previous research has shown that the PI3K-Akt signaling pathway is significantly implicated in the onset and progression of GC. This pathway contributes to the advancement of GC by inhibiting apoptosis, metastasis, EMT, and angiogenesis[26-31]. To verify CALD1's involvement in this pathway, we chose HGC27 cells, which exhibit low CALD1 expression, for overexpression experiments. These experiments were conducted both independently and in combination with PI3K-Akt pathway inhibition. Our results indicated that CALD1 upregulation enhanced expression of PI3K-Akt pathway members, PI3K, p-AKT, and p-mTOR, reduced PTEN expression, and promoted cell viability, migra
However, our study has limitations. Although the study suggests that CALD1 is involved in EMT and the PI3K-Akt pathway, the exact molecular mechanisms remain unknown, and its role in the tumor microenvironment requires further exploration. Further research needs more patients to be included to verify the conclusions obtained in this study.
In summary, our findings show that CALD1 is upregulated in GC tissues and cell lines, and that high CALD1 expression is associated with a poor prognosis in GC patients. Alterations in CALD1 expression result in changes in cell activity, invasion, migration, and the expression of EMT-related genes and proteins. The PI3K-Akt signaling pathway was dis
CALD1 is known for its abnormal expression in various malignant tumors, and this expression is linked to tumor growth and immune system infiltration. However, the specific functions and underlying mechanisms of CALD1 in the epithelial-mesenchymal transition (EMT) process in gastric cancer (GC) remain unclear.
The motivation behind this research is to explore and better comprehend how CALD1 functions in the context of GC.
This study aimed to investigate the role and mechanism of CALD1 in GC progression, invasion, and migration.
In this study, the relationship between CALD1 and GC, as well as the possible network regulatory mechanisms of CA
The bioinformatics analysis revealed that CALD1 expression was significantly elevated in GC tissues, particularly in the EMT type. Additionally, the CALD1 gene was found to be associated with the PI3K-Akt signaling pathway and other EMT components. Compared to gastric epithelial cell lines, GC cell lines showed higher levels of CALD1 expression. Suppressing CALD1 and the PI3K-Akt pathway resulted in reduced viability, invasion, and migration of GC cells. These experimental findings elucidate the role of CALD1 and the PI3K-Akt pathway in GC, laying a foundation for further molecular mechanism studies of this disease.
By influencing the PI3K-Akt pathway, CALD1 plays a pivotal role in advancing the EMT in GC.
This study, through bioinformatics analysis, as well as in vivo and in vitro experiments, has confirmed that CALD1 promotes the EMT in GC by affecting the PI3K-Akt signaling pathways. These findings underscore its significance in tumor progression and potential as a target for future therapeutic approaches. However, the investigation into the re
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68647] [Article Influence: 13729.4] [Reference Citation Analysis (201)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56682] [Article Influence: 7085.3] [Reference Citation Analysis (135)] |
| 3. | Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016;388:2654-2664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1282] [Cited by in RCA: 1505] [Article Influence: 150.5] [Reference Citation Analysis (1)] |
| 4. | Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ, Estève J, Ogunbiyi OJ, Azevedo E Silva G, Chen WQ, Eser S, Engholm G, Stiller CA, Monnereau A, Woods RR, Visser O, Lim GH, Aitken J, Weir HK, Coleman MP; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2711] [Cited by in RCA: 3702] [Article Influence: 462.8] [Reference Citation Analysis (1)] |
| 5. | Zhang L, Kang W, Lu X, Ma S, Dong L, Zou B. LncRNA CASC11 promoted gastric cancer cell proliferation, migration and invasion in vitro by regulating cell cycle pathway. Cell Cycle. 2018;17:1886-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 111] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 6. | Chen W, Huang S, Fan X, Gao Y. Human Epidermal Growth Factor Receptor 2 Targeting Specific T Cells Immunotherapy for Gastric Cancer. J Mod Med Oncol. 3:7. [DOI] [Full Text] |
| 7. | Meola J, Hidalgo Gdos S, Silva JC, Silva LE, Paz CC, Ferriani RA. Caldesmon: new insights for diagnosing endometriosis. Biol Reprod. 2013;88:122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Liu Y, Xie S, Zhu K, Guan X, Guo L, Lu R. CALD1 is a prognostic biomarker and correlated with immune infiltrates in gastric cancers. Heliyon. 2021;7:e07257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Li C, Yang F, Wang R, Li W, Maskey N, Zhang W, Guo Y, Liu S, Wang H, Yao X. CALD1 promotes the expression of PD-L1 in bladder cancer via the JAK/STAT signaling pathway. Ann Transl Med. 2021;9:1441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Zheng H, Bai Y, Wang J, Chen S, Zhang J, Zhu J, Liu Y, Wang X. Weighted Gene Co-expression Network Analysis Identifies CALD1 as a Biomarker Related to M2 Macrophages Infiltration in Stage III and IV Mismatch Repair-Proficient Colorectal Carcinoma. Front Mol Biosci. 2021;8:649363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Cheng Q, Tang A, Wang Z, Fang N, Zhang Z, Zhang L, Li C, Zeng Y. CALD1 Modulates Gliomas Progression via Facilitating Tumor Angiogenesis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Du Y, Jiang X, Wang B, Cao J, Wang Y, Yu J, Wang X, Liu H. The cancer-associated fibroblasts related gene CALD1 is a prognostic biomarker and correlated with immune infiltration in bladder cancer. Cancer Cell Int. 2021;21:283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 13. | Alnuaimi AR, Nair VA, Malhab LJB, Abu-Gharbieh E, Ranade AV, Pintus G, Hamad M, Busch H, Kirfel J, Hamoudi R, Abdel-Rahman WM. Emerging role of caldesmon in cancer: A potential biomarker for colorectal cancer and other cancers. World J Gastrointest Oncol. 2022;14:1637-1653. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Lan Q, Tan X, He P, Li W, Tian S, Dong W. TRIM11 Promotes Proliferation, Migration, Invasion and EMT of Gastric Cancer by Activating β-Catenin Signaling. Onco Targets Ther. 2021;14:1429-1440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 15. | Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2519] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 16. | Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4715] [Cited by in RCA: 6492] [Article Influence: 541.0] [Reference Citation Analysis (0)] |
| 17. | Yoon JY, Sy K, Brezden-Masley C, Streutker CJ. Histo- and immunohistochemistry-based estimation of the TCGA and ACRG molecular subtypes for gastric carcinoma and their prognostic significance: A single-institution study. PLoS One. 2019;14:e0224812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Williams ED, Gao D, Redfern A, Thompson EW. Controversies around epithelial-mesenchymal plasticity in cancer metastasis. Nat Rev Cancer. 2019;19:716-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 320] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 19. | Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2456] [Cited by in RCA: 2600] [Article Influence: 152.9] [Reference Citation Analysis (0)] |
| 20. | Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4877] [Cited by in RCA: 5161] [Article Influence: 215.0] [Reference Citation Analysis (6)] |
| 21. | Mayanagi T, Sobue K. Diversification of caldesmon-linked actin cytoskeleton in cell motility. Cell Adh Migr. 2011;5:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Thorsen K, Sørensen KD, Brems-Eskildsen AS, Modin C, Gaustadnes M, Hein AM, Kruhøffer M, Laurberg S, Borre M, Wang K, Brunak S, Krainer AR, Tørring N, Dyrskjøt L, Andersen CL, Orntoft TF. Alternative splicing in colon, bladder, and prostate cancer identified by exon array analysis. Mol Cell Proteomics. 2008;7:1214-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 180] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 23. | Al Saleh S, Al Mulla F, Luqmani YA. Estrogen receptor silencing induces epithelial to mesenchymal transition in human breast cancer cells. PLoS One. 2011;6:e20610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 24. | Nalluri SM, O'Connor JW, Virgi GA, Stewart SE, Ye D, Gomez EW. TGFβ1-induced expression of caldesmon mediates epithelial-mesenchymal transition. Cytoskeleton (Hoboken). 2018;75:201-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Zhang L, Liu J, Wang X, Li Z, Zhang X, Cao P, She X, Dai Q, Tang J, Liu Z. Upregulation of cytoskeleton protein and extracellular matrix protein induced by stromal-derived nitric oxide promotes lung cancer invasion and metastasis. Curr Mol Med. 2014;14:762-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Baghery Saghchy Khorasani A, Pourbagheri-Sigaroodi A, Pirsalehi A, Safaroghli-Azar A, Zali MR, Bashash D. The PI3K/Akt/mTOR signaling pathway in gastric cancer; from oncogenic variations to the possibilities for pharmacologic interventions. Eur J Pharmacol. 2021;898:173983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 27. | Wang YY, Zhou YQ, Xie JX, Zhang X, Wang SC, Li Q, Hu LP, Jiang SH, Yi SQ, Xu J, Cao H, Zhao EH, Li J. MAOA suppresses the growth of gastric cancer by interacting with NDRG1 and regulating the Warburg effect through the PI3K/AKT/mTOR pathway. Cell Oncol (Dordr). 2023;46:1429-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 28. | Wang Z, Lv J, Li X, Lin Q. The flavonoid Astragalin shows anti-tumor activity and inhibits PI3K/AKT signaling in gastric cancer. Chem Biol Drug Des. 2021;98:779-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Chen W, Zhang Y, Gu X, Qian P, Liu W, Shu P. Qi Ling decoction reduces gastric cancer cell metastasis by inhibiting MMP-9 through the PI3K/Akt signaling pathway. Am J Transl Res. 2021;13:4591-4602. [PubMed] |
| 30. | Dai J, Liu D, Chen L, Sun L. Effect of Ag-1031 on apoptosis in gastric cancer AGS cells and its effects on the PI3K/AKT/mTOR signaling pathway. Biotechnol Lett. 2020;42:2447-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Sun D, Zhang M, Wei M, Wang Z, Qiao W, Liu P, Zhong X, Liang Y, Chen Y, Huang Y, Yu W. Ox-LDL-mediated ILF3 overexpression in gastric cancer progression by activating the PI3K/AKT/mTOR signaling pathway. Aging (Albany NY). 2022;14:3887-3909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 32. | Zhao Z, Zhu Y. FAP, CD10, and GPR77-labeled CAFs cause neoadjuvant chemotherapy resistance by inducing EMT and CSC in gastric cancer. BMC Cancer. 2023;23:507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (1)] |
| 33. | Zhang C, Sun D, Li C, Liu Y, Zhou Y, Zhang J. Development of cancer-associated fibroblasts subtype and prognostic model in gastric cancer and the landscape of tumor microenvironment. Int J Biochem Cell Biol. 2022;152:106309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Assumpcao PP, Brazil; Petrillo A, Italy S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Xu ZH