INTRODUCTION
Globally, colorectal cancer (CRC) ranks third in prevalence among cancers, constituting about 10% of all newly diagnosed cancer cases. In 2022, the global incidence of CRC was estimated at 1.9 million new cases, with mortality rates reaching as high as 900000[1]. As age advances, the risk of developing CRC grows, with individuals aged 65 and older exhibiting substantially higher incidence rates compared to other age groups. However, in recent years, there has also been an observed rise in CRC incidence among younger populations[2]. Known risk factors for CRC include genetics, dietary habits (such as high-fat, low-fiber diets), obesity, smoking, alcohol consumption, and lack of physical activity[3]. Currently, the foremost therapy for early-stage CRC is surgical resection. It is worth noting that, in addition to surgery, chemotherapy and radiotherapy are often necessary for advanced CRC. Nevertheless, early-stage CRC typically presents with few noticeable symptoms, making it easily overlooked. Hence, the majority of CRC patients also require radiotherapy[4]. More terribly, a portion of CRC patients exhibit resistance to radiation therapy, leading to poor treatment outcomes. Approximately 20% of patients with locally advanced rectal cancer are inherently insensitive to radiotherapy. As for patients with metastatic CRC, the proportion of insensitivity may be even higher[5]. Studies indicate that the reasons for CRC's resistance to radiation therapy include genetic mutations and enhanced DNA repair capabilities. Mutations in genes such as KRAS and BRAF may render tumor cells insensitive to radiation[6]. Furthermore, some tumor cells possess highly efficient DNA damage repair capabilities, which can diminish the cytotoxic effects of radiation[7]. To overcome the challenge of radiation insensitivity, researchers have proposed various strategies, including the use of combined chemotherapy and screening for radiosensitizers, etc.[8]. Among these strategies, the screening of radiosensitizers has shown tremendous potential.
Evidence from research shows that spermine synthase (SMS) has a pivotal role in polyamine metabolism. Polyamine compounds such as spermine not only participate in the regulation of growth and development in organisms but also correlate with physiological processes like cell apoptosis and proliferation[9]. Spermine can also protect DNA from damage and plays a role in antioxidant stress and ion channel regulation[10]. It is known that SMS is a group of key enzymes associated with the synthesis of polyamines such as spermine, spermidine, and putrescine in organisms[11,12]. Polyamines and spermine are essential for maintaining cell growth and proliferation. Additionally, polyamines act as important scavengers of intracellular free radicals, alleviating oxidative stress in tumor cells[13]. In various tumor cells, the expression levels of SMS are significantly elevated, thereby promoting spermine synthesis. Elevated levels of spermine can accelerate the tumor cell proliferation, and increased expression of SMS can promote the proliferation of CRC[14]. Recent studies have revealed a correlation between the expression levels of SMS and cancer development. Fahrmann et al[15] discovered a correlation between plasma spermidine levels and distant metastasis risk in patients with triple-negative breast cancer. Velenosi et al[16] proposed that diacetylspermine could serve as a biomarker for predicting the development and progression of triple-negative breast cancer. Additionally, research indicates a down-regulation in spermine levels in prostate cancer tissue, and such a down-regulation is considered an indicator of the transition from normal prostate tissue to malignant tissue[17]. Other studies have found that SMS is upregulated in pancreatic cancer, promoting cancer cell proliferation and migration, and is associated with poorer overall survival[18]. Snezhkina et al[19] identified dysregulated spermine metabolism in CRC. Some studies propose that during the development of CRC, SMS collaborates with the MYC protein (an oncogene associated with cell proliferation) to suppress the expression of the Bim gene[14]. This collaboration is believed to sustain the survival of CRC cells and enhance their malignant behavior. Other studies have found that SMS is overexpressed in CRC, and targeting SMS inhibition induces lipid metabolism reprogramming and ferroptosis, significantly inhibiting the growth of CRC cells[20]. Notably, some research has demonstrated that inhibiting spermine biosynthesis can render cells more sensitive to radiation damage[21]. Mivechi et al[22] in 1986 claimed that inhibitors of ornithine decarboxylase such as alpha-difluoromethylornithine could increase radiation-induced inter-strand DNA crosslink damage, thereby enhancing the radiosensitivity of cancer cells. The aforementioned findings emphasize the importance of SMS in the occurrence and progression of CRC, suggesting its potential role as a key regulator of cellular sensitivity to radiotherapy. Therefore, this study aimed to clarify the link between SMS and the radiosensitivity of CRC cells, elucidating its mechanisms and providing insights for improving the treatment of CRC.
MATERIALS AND METHODS
Cell culture and grouping
Normal colonic epithelial cells FHC (CRL-1831), CRC cell line HCT116 (CRL-247), SW62, SW480, HT-29 and LoVo cells were both purchased from WHELAB. The culture of FHC cells was performed in a DMEM/F12 medium comprising 10% fetal bovine serum, 100 μg/mL streptomycin, and 100 U/mL penicillin; HCT116, SW62, SW480, HT-29 and LoVo cells culture were finished in a RPMI1640 medium under the same conditions. Cells were maintained in a humidified environment at 37 °C with 50 mL/L CO2. When cells reached 80%-90% confluence, they were rinsed twice via phosphate-buffered saline (PBS), detached through 2.5 g/L trypsin-EDTA solution, and passaged. Cell grouping was initiated at the third passage. HCT116 cells were transfected with SMS shRNA (sh-SMS) and negative control shRNA (shNC) using Lipofectamine 3000 (L3000001, ThermoFisher, USA). Over-expression of SMS was achieved by transfecting HCT116 cells with SMS over-expression vector (OE-SMS) or negative control vector (OE-NC). HCT116 cells transfected with sh-SMS and shNC were subjected to a 4 Gy X-ray dose. Subsequent biological assays were performed after completion of the radiation treatment. The sequence for sh-SMS was 5’-CCGGCGCTTTAAAGAACAGCCTTTACTCGAGTAAAGGCTGT TCTTTAAAGC GTTTTT-3’, and the sequence for shNC was 5’-CCGGCAACAAGATGAAGAGCACCAACTC GAGTTGGTGCTCTTCATCTTGTTG-3’.
MTT assay
In 96-well plates, HCT116 cells were plated at a density of 5000 cells per well, with three replicate wells per condition. The plates were then incubated in a humidified incubator at 37 °C with 50 mL/L CO2 until the cells adhered completely. Subsequently, the cells were transfected or subjected to radiation treatment. Following that, the plates were returned to the incubator. At 24, 48, and 72 hours post-treatment, each well received 20 μL of MTT solution (5 mg/mL, ml057897, mlbio, China), and the cells underwent another four hours of incubation. Following the removal of the culture medium, 150 μL of dimethyl sulfoxide was provided for dissolving the MTT crystals. Ultimately, the absorbance (OD) of each well was measured using a microplate reader (Varioskan LUX, ThermoFisher, United States) at a wavelength of 490 nm.
Colony formation assay
HCT116 cells in the logarithmic growth phase were collected for transfection or radiation treatment. Following two washes utilizing PBS, cells were digested via 2.5 g/L trypsin-EDTA solution and subsequently cleaned twice with PBS to terminate digestion. Later, the cell samples were suspended in a complete medium containing 3-4 g/L agarose to create single-cell suspensions. Next, 2 mL of the cell suspension, with a density of 1000 cells per well, was supplemented into each well of a 6-well plate. The plate was then cultured in a humidified atmosphere at 37 °C with 50 mL/L CO2 for 2-3 weeks. Following culture, the cells were gently washed via PBS, stained utilizing 5 g/L crystal violet for 30 minutes, and then washed again with PBS. Lastly, cell colonies with a diameter ≥ 50 μm were observed and computed under an inverted microscope (BDS400, OPTEC, China).
Flow cytometry
HCT116 cells were cultured in a humidified atmosphere at 37 °C with 50 mL/L CO2, followed by transfection or radiation treatment. After treatment, cells were detached using trypsin and collected into centrifuge tubes. They were then stained using fluorescein isothiocyanate (FITC)-conjugated Annexin V (Annexin V-FITC) and propidium iodide (PI) from the Annexin V Apoptosis Detection Kit (E606336, Sangon, China). Annexin V-FITC bound to exposed phosphatidylserine on the outer membrane of early apoptotic cells, while PI entered late apoptotic/necrotic cells with compromised membranes. Finally, a flow cytometer (CytoFLEX, Beckman, Germany) was utilized to analyze the cells stained to detect and analyze apoptotic cells.
Comet assay
The comet assay, utilizing a Comet Assay Kit (C2041M, Beyotime, China), was employed to assess the level of DNA damage in HCT116 cells following transfection or radiation treatment. Cells were detached using trypsin and collected into centrifuge tubes. Low-melting agarose was added, and the mixture was spread onto slides to form a gel. A high-salt solution was applied to remove cell membranes and most proteins, followed by alkaline buffer to unwind DNA strands. Under an electric field (voltage 25 V), damaged DNA fragments migrated towards the anode, forming a "comet" shape. Subsequently, DNA was stained using PI (Ex/Em = 535/617 nm), and comet tail DNA level and comet tail length were observed under a fluorescence microscope (BX43, Olympus, Japan).
Immunofluorescence
The treated HCT116 cells were washed twice through PBS and fixed via 40 g/L paraformaldehyde for 30 minutes to preserve cell structure and antigenicity. Upon two PBS washes, the cells were permeabilized via 4 g/L Triton X-100 for 30 minutes, given two times of washes via PBS again, and then incubated with 50 g/L bovine serum albumin to block non-specific binding sites. Afterwards, the cells were incubated overnight at a low temperature with Alexa Fluor® 488 Rabbit monoclonal anti-LC3 antibody (1:500, Ex/Em = 495 nm/519 nm, ab225382, Abcam, United Kingdom). After washing twice by virtue of PBS, cell samples were stained with a nuclear dye (DAPI, Ex/Em = 364 nm/454 nm, C1005, Beyotime, China) for 5 minutes and washed twice with PBS. Eventually, LC3 puncta were observed under a fluorescence microscope (BX43, Olympus, Japan), and the proportion of LC3-positive cells was analyzed.
Western blot
FHC, HCT116, SW62, SW480, HT-29 and LoVo cells, along with HCT116 cells transfected or treated with radiation, were lysed using RIPA buffer. The protein concentration was determined utilizing a bicinchoninic acid assay kit. Upon mixing with SDS-PAGE loading buffer, the samples were denatured at 95 °C for 5 minutes. Denatured protein samples were loaded onto SDS-PAGE gels and isolated by electrophoresis. Following this, the samples were shifted from the gel to the polyvinylidene fluoride membrane by electroblotting. Then, 50 g/L bovine serum albumin solution was offered to block the membrane for 1 hour. Later, the membrane was subject to an overnight incubation with primary antibodies specific to the target proteins, including SMS (1:1000, ab151547, Abcam, United Kingdom), γH2AX (1:1000, ab124781, Abcam, United Kingdom), p-γH2AX (1:1000, ab81299, Abcam, United Kingdom), ATR (1:1000, ab2905, Abcam, United Kingdom), p-ATR (1:1000, ab289363, Abcam, United Kingdom), P53 (1:1000, ab26, Abcam, United Kingdom), LC3-I (1:1000, ab52628, Abcam, United Kingdom), LC3-II (1:1000, ab63817, Abcam, United Kingdom), p62 (1:1000, ab109012, Abcam, United Kingdom), Beclin 1 (1:1000, ab207612, Abcam, United Kingdom), mammalian target of rapamycin (mTOR, 1:1000, ab134903, Abcam, United Kingdom), p-mTOR (1:1000, ab109268, Abcam, United Kingdom), S6K1 (1:1000, ab32359, Abcam, United Kingdom), p-S6K1 (1:1000, ab59208, Abcam, United Kingdom), S6 (1:1000, ab250753, Abcam, United Kingdom), p-S6 (1:1000, ab2571, Abcam, United Kingdom), β-actin (1:1000, ab8227, Abcam, United Kingdom). Subsequently, the membrane was provided an incubation with HRP-conjugated secondary antibodies, including HRP Anti-Rabbit IgG antibody (1:5000, ab288151, Abcam, United Kingdom) and HRP Goat Anti-Mouse IgG (1:5000, ab6708, Abcam, United Kingdom). Further, a chemiluminescent substrate solution (P0211, Beyotime, China) was offered to initiate enzyme-linked secondary antibody reaction. The luminescence of the proteins was scanned using a ChemiDoc™ MP Imaging System (BioRed, United States).
Statistical analysis
The experiments were conducted with three independent replicates. Independent sample t-tests (between two groups) or one-way analysis of variance (among multiple groups) were performed using SPSS version 25. Data were displayed as mean ± SEM, with a significance threshold of P < 0.05. In addition, graphs were generated using GraphPad Prism 8.
RESULTS
Elevated SMS expression promotes proliferation and reduces apoptosis in CRC cells
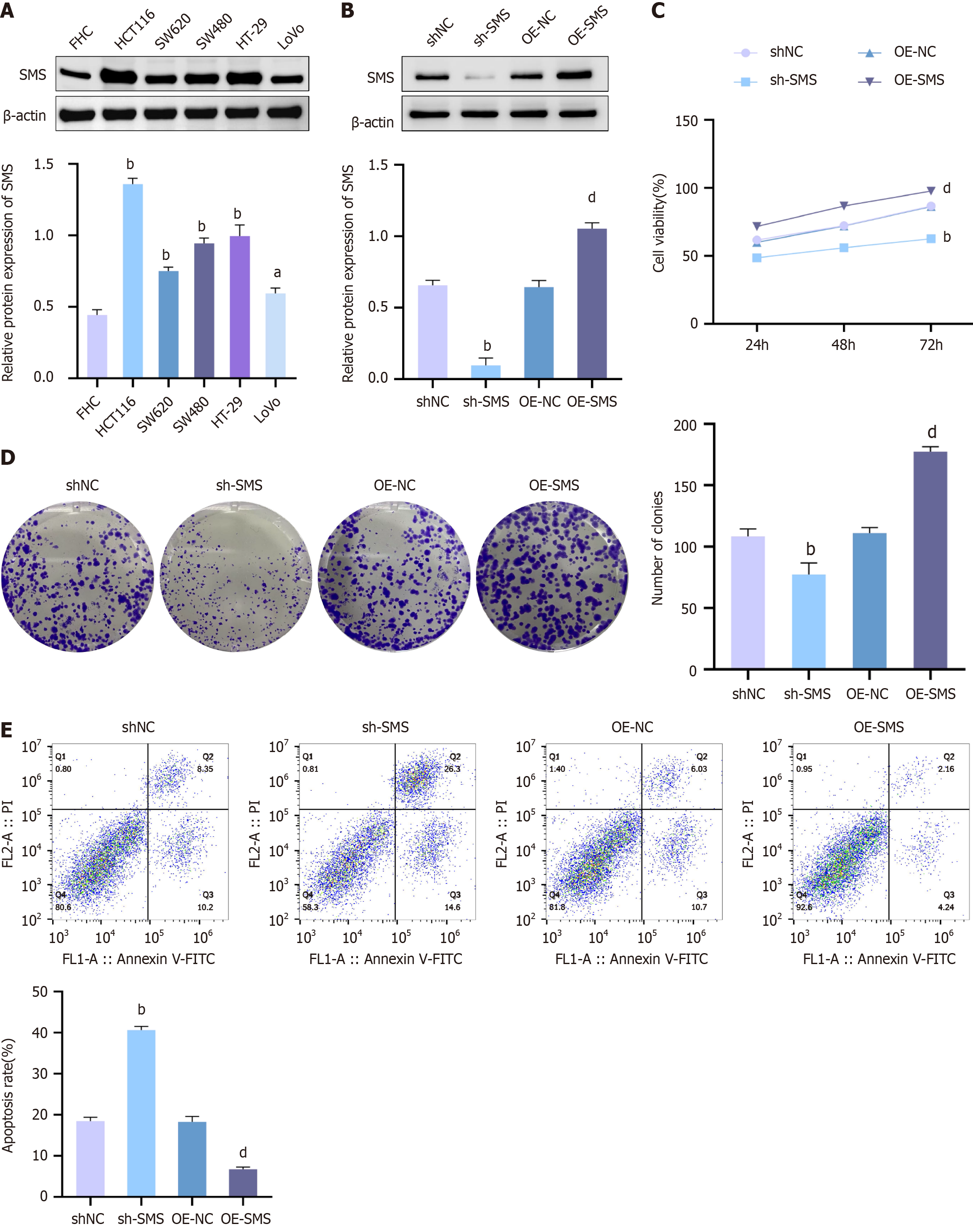
Initially, a detection was performed on the expression of SMS in normal colonic epithelial cells (FHC) and the CRC cell line HCT116, SW62, SW480, HT-29 and LoVo. The detection results disclosed that the protein expression levels of SMS were notable elevated in HCT116, SW62, SW480, HT-29 and LoVo cells in contrast to FHC cells. Additionally, SMS expression in HCT116 cells was higher than in SW62, SW480, HT-29, and LoVo cells (Figure 1A). For observing the impact of SMS expression levels on cell radiosensitivity, we established HCT116 cell groups with SMS knockdown or over-expression (Figure 1B). Subsequently, we observed the influence of diverse SMS expression levels on cell viability, colony formation ability, and apoptosis levels. The observational outcomes showed that relative to the shNC group, the viability and colony formation ability of HCT116 cells in the sh-SMS group were remarkably cut down, but the apoptosis level was increased. In contrast, compared to the OE-NC group, the viability and colony formation ability of HCT116 cells in the OE-SMS group were increased, while the apoptosis level was significantly decreased (Figure 1C-E). These results indicated that increased expression of SMS in HCT116 promoted cell proliferation and inhibited apoptosis.
Figure 1 Up-regulation of spermine synthase in colorectal cancer cells HCT116 promotes cell proliferation and inhibits apoptosis.
A and B: Western blot analysis for spermine synthase (SMS) protein expression levels in FHC, HCT116, SW62, SW480, HT-29 and LoVo cells groups (A), as well as in negative control shRNA (shNC), SMS shRNA (sh-SMS), negative control over-expression vector (OE-NC), and OE-SMS HCT116 cell groups (B); C: MTT assay to detect the viability of cells in shNC, sh-SMS, OE-NC, and OE-SMS HCT116 groups; D: Colony formation assay to assess the colony formation ability of cells in shNC, sh-SMS, OE-NC, and OE-SMS HCT116 groups; E: Flow cytometry analysis of cell apoptosis levels in shNC, sh-SMS, OE-NC, and OE-SMS HCT116 groups. aP< 0.05, bP < 0.01 vs FHC, shNC; dP < 0.01 vs OE-NC; SMS: Spermine synthase; sh-SMS: Spermine synthase shRNA; shNC: Negative control shRNA; OE-SMS: Spermine synthase over-expression vector; OE-NC: Negative control over-expression vector.
Down-regulation of SMS enhances the radiosensitivity of HCT116 cells, inhibits cell proliferation, and promotes apoptosis
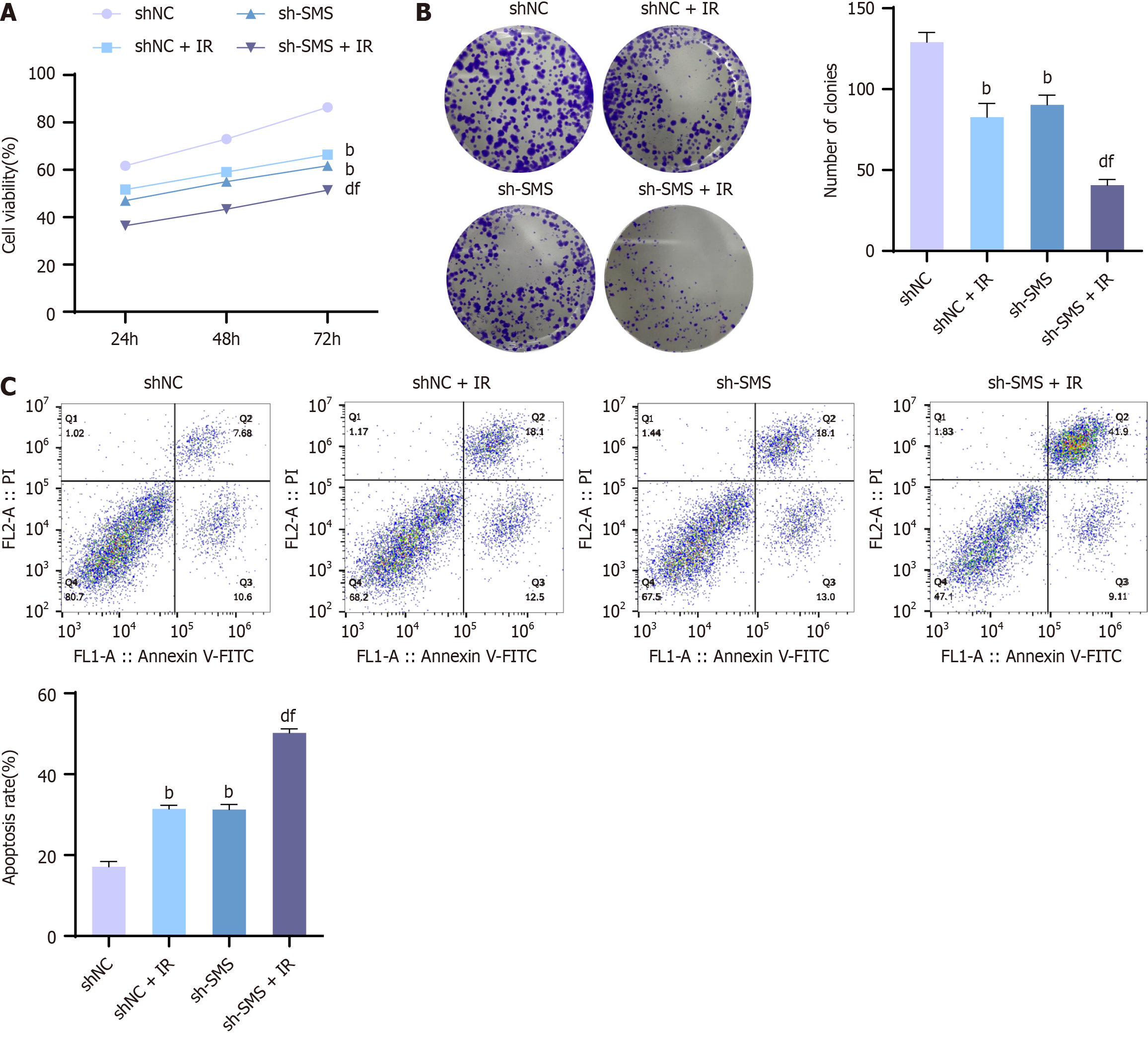
Although it is known that the activity of SMS can affect the sensitivity of CRC cells to radiotherapy, increased activity of SMS can lead to increased resistance of CRC cells to radiation. Additionally, SMS is involved in various reactions, but the mechanism of action of SMS on radiotherapy sensitivity is not yet clear. Therefore, we observed the effect of down-regulating SMS expression on cellular radiosensitivity. After altering the expression levels of SMS, cells were treated utilizing a dose of 4 Gy X-rays, and cell colony formation ability, viability, and apoptosis levels were assessed. The assessment outcomes exhibited that X-rays reduced cell viability and colony formation ability but encouraged apoptosis. Briefly, compared to the shNC + Ionizing radiation (IR) group, the viability and colony formation ability of HCT116 cells in the sh-SMS + IR group were significantly reduced, while the apoptosis levels were increased (Figure 2). Thus, down-regulation of SMS enhanced the radiosensitivity of HCT116 cells, inhibited cell proliferation, and promoted apoptosis.
Figure 2 Down-regulation of spermine synthase enhances the radiosensitivity of HCT116 cells, inhibits cell proliferation, and promotes apoptosis.
A: MTT assay to detect the viability of HCT116 cells in the negative control shRNA (shNC) group, shNC + IR group, spermine synthase shRNA (sh-SMS) group, and sh-SMS + IR group; B: Colony formation assay to assess the colony formation ability of HCT116 cells in the shNC group, shNC + IR group, sh-SMS group, and sh-SMS + IR group; C: Flow cytometry analysis to measure the apoptosis levels of HCT116 cells in the shNC group, shNC + IR group, sh-SMS group, and sh-SMS + IR group. bP < 0,01 vs shNC, dP < 0.01 vs shNC + IR, fP < 0.01 vs sh-SMS; SMS: Spermine synthase; sh-SMS: Spermine synthase shRNA; shNC: Negative control shRNA; OE-SMS: Spermine synthase over-expression vector; OE-NC: Negative control over-expression vector.
Knocking down SMS enhances the radiosensitivity of HCT116 cells by promoting DNA damage
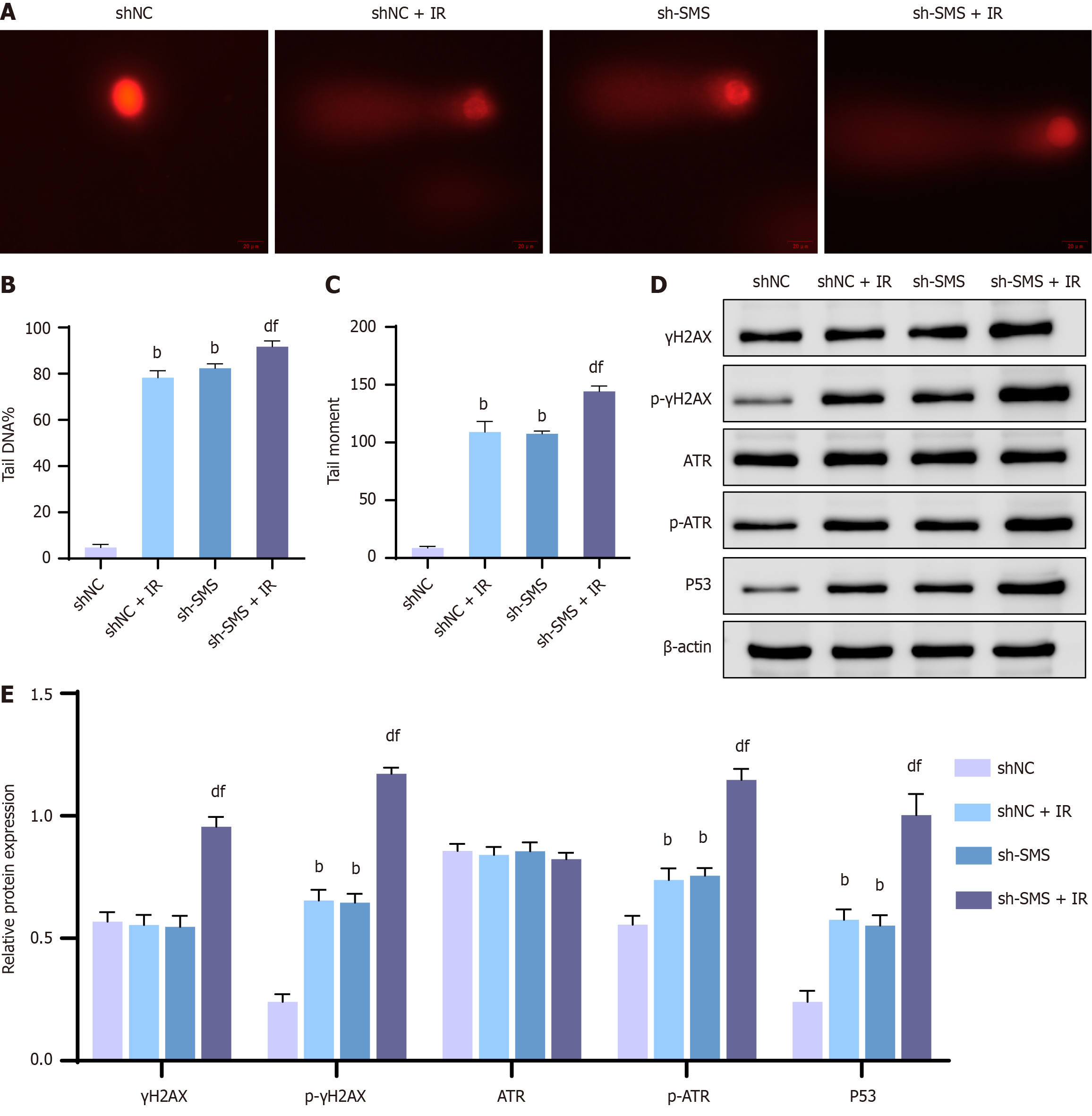
Research has already indicated that knocking down SMS leads to DNA damage and impedes DNA repair processes[23,24]. To examine the relationship between SMS and radiation-induced DNA damage in cancer cells, HCT116 cells were treated via a dose of 4 Gy X-rays following SMS knockdown. The comet assay was employed to determine the extent of DNA damage in HCT116 cells, and the western blot assay was recruited to analyze the expression of DNA damage-related proteins (γH2AX, p-γH2AX, ATR, p-ATR, P53). Our comet assay results revealed an increase in DNA damage levels in HCT116 cells following down-regulation of SMS expression, including notable elevations in comet tail DNA levels and comet tail moments. Compared to the shNC + IR or sh-SMS groups, the sh-SMS + IR group exhibited markedly elevated DNA damage levels in HCT116 cells, along with increased comet tail DNA levels and comet tail moments (Figure 3A-C). Moreover, knocking down SMS expression resulted in significant up-regulation of p-γH2AX, p-ATR, and P53 protein levels in HCT116 cells. Importantly, when compared to the shNC + IR or sh-SMS groups, the sh-SMS + IR group displayed substantially raised γH2AX, p-γH2AX, p-ATR, and P53 protein levels in HCT116 cells (Figure 3D and E). In a nutshell, knocking down SMS enhanced the radiosensitivity of HCT116 cells through supporting DNA damage.
Figure 3 Knockdown of spermine synthase enhances the radiosensitivity of HCT116 cells by advancing DNA damage.
A-C: Comet assay for assessing DNA damage levels in HCT116 cells of the negative control shRNA (shNC), shNC + IR, spermine synthase shRNA (sh-SMS), and sh-SMS + IR groups (A), and analysis of comet tail DNA levels (B) and comet tail moments (C); D and E: Western blot analysis of γH2AX, p-γH2AX, ATR, p-ATR, and P53 protein levels in HCT116 cells of the shNC group, shNC + IR group, sh-SMS group, and sh-SMS + IR group. bP < 0,01 vs shNC, dP < 0.01 vs shNC + IR, fP < 0.01 vs sh-SMS; SMS: Spermine synthase; sh-SMS: Spermine synthase shRNA; shNC: Negative control shRNA; OE-SMS: Spermine synthase over-expression vector; OE-NC: Negative control over-expression vector.
Knocking down SMS enhances the radiosensitivity of HCT116 cells by promoting autophagy
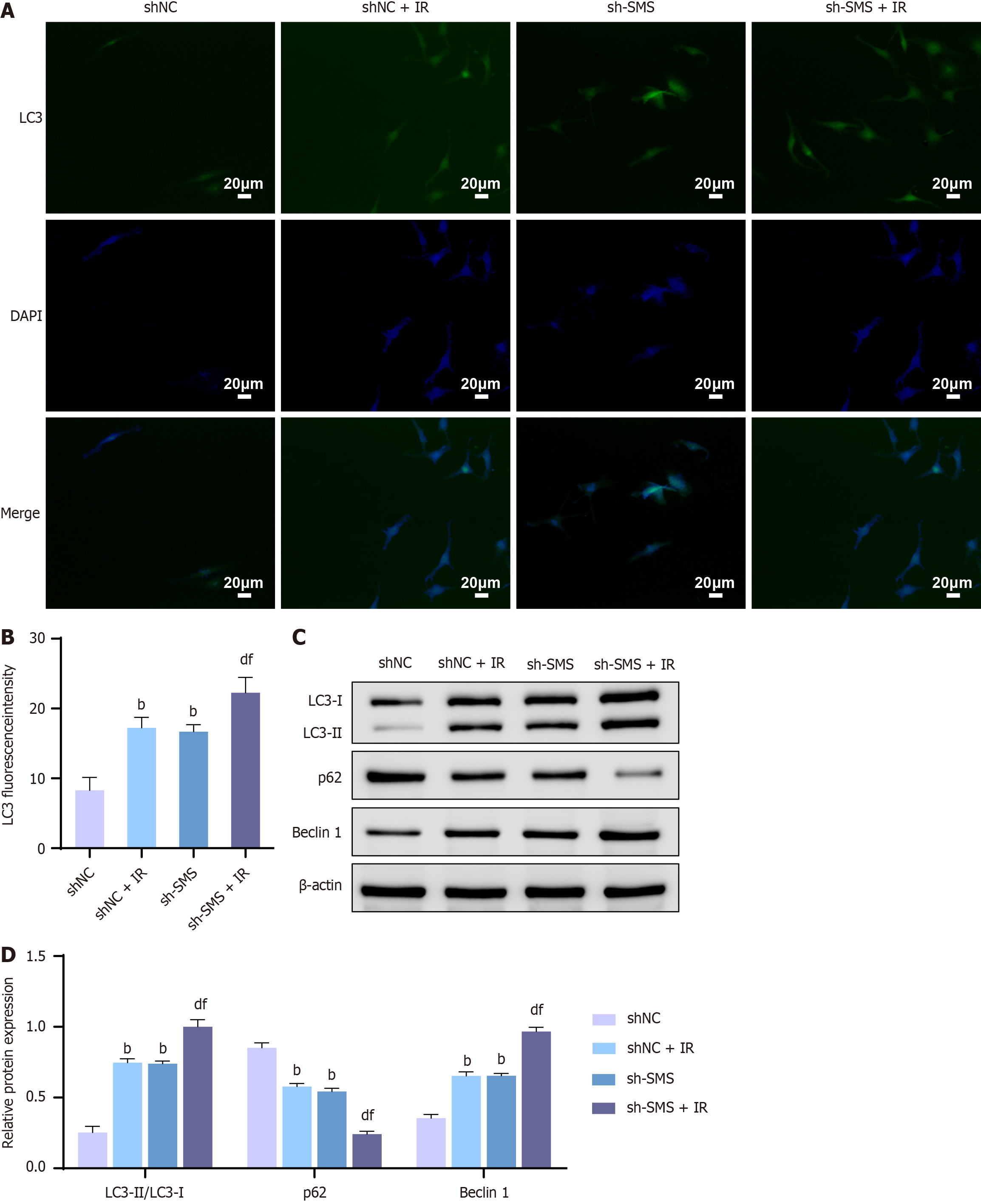
While the correlation between SMS and DNA damage has been established, autophagy also serves as a mechanism of cell death induced by radiation therapy[25,26]. It is worth noting that the relationship between SMS and cellular autophagy remains controversial[27,28]. Therefore, immunofluorescence and western blot analysis of autophagy-related protein expression were performed to observe the level of cellular autophagy after knocking down SMS expression. Immunofluorescence results revealed an increase in the number of LC3 puncta and the proportion of LC3-positive cells following SMS knockdown. Moreover, compared to the shNC + IR or sh-SMS groups, the sh-SMS + IR group displayed a marked increase in the number of LC3 puncta and the proportion of LC3-positive cells (Figure 4A and B). Additionally, western blot analysis revealed an escalation in the protein levels of LC3-I, LC3-II, and Beclin 1, along with a down-regulation in p62 protein levels, and an up-regulation in the LC3-II/LC3-I ratio following SMS knockdown. Importantly, compared to the shNC + IR or sh-SMS groups, the sh-SMS + IR group exhibited significantly elevated protein levels of LC3-I, LC3-II, and Beclin 1, decreased p62 protein levels, and down-regulated ratio of LC3-II/LC3-I (Figure 4C and D). These results indicated that knocking down SMS enhanced the radiosensitivity of HCT116 cells by promoting autophagy.
Figure 4 Knockdown of spermine synthase enhances the radiosensitivity of HCT116 cells by promoting autophagy.
A and B: Immunofluorescence detection for LC3 puncta in HCT116 cells of the negative control shRNA (shNC) group, shNC + IR group, spermine synthase shRNA (sh-SMS) group, and sh-SMS + IR group; C and D: Western blot analysis for protein levels of LC3-I, LC3-II, p62, and Beclin 1 in HCT116 cells of the shNC group, shNC + IR group, sh-SMS group, and sh-SMS + IR group. bP < 0.01 vs shNC, dP < 0.01 vs shNC + IR, fP < 0.01 vs sh-SMS; SMS: Spermine synthase; sh-SMS: Spermine synthase shRNA; shNC: Negative control shRNA; OE-SMS: Spermine synthase over-expression vector; OE-NC: Negative control over-expression vector.
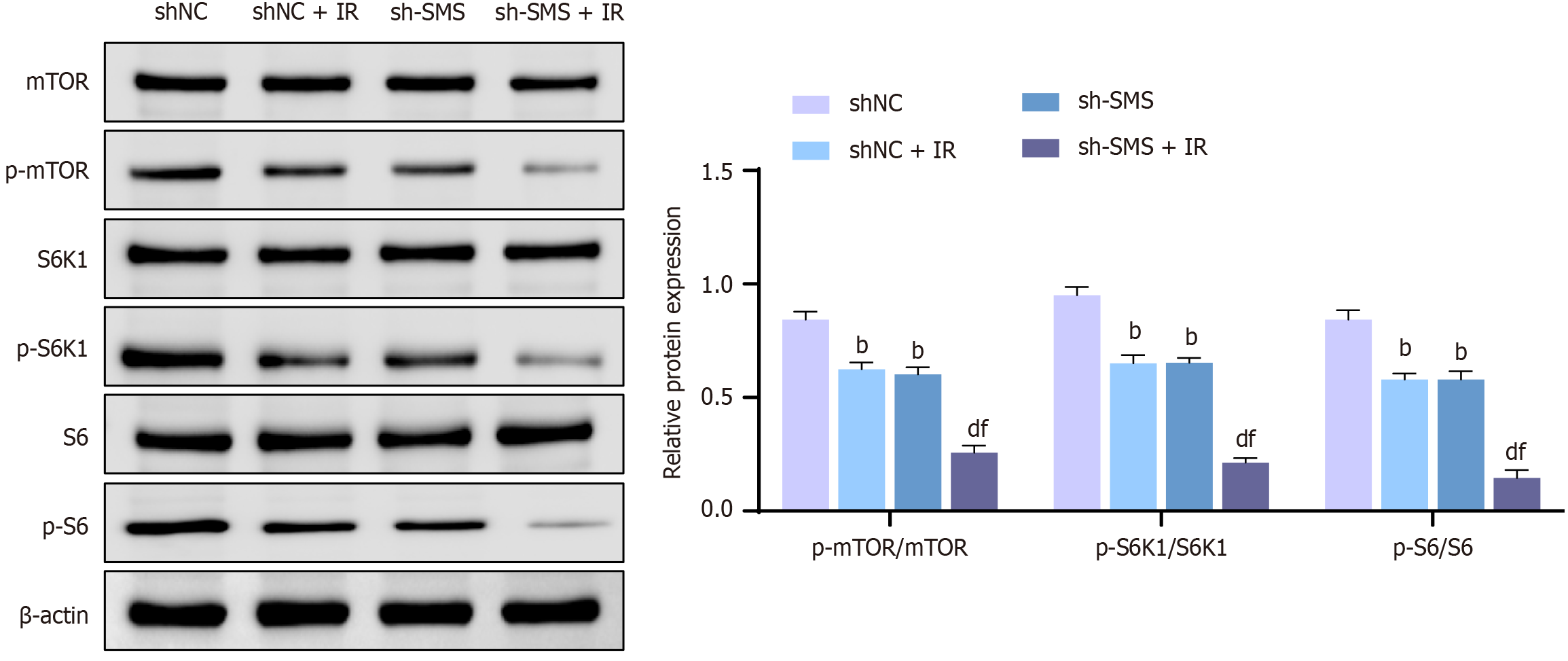
Knocking down SMS enhances the radiosensitivity of HCT116 cells by suppressing the mTOR pathway
The aforementioned research suggests that spermine negatively regulates cellular autophagy. Studies have shown that SMS can modulate autophagy-related signaling through the mTOR pathway[29,30]. Therefore, we hypothesized that the mTOR pathway may be a key regulator of SMS-mediated cellular autophagy. According to western blot results, knocking down SMS expression lowered the p-mTOR, p-S6K1, and p-S6 protein expression levels in HCT116 cells, resulting in lessened ratios of p-mTOR/mTOR, p-S6K1/S6K1, and p-S6/S6. Furthermore, compared to the shNC + IR or sh-SMS groups, the sh-SMS + IR group exhibited lower protein expression levels of p-mTOR, p-S6K1, and p-S6, along with down-regulated ratios of p-mTOR/mTOR, p-S6K1/S6K1, and p-S6/S6 (Figure 5). These findings indicated that knocking down SMS enhanced the radiosensitivity of HCT116 cells by suppressing the mTOR pathway.
Figure 5 Knocking down spermine synthase enhances radiosensitivity of HCT116 cells by suppressing the mammalian target of rapamycin pathway.
Western blot analysis for detecting the protein expression levels of mammalian target of rapamycin (mTOR), p-mTOR, S6K1, p-S6K1, S6, and p-S6 in HCT116 cells under the following conditions: Negative control shRNA (ShNC), shNC + IR, spermine synthase shRNA (sh-SMS), and sh-SMS + IR. bP < 0.01 vs shNC, dP < 0.01 vs shNC + IR, fP < 0.01 vs sh-SMS; SMS: Spermine synthase; sh-SMS: Spermine synthase shRNA; shNC: Negative control shRNA; OE-SMS: Spermine synthase over-expression vector; OE-NC: Negative control over-expression vector; mTOR: Mammalian target of rapamycin.
DISCUSSION
As a pivotal part of comprehensive CRC management, radiotherapy effectively shrinks tumors and prepares patients for further surgical interventions[31,32]. Postoperatively, radiotherapy can effectively eliminate residual cancer cells and reduce the risk of recurrence. Moreover, as for patients with advanced CRC who are unable to undergo surgery, radiotherapy can improve their quality of life and prognosis by alleviating symptoms[33]. However, the current radiotherapy outcomes for patients with CRC are not satisfactory, and adjuvant therapies are needed to improve the efficacy of radiotherapy[34]. In this study, the SMS expression level significantly impacted the radiosensitivity of CRC cells, suggesting its potential as a potential therapeutic target to enhance radiosensitivity.
SMS, an enzyme that converts spermidine to spermine, is involved in diverse physiological processes such as cell proliferation, differentiation, and apoptosis[11]. Recent research has displayed that SMS over-expression is associated with tumorigenesis, progression, invasion, and metastasis in various cancers[12]. SMS expression is highest in the prostate, being approximately 10-20 times higher than in other tissues[35]. Prostate cancer tissues exhibit significantly higher SMS expression compared to adjacent normal tissues, and SMS levels correlate with tumor stage and poor prognosis[36]. Additionally, SMS over-expression has been observed in lung, gastric, liver, bladder, and ovarian cancers[37,38]. Notably, research has demonstrated elevated SMS expression in CRC tissues as opposed to adjacent normal tissues, with a positive correlation to tumor malignancy[14]. These findings indicate that SMS can serve as a potential therapeutic target for CRC. In line with previous findings, our study demonstrated a significant up-regulation of SMS expression in the CRC cell line HCT116 compared to normal colonic epithelial cells (FHC). Notably, reducing SMS expression remarkably suppressed HCT116 cell viability and induced apoptosis. These outcomes strongly support the oncogenic role of SMS in CRC. While reducing SMS expression has been shown to induce chemoresistance in CRC cells[39], our study unveiled a novel role for SMS in regulating radiosensitivity. Furthermore, our research demonstrated that silencing SMS expression significantly enhanced the radiosensitivity of HCT116 cells. Consequently, SMS can be a critical determinant of cancer cell responsiveness to both radiotherapy and chemotherapy.
SMS catalyzes the conversion of spermidine to spermine, a polyamine with potent antioxidant properties that can alleviate oxidative DNA damage[40]. Spermine also plays a crucial role in DNA repair, particularly in double-strand break and base excision repair pathways. Upon DNA damage, SMS is activated and rapidly mobilized to the lesion site, facilitating DNA repair. Additionally, SMS maintains chromosomal structure and function, preventing DNA breaks and aberrations[41]. Given these pro-survival functions, SMS inhibitors have been extensively investigated to enhance radiotherapy efficacy[42]. For instance, eflornithine and difluoromethylornithine, SMS inhibitors, have demonstrated radiosensitizing effects in glioma and CRC cells. These inhibitors augment radiosensitivity by impairing the ability of cancer cells to repair radiation-induced DNA damage[43-46]. In line with these observations, our study provided compelling evidence that SMS knockdown sensitized HCT116 cells to radiotherapy by exacerbating DNA damage.
Autophagy, a programmed cell death pathway, plays a crucial role in influencing radiotherapy sensitivity[47]. However, the relationship between SMS and autophagy remains controversial. Some studies suggest that SMS promotes autophagy through lipid metabolism, facilitating autophagosome formation[48]. Moreover, in neurodegenerative disease models, decreased SMS activity is often associated with autophagy impairment. Conversely, other studies propose an inverse correlation between SMS and autophagy. SMS may suppress autophagy by regulating the NAD+/NADH ratio, inhibiting AMPK activity, and activating the mTOR signaling pathway[49,50]. Additionally, SMS can induce the activation of transcription factors such as NF-κB and HIF-1α, which can repress the expression of autophagy-related genes[51,52]. In our study, upon silencing SMS, the protein levels of LC3-Ⅰ, LC3-Ⅱ, and Beclin 1 rose, whereas p62 Levels fell, indicating that SMS suppressed cellular autophagy. Notably, SMS silencing markedly inhibited the phosphorylation of mTOR, S6K1, and S6 (p-mTOR, p-S6K1, p-S6), suggesting a positive regulatory relationship between SMS and mTOR. This implied that SMS may enhance chemotherapy resistance by suppressing autophagy through activating the mTOR signaling pathway.
It is important to note that this study did not include in vivo experiments to examine the effects of SMS inhibition on CRC chemosensitivity. Moreover, the specific molecular mechanisms connecting SMS to DNA damage and the mTOR signaling pathway are not yet fully understood. Besides, our research did not investigate whether SMS knockdown might increase radiotherapy resistance. These gaps necessitate further investigation through well-designed experiments. In addition, our research was limited to a single CRC cell line, which somewhat restricts the generalizability of our findings.