Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4675
Revised: August 31, 2024
Accepted: September 19, 2024
Published online: December 15, 2024
Processing time: 113 Days and 14.2 Hours
Numerous studies have indicated that the temozolomide and capecitabine regimen (TEMCAP) exhibits a certain level of efficacy in treating advanced, well-differentiated gastroenteropancreatic neuroendocrine tumors (GEP-NET). Ho
To evaluate overall survival (OS) in patients diagnosed with advanced GEP-NET treated with TEMCAP at the Instituto Nacional de Enfermedades Neoplásicas (INEN) in Lima-Perú.
A retrospective review was conducted to identify patients with GEP-NEN treated with the TEMCAP regimen between 2011 and 2021 at the INEN. A total of thirty-eight patients were included in the final analysis: Thirty-five received TEMCAP as a first-line treatment, and three as a second-line treatment. The primary objective was to evaluate OS. The efficacy and safety of TEMCAP were assessed until the occurrence of unacceptable toxicity or disease progression. Survival outcomes were estimated using the Kaplan-Meier method.
The median age of the patients was 52 years (range 24-77 years), and 53.3% were female. The most common symptoms at diagnosis were abdominal pain in 31 patients (81.6%). Primary tumors included 12 in the rectum (31.6%), 11 in the pancreas (28.9%), 3 in the ileum (7.9%), 2 in the mesentery (5.3%), 2 in the small intestine (5.3%), 1 in the appendix (2.6%), 1 in the stomach (2.6%) and 6 cases of liver metastasis of unknown primary (15.8%). Five were neuroendocrine tumors (NET) G1 (13.2%), 33 were NET G2 (86.8%), five had Ki67 < 3% (13.2%), and 33 had Ki67 between 3% and 20% (86.8%). TEMCAP was administered to 35 (92.1%) patients as first-line treatment. OS at 12, 36, and 60 months was estimated in 80%, 66%, and 42%, respectively, with a median OS of 49 months.
TEMCAP therapy is a viable first-line option regarding efficacy and tolerability in areas where standard therapy is inaccessible.
Core Tip: In this study, patients diagnosed with advanced gastroenteropancreatic neuroendocrine tumors who were treated with the temozolomide and capecitabine regimen exhibited a median overall survival of 49 months, with 42% surviving at 60 months. The regimen was well-tolerated, and most patients experienced stable disease. These findings suggest that this treatment could be viable in settings where standard therapies are unavailable or inaccessible, although further prospective studies are needed for confirmation.
- Citation: Cruz-Diaz WE, Paitan V, Medina J, Flores R, Haro-Varas J, Mantilla R, Castro-Oliden V. Temozolomide and capecitabine regimen as first-line treatment in advanced gastroenteropancreatic neuroendocrine tumors at a Latin American reference center. World J Gastrointest Oncol 2024; 16(12): 4675-4684
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4675.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4675
Neuroendocrine tumors (NEN) represent a diverse array of neoplasms arising from cells within the endocrine and nervous systems, and exhibit a broad spectrum of behaviors. While historically considered rare diseases, their prevalence has been increasing. In England, NEN are the 10th most prevalent cancer and the second most common gastrointestinal cancer, only preceded by colorectal cancer[1]. NEN have witnessed a notable surge over the past two decades; the age-adjusted incidence per 100000 persons increased from 4.90 in 2000 to 8.19 in 2018[2]. With regard to gastroenteropancreatic neuroendocrine tumors (GEP-NET) in the United States, the incidence is reported to be 3.56 cases per 100000 individuals[3].
The information published regarding NET in Latin American countries remains largely unreported, and clinical literature is highly scarce. An observational study in Argentina documented 532 NET cases, including 461 GEP-NET and 71 bronchial NET[4]. A NET registry from Brazil has compiled baseline data on the initial 1000 patients enrolled across 32 centers spanning all country regions. It categorized GEP-NET as the second most prevalent type, constituting 20.2% of cases, trailing only thoracic NEN[5]. In a retrospective review at the Instituto Nacional de Enfermedades Neoplásicas (INEN), 367 NEN were reported between 2010 and 2014. Gastroenteropancreatic NEN were the most prevalent, with 152 cases (44.84%), followed by thoracic NEN, with 75 cases (22.12%)[6].
The clinical practice guideline for medical management of GEP-NET at the INEN reported 650 cases of NEN between 2009 and 2018, with an average age of 55 years. The most frequent sites were the rectum (15%), lung (9.84%), stomach (8.3%), neuroendocrine Merkel cells (9.07%), and unknown primary (9.07%)[7]. According to the WHO classification for NEN, they are categorized into well-differentiated low-grade G1 (Ki67 < 3%), intermediate-grade well-differentiated G2 (Ki67 3%-20%), and high-grade well-differentiated G3 (Ki67 > 20%), based on the Ki67 proliferation index. G3 tumors are divided into well-differentiated high-grade or poorly differentiated high-grade neuroendocrine carcinomas (GEP-NEC)[8]. Of all NEN, approximately 80%-90% are well-differentiated[9]. GEP-NET can be classified according to their origin into two main groups: Pancreatic NET (pan-NET) and non-pan-NET. Furthermore, they can be classified based on hormone production in functioning and non-functioning tumors. Most GEP-NET are non-functioning; 20% of intestinal NET are functioning tumors, while pan-NET are functioning in 10%-30% of cases[9]. For non-functioning NET, early detection can be challenging unless the tumor has grown sufficiently large to cause symptoms.
Medical treatment options for advanced GEP-NET with antiproliferative effects include targeted drugs and systemic chemotherapy. Regarding somatostatin analogs (SSA), the CLARINET trial, which compared lanreotide to placebo, estimated progression-free survival (PFS) rates at 24 months of 65.1% in the experimental arm and 33% in the placebo group[10]. These results were confirmed in the open-label extension study[11]. In the phase III PROMID trial, the median time to progression in the octreotide long-acting release (LAR) and placebo groups was 14.3 and 6 months, respectively[12]. However, the updated trial did not show a difference in OS[13]. Everolimus was shown to prolong PFS compared to placebo in previously treated GEP-NET patients[14,15]. Despite these findings, its efficacy in patients with GEP-NET associated with carcinoid syndrome remains unclear[16]. Sunitinib, a multitargeted tyrosine kinase inhibitor, demon
Due to their high cost, these new agents are only available to some patients, especially in resource-limited countries. The restriction on access to first-line treatments at our institution creates an urgent need to investigate other effective alternatives. This study aims to assess the efficacy of the TEMCAP regimen as first-line therapy in patients with advanced GEP-NET in a Latin American population.
A retrospective review was conducted to identify patients with GEP-NEN who were treated with the TEMCAP regimen at any point during their disease between 2011 and 2021. The registry data were provided by the Epidemiology and Statistics Department of the INEN. A total of forty-five GEP-NEN patients were identified, of which nine were excluded due to a diagnosis of NEC. Consequently, thirty-eight patients were included in the final analysis: Thirty-five received TEMCAP as a first-line treatment, and three received TEMCAP as a second-line treatment.
Inclusion criteria: Patients with a diagnosis of unresectable, metastatic, or recurrent GEP-NET; histologic grade 1 or 2; Ki67 index less than 20%.
Exclusion criteria: Patients with GEP-NEC, GEP-NET histologic grade 3, a Ki67 index greater than or equal to 20%, and incomplete medical records.
The primary objective was to evaluate OS in the entire population. Secondary objectives included assessing PFS, eva
The TEMCAP regimen consisted of capecitabine 750 mg/m² twice daily on days 1–14, followed by Temozolomide 200 mg/m² on days 10 to 14 in 28-day cycles until unacceptable toxicity or disease progression. The response was assessed by computed tomography (CT) according to RECIST 1.1 criteria. Toxicity was evaluated according to Common Terminology Criteria for Adverse Events version 5.0 in all 38 patients.
A descriptive analysis was performed on qualitative variables using frequencies and percentages. Quantitative variables were summarized using measures of central tendency (mean, minimum, and maximum for normally distributed va
The median age was 52 years (24-77 years); 55.3% were females, and 44.7% were males. Lima was the most common region of origin (36.8%), followed by Huánuco (13.2%). The majority (92.1%) had an Eastern Cooperative Oncology Group (ECOG) performance status of 1. The median body mass index (BMI) was 23.438 kg/m2. The most common symptom was abdominal pain (81.6%), followed by rectal bleeding (31.6%), weight loss (23.7%), diarrhea (13.2%), emesis (10.5%), and flushing (5.3%). Demographic and clinical features are shown in Table 1. According to clinical staging, 15.8% were in stage III, 81.6% in stage IV, and one patient was in an unspecified stage. Two (5.3%) patients had unresectable tumors, 5 (13.2%) had recurrent disease, and 31 (81.6%) had metastatic disease at presentation. Among the 38 patients, the primary tumor locations were distributed as follows: Twelve (31.6%) were rectal tumors, eleven (28.9%) were pancreatic tumors (28.9%), three (7.9%) were ileum tumors, two (5.3%) were mesenteric tumors, two (5.3%) were small intestine tumors, one (2.6%) was an appendix tumor, one (2.6%) was a stomach tumor and six (15.8%) were liver metastases from an unknown primary.
| Feature | n = 38 |
| Age at diagnosis (years) | |
| Median (min-max) | 52 (24-77) |
| Sex | |
| Female | 21 (55.3) |
| Male | 17 (44.7) |
| Region of birth | |
| Lima | 14 (36.8) |
| Huánuco | 5 (13.2) |
| Ancash | 3 (7.9) |
| Ica | 3 (7.9) |
| Junín | 3 (7.9) |
| Lambayeque | 2 (5.3) |
| Ayacucho | 2 (5.3) |
| Cajamarca | 2 (5.3) |
| Amazonas | 1 (2.6) |
| Cusco | 1 (2.6) |
| Puno | 1 (2.6) |
| Tacna | 1 (2.6) |
| BMI, kg/m2 | |
| Median (IQR) | 23.438 (20.65-26.279) |
| ECOG scale | |
| 0 | 1 (2.6) |
| 1 | 35 (92.1) |
| 2 | 2 (5.3) |
| Symptoms | |
| Abdominal pain | 31 (81.6) |
| Rectal bleeding | 12 (31.6) |
| Weight loss | 9 (23.7) |
| Diarrhea | 5 (13.2) |
| Emesis | 4 (10.5) |
| Flushing | 2 (5.3) |
TEMCAP was administered to 35 patients (92.1%). The median number of chemotherapy cycles at first-line was nine. Fourteen patients (36.8%) received a second-line treatment. Among these patients, the most commonly used regimen was capecitabine in four patients (28.6%), followed by capecitabine/oxaliplatin and TEMCAP in three patients (21.4%) each, cisplatin/etoposide in one patient (7.1%), dacarbazine in one patient (7.1%), gemcitabine/oxaliplatin in one patient (7.1%), and temozolomide in one patient (7.1%). Characteristics of systemic therapy are shown in Table 2.
| Feature | n = 38 |
| First-line treatment | |
| Temozolomide/capecitabine | 35 (92.1) |
| CAPOX | 1 (2.6) |
| Cisplatin/etoposide | 1 (2.6) |
| Interferon alpha | 1 (2.6) |
| First-line cycles | |
| Median (IQR) | 9 (6-22.75) |
| Duration of first-line treatment, days | |
| Median (IQR) | 331 (133.25-606) |
| Second-line treatment | |
| Yes | 14 (36.8) |
| No | 24 (63.2) |
| Second-line regimen, n = 14 | |
| Capecitabine | 4 (28.6) |
| CAPOX | 3 (21.4) |
| TEMCAP | 3 (21.4) |
| Cisplatin/etoposide | 1 (7.1) |
| Dacarbazine (DTIC) | 1 (7.1) |
| GEMOX | 1 (7.1) |
| Temozolomide | 1 (7.1) |
The responses of the 38 patients according to RECIST 1.1 were as follows: One (2.6%) patient had a complete response (CR), two (5.3%) had a partial response (PR), sixteen (42.1%) had stable disease (SD), fifteen (39.5%) had progressive disease (PD), and four (10.5%) were without RECIST evaluation. The ORR was 7.9%, and the DCR was 50%. Regarding the pan-NET subgroup, there was one CR, two PR, three SD, four PD, and one case without response evaluation; the ORR was 27.2%, and the DCR was 54.5%.
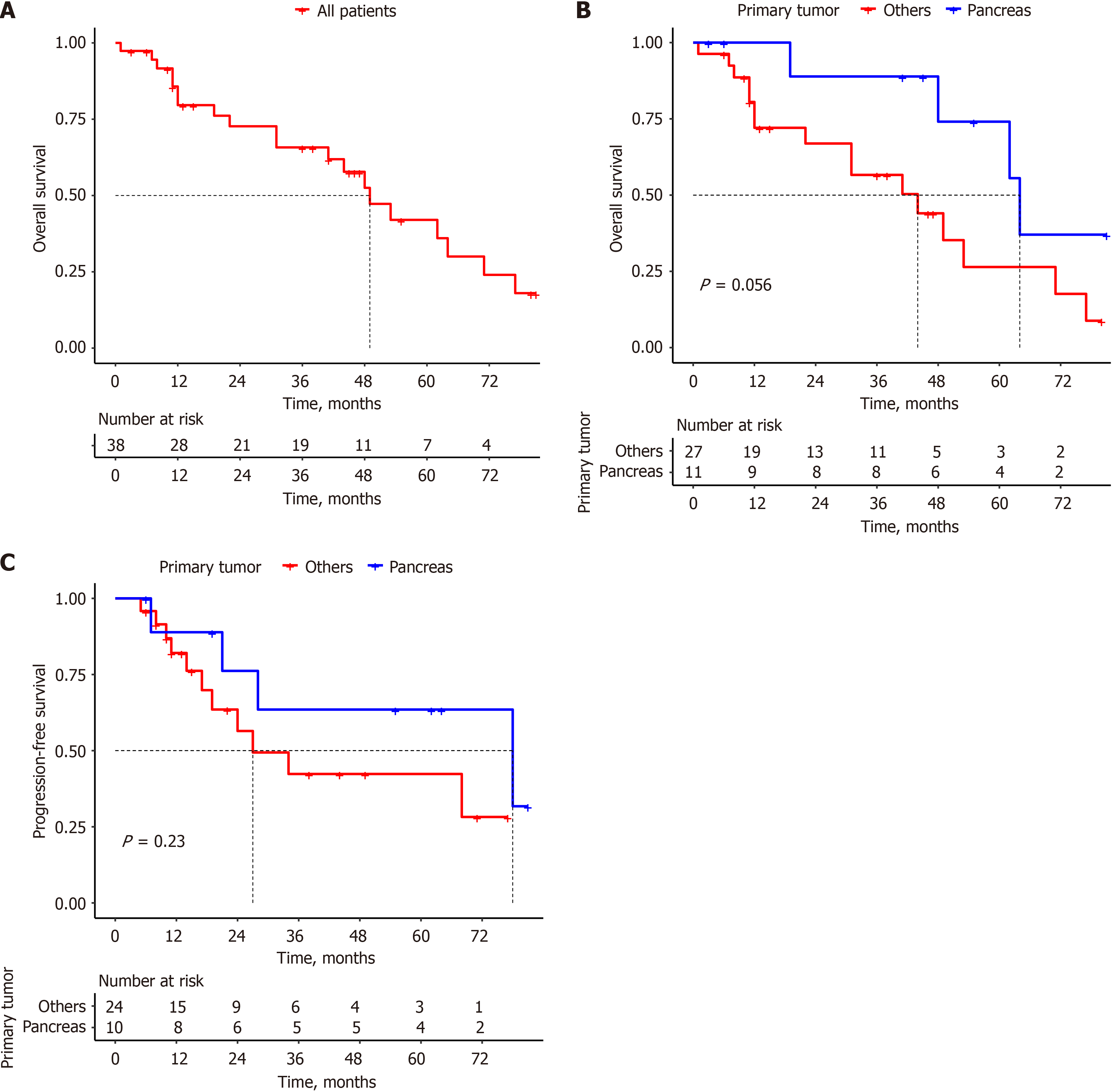
With a median follow-up of 33.5 months (range 1–81 months), the estimated OS rates at 12, 36, and 60 months were 80%, 66%, and 42%, respectively, with a median OS of 49 months for the entire population (Figure 1A). The median OS in the pan-NET group was 64 months, compared to 44 months in the non-pan-NET group, with a P-value of 0.056, which was not statistically significant (Figure 1B).
Among the total population (n = 34; RECIST criteria not evaluated in 4 patients), 15 (44.1%) patients experienced PD, with a median follow-up time for PFS of 20 months (range 5-81 months). The estimated PFS at 12, 36, and 60 months was 84.2%, 49.9%, and 49.9%, respectively, with a median PFS of 34 months. In the pan-NET group, the median PFS was 78 months, compared to 27 months in the non-pan-NET group (Figure 1C).
The most common adverse event of any grade was neutropenia, with 7 (18.4%) recorded events, followed by hand-foot syndrome with 6 (15.8%) events, and hypertransaminasemia and nausea with 5 (13.2%) events each. The total number of adverse events is shown in Table 3. Regarding adverse events grade 3 and 4, neutropenia occurred in three patients (7.9%), two (5.3%) experiencing grade 3 and one (2.6%) grade 4; Thrombocytopenia was observed in four patients (10.6%), two (5.3%) experiencing grade 3 and two (5.3%) grade 4; anemia was reported in two patients (5.3%), one (2.6%) experiencing grade 3 and one (2.6%) grade 4. Additionally, nausea of grade 3 was documented in one patient (2.6%).
| Grade | ||||
| 1 | 2 | 3 | 4 | |
| Neutropenia | 3 (7.9) | 1 (2.6) | 2 (5.3) | 1 (2.6) |
| Hand-foot syndrome | 5 (13.2) | 1 (2.6) | ||
| Hypertransaminasemia | 3 (7.9) | 2 (5.3) | ||
| Nausea | 4 (10.5) | 1 (2.6) | ||
| Peripheral neuropathy | 4 (10.5) | 1 (2.6) | ||
| Vomiting | 4 (10.5) | 1 (2.6) | ||
| Anemia | 2 (5.3) | 1 (2.6) | 1 (2.6) | |
| Asthenia | 4 (10.5) | |||
| Constipation | 4 (10.5) | |||
| Thrombocytopenia | 2 (5.3) | 2 (5.3) | ||
| Anorexia | 3 (7.9) | |||
| Diarrhea | 2 (5.3) | |||
| Sialorrhea | 2 (5.3) | |||
| Hyperbilirubinemia | 1 (2.6) | |||
We observed a median OS of 49 months in our study, similar to the findings of other studies that retrospectively eva
Prospective data evaluating TEMCAP are scarce. The first prospective trial was phase II ECOG-ACRIN E2211, which compared TEMCAP vs temozolomide in advanced pan-NET. This trial met its primary endpoint with a PFS of 22.7 months in the TEMCAP arm vs 14.4 months in the temozolomide arm. Although the median OS was 4.9 months superior in the TEMCAP arm, it did not achieve statistical significance[26].
In our study, we observed an ORR of 7.9% with one (2.6%) CR and two (5.3%) PR; the DCR was 50%, including 16 patients (42.1%) with SD. Our DCR results are very similar to those in other retrospective studies. Crespo et al[22] evaluated TEMCAP in 65 patients with GEP-NET (70.8% had pan-NET) and the DCR was 47.7%, with two CR (3.1%), 29 PR (44.6%), and 27 (41.5%) SD. Fine et al[19] evaluated 18 patients with well-differentiated NET metastatic to the liver who had failed front-line therapy. The ORR was 61%, and the DCR was 83.2%. Abbasi et al[27] evaluated 21 patients (14 with pan-NET and 7 with carcinoid tumors) who failed treatment with SSA and platinum-based chemotherapy combined with etoposide and reported a DCR of 80%. The systematic review by Arrivi et al[25] evaluated 1,818 patients from 42 articles with advanced NEN of gastroenteropancreatic, lung, and unknown origin. The ORR was 77%, with a median OS ranging from 8 to 103 months. ORR and DCR appear more critical as surrogates of the PFS and OS for the TEMCAP regimen in GEP-NET.
Our results for pan-NET showed an ORR of 27.2% and a DCR of 54.5%, consistent with what has been described in the literature. Pan-NET have historically better chemotherapy responses than non-pan-NET. Our study also showed this trend, with OS in pan-NET being 20 months longer than non-pan-NET. The PSF for pan-NET in our series was 78 months compared to 27 months in non-pan-NET. Notably, of the 11 patients with pan-NET, 10 were evaluated according to RECIST 1.1, of which 4 showed disease progression; the remaining patients are still alive and continue to be followed up.
A meta-analysis revealed a lower ORR in non-pan-NET than in pan-NET patients; however, this difference was not statistically significant when high-risk bias studies were excluded[28]. In a cohort of 101 patients, which included 53 with pan-NEN and 44 with carcinoid tumors treated with temozolomide-based chemotherapy, an ORR of 34% was observed in pan-NEN compared to 2% in carcinoid tumors[29]. Patients with pan-NET who require clinically meaningful tumor shrinkage may benefit more from chemotherapeutic regimens.
Anemia, neutropenia, and thrombocytopenia were the most common grade 3-4 adverse events observed in our study, consistent with findings from other trials. Crespo et al[22] reported neutropenia in 7.7% of patients, while in the sys
We must consider some limitations in our study inherent to all retrospective analyses, particularly the potential for selection bias. Additionally, the small sample size of 38 patients may have contributed to the lack of statistical significance in some outcomes. Despite these limitations, our data support the effectiveness of the TEMCAP regimen in patients with advanced GEP-NET.
This is the first retrospective study in Peru to evaluate the use of TEMCAP for advanced GEP-NET. The findings suggest that TEMCAP could be a viable first-line treatment in regions where standard therapies are not readily accessible, particularly for grade 2 tumors. A notable 42% OS rate at 60 months was observed. Prospective studies are needed to determine its value as a treatment option in this setting.
| 1. | White BE, Rous B, Chandrakumaran K, Wong K, Bouvier C, Van Hemelrijck M, George G, Russell B, Srirajaskanthan R, Ramage JK. Incidence and survival of neuroendocrine neoplasia in England 1995-2018: A retrospective, population-based study. Lancet Reg Health Eur. 2022;23:100510. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 95] [Reference Citation Analysis (0)] |
| 2. | Wu P, He D, Chang H, Zhang X. Epidemiologic trends of and factors associated with overall survival in patients with neuroendocrine tumors over the last two decades in the USA. Endocr Connect. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 3. | Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, Shih T, Yao JC. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3:1335-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1510] [Cited by in RCA: 2663] [Article Influence: 295.9] [Reference Citation Analysis (5)] |
| 4. | O'Connor JM, Marmissolle F, Bestani C, Pesce V, Belli S, Dominichini E, Mendez G, Price P, Giacomi N, Pairola A, Loria FS, Huertas E, Martin C, Patane K, Poleri C, Rosenberg M, Cabanne A, Kujaruk M, Caino A, Zamora V, Mariani J, Dioca M, Parma P, Podesta G, Andriani O, Gondolesi G, Roca E. Observational study of patients with gastroenteropancreatic and bronchial neuroendocrine tumors in Argentina: Results from the large database of a multidisciplinary group clinical multicenter study. Mol Clin Oncol. 2014;2:673-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 5. | Younes RN; GETNE (Grupo de Estudo de Tumores Neuroendócrinos). Neuroendocrine tumors: a registry of 1,000 patients. Rev Assoc Med Bras (1992). 2008;54:305-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | 19th Annual ENETS Conference for the Diagnosis and Treatment of Neuroendocrine Tumor Disease, 10 - 11 March 2022. J Neuroendocrinol. 2022;34:e13108. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Instituto Nacional de Enfermedades Neoplásicas (INEN). Resolución Jefatural N° 026-2021-J-INEN. 2021. [cited 3 September 2024]. Available from: https://portal.inen.sld.pe/wp-content/uploads/2021/02/RJ-026-2021-J-INEN.pdf. |
| 8. | Rindi G, Mete O, Uccella S, Basturk O, La Rosa S, Brosens LAA, Ezzat S, de Herder WW, Klimstra DS, Papotti M, Asa SL. Overview of the 2022 WHO Classification of Neuroendocrine Neoplasms. Endocr Pathol. 2022;33:115-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 618] [Article Influence: 154.5] [Reference Citation Analysis (2)] |
| 9. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 790] [Article Influence: 131.7] [Reference Citation Analysis (0)] |
| 10. | Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L, Rindi G, Langley A, Martinez S, Blumberg J, Ruszniewski P; CLARINET Investigators. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1142] [Cited by in RCA: 1352] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 11. | Caplin ME, Pavel M, Phan AT, Ćwikła JB, Sedláčková E, Thanh XT, Wolin EM, Ruszniewski P; CLARINET Investigators. Lanreotide autogel/depot in advanced enteropancreatic neuroendocrine tumours: final results of the CLARINET open-label extension study. Endocrine. 2021;71:502-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R; PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656-4663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1609] [Cited by in RCA: 1789] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 13. | Rinke A, Wittenberg M, Schade-Brittinger C, Aminossadati B, Ronicke E, Gress TM, Müller HH, Arnold R; PROMID Study Group. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 14. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG, Tomassetti P, Pavel ME, Hoosen S, Haas T, Lincy J, Lebwohl D, Öberg K; RAD001 in Advanced Neuroendocrine Tumors, Third Trial (RADIANT-3) Study Group. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2162] [Article Influence: 144.1] [Reference Citation Analysis (0)] |
| 15. | Yao JC, Fazio N, Singh S, Buzzoni R, Carnaghi C, Wolin E, Tomasek J, Raderer M, Lahner H, Voi M, Pacaud LB, Rouyrre N, Sachs C, Valle JW, Fave GD, Van Cutsem E, Tesselaar M, Shimada Y, Oh DY, Strosberg J, Kulke MH, Pavel ME; RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387:968-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 749] [Cited by in RCA: 943] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 16. | Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Öberg K, Van Cutsem E, Yao JC; RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 814] [Cited by in RCA: 768] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 17. | Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A, Chen JS, Hörsch D, Hammel P, Wiedenmann B, Van Cutsem E, Patyna S, Lu DR, Blanckmeister C, Chao R, Ruszniewski P. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2032] [Cited by in RCA: 1878] [Article Influence: 125.2] [Reference Citation Analysis (0)] |
| 18. | Singh S, Halperin DM, Myrehaug S, Herrmann K, Pavel M, Kunz PL, Chasen B, Capdevila J, Tafuto S, Oh D, Yoo C, Falk S, Halfdanarson TR, Folitar I, Zhang Y, Santoro P, Aimone P, de Herder WW, Ferone D. [177Lu]Lu-DOTA-TATE in newly diagnosed patients with advanced grade 2 and grade 3, well-differentiated gastroenteropancreatic neuroendocrine tumors: Primary analysis of the phase 3 randomized NETTER-2 study. J Clin Oncol. 2024;42:LBA588-LBA588. [DOI] [Full Text] |
| 19. | Fine RL, Gulati AP, Krantz BA, Moss RA, Schreibman S, Tsushima DA, Mowatt KB, Dinnen RD, Mao Y, Stevens PD, Schrope B, Allendorf J, Lee JA, Sherman WH, Chabot JA. Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol. 2013;71:663-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 191] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 20. | Strosberg JR, Fine RL, Choi J, Nasir A, Coppola D, Chen DT, Helm J, Kvols L. First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer. 2011;117:268-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 555] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 21. | Cives M, Ghayouri M, Morse B, Brelsford M, Black M, Rizzo A, Meeker A, Strosberg J. Analysis of potential response predictors to capecitabine/temozolomide in metastatic pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2016;23:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 22. | Crespo G, Jiménez-Fonseca P, Custodio A, López C, Carmona-Bayonas A, Alonso V, Navarro M, Aller J, Sevilla I, Grande E, Gajate P, Alonso-Gordoa T, Matos I, Capdevila J, Nieto B, Barriuso J. Capecitabine and temozolomide in grade 1/2 neuroendocrine tumors: a Spanish multicenter experience. Future Oncol. 2017;13:615-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | de Mestier L, Walter T, Brixi H, Evrard C, Legoux JL, de Boissieu P, Hentic O, Cros J, Hammel P, Tougeron D, Lombard-Bohas C, Rebours V, Ruszniewski P, Cadiot G. Comparison of Temozolomide-Capecitabine to 5-Fluorouracile-Dacarbazine in 247 Patients with Advanced Digestive Neuroendocrine Tumors Using Propensity Score Analyses. Neuroendocrinology. 2019;108:343-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 24. | Lamarca A, Barriuso J, Mccallum L, Papaxoinis G, Nasralla M, Nuttall C, Frizziero M, Kordatou Z, Mcnamara M, Hubner R, Manoharan P, Mansoor W, Valle J. Temozolomide-capecitabine (TemCap) chemotherapy for neuroendocrine neoplasms (NENs): Time to maximum response and optimal treatment duration. Ann Oncol. 2017;28:v151. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Arrivi G, Verrico M, Roberto M, Barchiesi G, Faggiano A, Marchetti P, Mazzuca F, Tomao S. Capecitabine and Temozolomide (CAPTEM) in Advanced Neuroendocrine Neoplasms (NENs): A Systematic Review and Pooled Analysis. Cancer Manag Res. 2022;14:3507-3523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Kunz PL, Graham NT, Catalano PJ, Nimeiri HS, Fisher GA, Longacre TA, Suarez CJ, Martin BA, Yao JC, Kulke MH, Hendifar AE, Shanks JC, Shah MH, Zalupski MM, Schmulbach EL, Reidy-Lagunes DL, Strosberg JR, O'Dwyer PJ, Benson AB 3rd. Randomized Study of Temozolomide or Temozolomide and Capecitabine in Patients With Advanced Pancreatic Neuroendocrine Tumors (ECOG-ACRIN E2211). J Clin Oncol. 2023;41:1359-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 134] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 27. | Abbasi S, Kashashna A, Albaba H. Efficacy of capecitabine and temozolomide combination in well-differentiated neuroendocrine tumors: Jordan experience. Pancreas. 2014;43:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Lamarca A, Elliott E, Barriuso J, Backen A, McNamara MG, Hubner R, Valle JW. Chemotherapy for advanced non-pancreatic well-differentiated neuroendocrine tumours of the gastrointestinal tract, a systematic review and meta-analysis: A lost cause? Cancer Treat Rev. 2016;44:26-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 29. | Kulke MH, Hornick JL, Frauenhoffer C, Hooshmand S, Ryan DP, Enzinger PC, Meyerhardt JA, Clark JW, Stuart K, Fuchs CS, Redston MS. O6-methylguanine DNA methyltransferase deficiency and response to temozolomide-based therapy in patients with neuroendocrine tumors. Clin Cancer Res. 2009;15:338-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 311] [Cited by in RCA: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 30. | Chan JA, Stuart K, Earle CC, Clark JW, Bhargava P, Miksad R, Blaszkowsky L, Enzinger PC, Meyerhardt JA, Zheng H, Fuchs CS, Kulke MH. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30:2963-2968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 212] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 31. | Kulke MH, Stuart K, Enzinger PC, Ryan DP, Clark JW, Muzikansky A, Vincitore M, Michelini A, Fuchs CS. Phase II study of temozolomide and thalidomide in patients with metastatic neuroendocrine tumors. J Clin Oncol. 2006;24:401-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 359] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/