Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4614
Revised: August 13, 2024
Accepted: September 5, 2024
Published online: December 15, 2024
Processing time: 112 Days and 0.7 Hours
Rectal cancer has become one of the leading malignancies threatening people’s health. For locally advanced rectal cancer (LARC), the comprehensive strategy combining neoadjuvant chemoradiotherapy (NCRT), total mesorectal excision (TME), and adjuvant chemotherapy has emerged as a standard treatment regimen, leading to favorable local control and long-term survival. However, in recent years, an increasing attention has been paid on the exploration of organ preservation strategies, aiming to enhance quality of life while maintaining optimal oncological treatment outcomes. Local excision (LE), compared with low anterior resection (LAR) or abdominal-perineal resection (APR) was introduced dating back to 1970’s. LE has historically been linked to a heightened risk of recurrence compared to TME, potentially due to occult lymph node metastasis and intraluminal recurrence. Recent evidence has demonstrated that LE might be an alternative approach, instead of LAR or APR, in cases with favorable tumor regression after NCRT with potentially better quality of life. Therefore, a retrospective analysis of clinicopathological data from mid-low LARC patients who underwent LE after NCRT was conducted, aiming to evaluate the treatment's efficacy, safety, and oncologic prognosis.
To explore the safety, efficacy, and long-term prognosis of LE in patients with mid-low rectal cancer who had a good response to NCRT.
Patients with LE between 2012 to 2021 were retrospectively collected from the rectal cancer database from Gastro-intestinal Ward III in Peking University Cancer Hospital. The clinicopathological features, postoperative complications, and long-term prognosis of these patients were analyzed. The Kaplan-Meier method was used to create cancer-specific survival curve, and the log-rank test was used to compare the differences regarding outcomes.
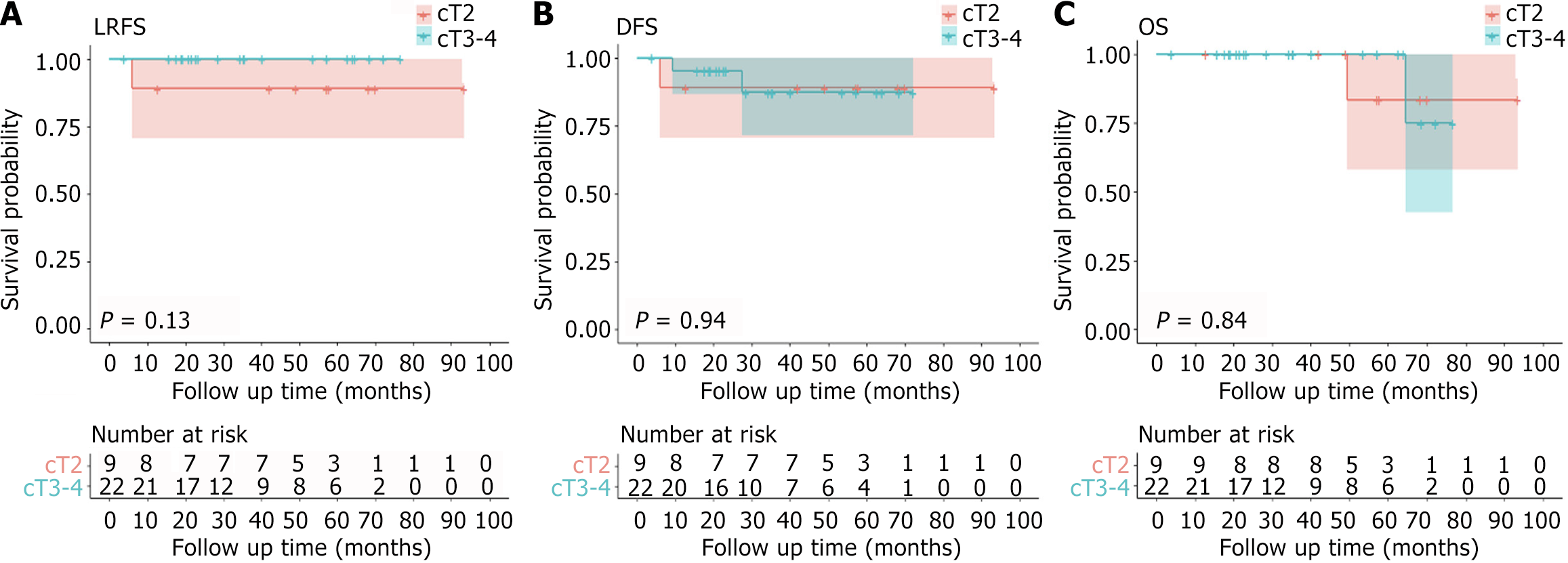
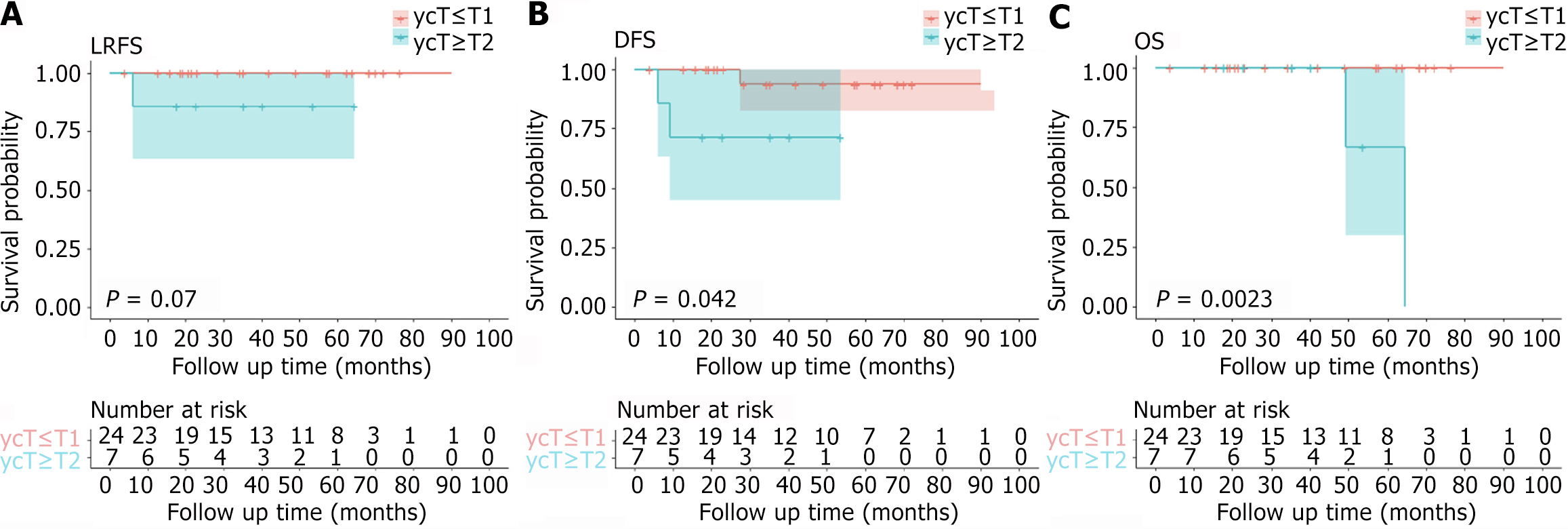
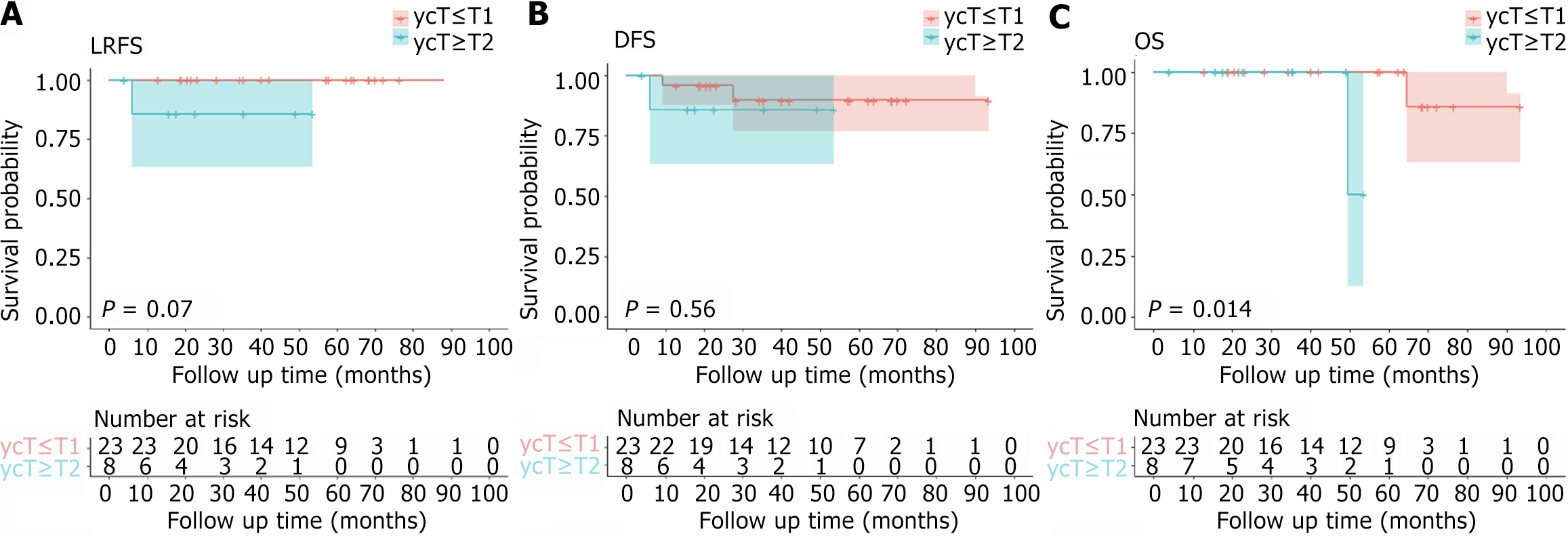
A total of 33 patients were included in this study. The median interval between NCRT and surgery was 25.4 (range: 8.7-164.4) weeks. The median operation time was 57 (20.0-137.0) minutes. The initial clinical T staging (cT): 9 (27.3%) patients were cT2, 19 (57.6%) patients were cT3, and 5 (15.2%) patients were cT4; The initial N staging (cN): 8 patients (24.2%) were cN negative, 25 patients (75.8%) were cN positive; The initial M stage (cM): 2 patients (6.1%) had distant metastasis (ycM1), 31 (93.9%) patients had no distant metastasis (cM0). The pathological results: 18 (54.5%) patients were pathological T0 stage (ypT0), 6 (18.2%) patients were ypT1, 7 (21.2%) patients were ypT2, and 2 (6.1%) patients were ypT3. For 9 cT2 patients, 5 (5/9, 55.6%) had a postoperative pathological result of ypT0. For 19 cT3 patients, 11 (57.9%) patients were ypT0, and 2 (40%) were ypT0 in 5 cT4 patients. The most common complication was chronic perineal pain (71.4%, 5/7), followed by bleeding (43%, 3/7), stenosis (14.3%, 1/7), and fecal incontinence (14.3%, 1/7). The median follow-up time was 42.0 (4.0-93.5) months. For 31 patients with cM0, the 5-year disease-free survival (DFS) rate, 5-year local recurrence-free survival (LRFS) rate, and 5-year overall survival (OS) rate were 88.4%, 96.7%, and 92.9%, respectively. There were significant differences between the ycT groups concerning either DFS (P = 0.042) or OS (P = 0.002) in the Kaplan-Meier analysis. The LRFS curve of ycT ≤ T1 patients was better than that of ycT ≥ T2 patients, and the P value was very close to 0.05 (P = 0.070). The DFS curve of patients with ypT ≤ T1 was better than that of patients with ypT ≥ T2, but the P value was not statistically significant (P = 0.560). There was a significant difference between the ypT groups concerning OS (P = 0.014) in the Kaplan-Meier analysis. The LRFS curve of ypT ≤ T1 patients was better than that of ypT ≥ T2 patients, and the P value was very close to 0.05 (P = 0.070). Two patients with initial cM1 were alive at the last follow-up.
LE for rectal cancer with significant tumor regression after NCRT can obtain better safety, efficiency, and oncological outcome. Minimally invasive or nonsurgical treatment with patient participation in decision-making can be performed for highly selected patients. Further investigation from multiple centers will bring better understanding of potential advantages regarding local resection.
Core Tip: This retrospective study explores the safety, efficacy, and long-term prognosis of local excision (LE) in patients with middle-low rectal cancer who responded well to neoadjuvant chemoradiotherapy. The findings demonstrate that LE can achieve high rates of organ preservation and favorable oncological outcomes, including a 5-year disease-free survival rate of 88.4% and overall survival rate of 92.9%. Complications were manageable and non-severe. This study supports the potential of minimally invasive treatments in selected patients, highlighting the importance of patient participation in treatment decisions.
- Citation: Chen N, Li CL, Wang L, Yao YF, Peng YF, Zhan TC, Zhao J, Wu AW. Local excision for middle-low rectal cancer after neoadjuvant chemoradiation: A retrospective study from a single tertiary center. World J Gastrointest Oncol 2024; 16(12): 4614-4624
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4614.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4614
Rectal cancer has attracted more and more attention as a tumor worldwide. Neoadjuvant chemoradiotherapy (NCRT) followed by total mesorectal excision (TME) and adjuvant chemotherapy has been widely used as a classic treatment strategy for patients with locally advanced rectal cancer (LARC), which has shown good local control rate and survival results[1,2]. However, for mid-low rectal cancer, the traditional low anterior resection (LAR) or abdominal-perineal resection (APR) treatment has a series of disadvantages, such as LAR syndrome or permanent stoma[3,4]. In recent years, organ preservation strategy has been recognized by more and more surgeons. Morson et al[5] introduced their experience of the traditional transanal approach and surgical criteria for local excision (LE) of early rectal cancer in 1977. Since then, the application of LE for early rectal cancer has gradually increased, and patients have high function preservation and prognosis results[6-8]. However, for more advanced tumors, LE has long been associated with a higher risk of recurrence than TME, which is considered to be related to occult lymph node metastasis and intraluminal recurrence[9,10]. For LARC, strengthening the intensity of preoperative treatment, such as total neoadjuvant therapy treatment strategy or consolidation chemotherapy, brings better tumor treatment response, and some patients may achieve clinical complete response (cCR) or near-cCR status[11,12]. To date, LE, known as the total mesorectal neglect strategy, for these well-responding rectal cancer patients is still in the exploratory stage[13,14]. The ACOSOG Z6041 study showed that for patients with early rectal cancer, NCRT combined with LE can obtain a better oncologic prognosis and a higher quality of life and anal function[15]. However, some studies have also reported that LE after NCRT has a high incidence of complications and a variable recurrence rate[16,17]. A meta-analysis showed no difference in the risk of postoperative complications between radical surgery and transanal endoscopic microsurgery[18]. Based on these inconsistent results, we retrospectively analyzed the clinicopathological data of patients with mid-low LARC who underwent LE after NCRT in our center and explored the efficiency, safety, and oncological prognosis.
The study subjects were rectal cancer patients with a follow-up period of more than 3 years, therefore, databased was formed focusing patients with LE between 2012 to 2021 were retrospectively collected from the rectal cancer database at Peking University Cancer Hospital., and local resection was chosen as the surgical treatment. The exclusion criterion was total mesorectal resection. Screening criteria: (1) Patients with mid-low rectal cancer treated with intensity-modulated radiation therapy (IMRT); and (2) Patients who underwent transanal LE or transanal minimally invasive surgery (TAMIS). The study was approved by the medical ethics committee of the Peking University Cancer Hospital, and informed consent was waived (2015KT31/2017KT104).
The IMRT regimen consisted of 22 fractions of 2.3 Gy (gross tumor volume, GTV) and 1.9 Gy (clinical target volume, CTV), which has been described in our previous report[19,20]: The total dose of 50.6 Gy (GTV)/41.8 Gy (CTV) was administered 5 times per week over a period of 30 days. IMRT was administered using the Varian Rapid Arc system. The GTV was defined as the primary tumor, including the mesorectum. The CTV was defined as the primary tumor, mesorectal region, presacral region, mesorectal lymph nodes, lateral lymph nodes, internal iliac lymph node chain, or pelvic wall area. Capecitabine treatment was administered concurrently with IMRT at a dose of 825 mg/m2 orally, twice per day.
The patient was given general anesthesia. According to the location of the tumor, the lithotomy position (the tumor was located in the posterior wall of the rectum) or the jackknife position (the tumor was located in the anterior wall of the rectum) was used. Transanal LE or TAMIS surgery was performed by a skilled surgeon. Local full-thickness resection was performed using an ultrasonic scalpel or electric scalpel at a distance of 1cm from the tumor. The surgical wound was closed by continuous suture with barbed suture or intermittent suture with 4-0 absorbable sutures to ensure complete suture and no active bleeding. After flattening, the surgical specimen was fixed on a soft plate with a pin and sent to the pathology department for paraffin pathological examination. Negative margins were defined as microscopically confirmed full-thickness resections with a circumferential resection margin of 1 mm or more.
Patients were regularly followed up every 3 months for the first 2 years and every 6 months thereafter for 3 years. After 5 years, follow-up visits were performed once a year until death or loss of follow-up. Follow-up examinations included digital rectal examination, serum tumor markers, thoracoabdominal/pelvic computed tomography or magnetic resonance imaging, and enteroscopy. Local recurrence-free survival (LRFS) was defined as the time from surgery to the local recurrence, final follow-up, or death (without recurrence or metastasis). Disease-free survival (DFS) was defined as the time from surgery to the first recurrence (local or distant), final follow-up, or death (without recurrence or metastasis). Overall survival (OS) was defined as the time from surgery to death from any cause or final follow-up. The follow-up information was obtained through telephone communication or inquiry into outpatient medical records.
Statistical analyses were performed using R software (4.0.4, R Foundation for Statistical Computing, Vienna, Austria). The ‘survival’ and ‘survminer’ package were used for survival analysis, and the ‘ggplot2’ package was used for plotting. The clinicopathological characteristics of patients were descriptive. The measurement variables were expressed as means and standard deviations. Count variables were expressed as percentages. The Kaplan-Meier method was used to draw the tumor-specific survival curve. The Log-rank test was used to examine differences in outcomes. Differences with P-values < 0.05 were considered statistically significant.
A total of 33 patients were included in this study, and the basic information is shown in Table 1. There were 16 (48.5%) males and 17 (51.5%) females, aged 61.0 (28.0-75.0) years; The median distance between the lower edge of the tumor and the anal verge was 3 (1.0-10.0) cm. The median interval time between NCRT and surgery was 25.4 (8.7-164.4) weeks. The median operation time was 57 (20.0-137.0) min. Four patients underwent salvage APR surgery, and organ preservation was achieved in 29/33 (87.9%) patients. The initial clinical T staging (cT): 9 (27.3%) patients were cT2, 19 (57.6%) patients were cT3, and 5 (15.2%) patients were cT4; The initial N staging (cN): 8 patients (24.2%) were cN negative, 25 patients (75.8%) were cN positive; The initial M stage (cM): 2 patients (6.1%) had distant metastasis (cM1), 31 (93.9%) patients had no distant metastasis (cM0).
| Variables | n (%) or median (range) |
| Age (years) | 61.0 (28.0-75.0) |
| Gender | |
| Male | 16 (48.5) |
| Female | 17 (51.5) |
| Diameter of lesion (cm) | 2.0 (1.0-3.0) |
| Distance from the anal verge (cm) | 3.0 (1.0-10.0) |
| cT baseline | |
| 1 | 0 (0.0) |
| 2 | 9 (27.3) |
| 3 | 19 (57.6) |
| 4 | 5 (15.2) |
| cN baseline | |
| Negative | 8 (24.2) |
| Positive | 25 (75.8) |
| cM baseline | |
| M0 | 31 (93.9) |
| M1 | 2 (6.1) |
| ycT | |
| 0 | 21 (63.6) |
| 1 | 5 (15.2) |
| 2 | 6 (18.2) |
| 3 | 1 (3.0) |
| 4 | 0 (0.0) |
| ypT | |
| 0 | 18 (54.5) |
| 1 | 6 (18.2) |
| 2 | 7 (21.2) |
| 3 | 2 (6.1) |
| 4 | 0 (0.0) |
| Differentiation | |
| G1 | 3 (9.1) |
| G2 | 29 (87.9) |
| G3 | 1(3.0) |
| Baseline CEA | |
| Normal | 32 (97.0) |
| Abnormal | 1 (3.0) |
| Interval between NCRT and surgery (week) | 25.4 (8.7-164.4) |
| Chemotherapy | |
| Cap | 13 (39.4) |
| CapeOx | 20 (60.6) |
| Surgical duration (minute) | 57.0 (20.0-137.0) |
| Blood loss (mL) | 10.0 (5.0-50.0) |
| Postoperative hospital stay (days) | 4.0 (1.0-11.0) |
| Complication | |
| No complication | 26 (78.8) |
| With complication | 7 (21.2) |
| CD grade | |
| Grade I | 1 (3) |
| Grade II | 6 (18.2) |
| Grade III-IV | 0 (0.0) |
| Recurrence | |
| No | 31 (93.9) |
| Yes | 2 (6.1) |
| Metastasis (cM0, n = 31) | |
| No | 28 (90.3) |
| Yes | 3 (9.7) |
| Follow-up time (month) | 42.0 (4.0-93.5) |
The pathological results: 18 (54.5%) patients were pathological T0 stage (ypT0), 6 (18.2%) patients were ypT1, 7 (21.2%) patients were ypT2, and 2 (6.1%) patients were ypT3. Table 2 shows the treatment response of the patients. For 9 cT2 patients, 5 (5/9, 55.6%) had a postoperative pathological result of ypT0. For 19 cT3 patients, 11 (57.9%) patients were ypT0, and 2 (40%) were ypT0 in 5 cT4 patients.
| Pre-cT | ypT after local excision | |||
| ypT0 | ypT1 | ypT2 | ypT3 | |
| cT2 (n = 9) | 5 (55.6) | 2 (22.2) | 2 (22.2) | 0 (0.0) |
| cT3 (n = 19) | 11 (57.9) | 3 (15.8) | 4 (21.1) | 1 (5.3) |
| cT4 (n = 5) | 2 (40.0) | 1 (20.0) | 1 (20.0) | 1 (20.0) |
Table 3 shows the perioperative complications of patients. The overall postoperative complication rate was 21.2% (7/33). The most common complication was bleeding (43%, 3/7), followed by chronic perineal pain and stenosis.
| Gender | Age | Neoadjuvant | Interval from NCRT to local excision (week) | Type of complications | Clavien-Dindo grades |
| Male | 74 | NCRT | 69 | Bleeding | II |
| Male | 68 | NCRT | 18 | Bleeding | I |
| Female | 59 | NCRT | 11 | Perineal pain | II |
| Female | 71 | NCRT | 11 | Perineal pain | II |
| Male | 40 | NCRT + CapOx | 24 | Perineal pain + stricture | II |
| Male | 56 | NCRT + CapOx | 59 | Pain + fecal incontinence | II |
| Female | 66 | NCRT + CapOx | 32 | Bleeding + pain | II |
The median follow-up time was 42.0 (4.0-93.5) months. For 31 patients with cM0, the 5-year DFS rate, 5-year LRFS rate, and 5-year OS rate were 88.4%, 96.7%, and 92.9%, respectively. Three patients (9.7%, 3/31) had disease progression, including 2 cases of distant metastasis (1 case of lung metastasis, 1 case of bone metastasis) and 1 case of local tumor recurrence and distant metastasis (sacrum and lung metastasis). After local recurrence or distant metastasis, patients received systemic combined local treatment, one patient achieved no evidence of disease status, and two patients died (Shown in Table 4). Figure 1, Figure 2, and Figure 3 show the survival of patients with different cT stages, ycT stages, and ypT stages. There were no significant differences between the cT groups concerning DFS, LRFS or OS in the Kaplan-Meier analysis. There were significant differences between the ycT groups (ycT ≤ T1 vs ycT ≥ T2) concerning either DFS (P = 0.042) or OS (P = 0.002) in the Kaplan-Meier analysis. The LRFS curve of ycT ≤ T1 patients was better than that of ycT ≥ T2 patients, and the P value was very close to 0.05 (P = 0.070). The DFS curve of patients with ypT ≤ T1 was better than that of patients with ypT ≥ T2, but the P value was not statistically significant (P = 0.560). There was a significant difference between the ypT groups (ypT ≤ T1 vs ypT ≥ T2) concerning OS (P = 0.014) in the Kaplan-Meier analysis. The LRFS curve of ypT ≤ T1 patients was better than that of ypT ≥ T2 patients, and the P value was very close to 0.05 (P = 0.070). Two patients with initial cM1 were alive at the last follow-up.
| Patient (sex/age) | Primary staging | NCRT | Interval time (week) | Response | Disease progression | Prognosis | ||||||||
| cT | cN | cM | High-risk factor (s) | mrTRG | ypT | Local recurrence | Distant metastasis | Treatment | DFS (month) | OS (month) | Status | |||
| Male/74 | T4 | N1 | cM0 | cT4 | NCRT | 8.7 | 0 | ypT0 | Negative | Bone | C | 9.3 | 64.6 | Dead |
| Female/57 | T3 | N2 | cM0 | EMVI+ | NCRT + CapOx2 | 9 | 0 | ypT0 | Negative | Lung | C+ resection | 27.5 | 76.5 | Alive (NED) |
| Female /61 | T2 | N2 | cM0 | N2 | NCRT | 101 | 3 | ypT0 | Positive | Sacrum and Lung | Resection | 6.1 | 49.4 | Dead |
| Female /36 | T4 | N1 | cM1 | cT4 | CRT + CapOx2 | 19 | 2 | ypT2 | Negative | Lung | C+ resection | 42.0 | Alive (NED) | |
| Female /30 | T4 | N2 | cM1 | cT4 | CRT+CapOx1 | 36 | 1 | ypT0 | Positive | Liver | Resection | 27.5 | Alive (NED) | |
There are few studies on LE after NCRT for rectal cancer, and there are significant differences in surgical indications, technical and pathological reports, postoperative complications, and survival[13,21,22]. The efficacy of LE in patients with different stages is inconsistent[23-26]. This study retrospectively collected the data of patients who underwent LE in our center and clarified the good organ preservation rate, oncological outcomes, and the safety and efficacy of LE after NCRT for rectal cancer. In addition, we found that for patients with initial cM1 and significant local tumor regression after preoperative treatment, LE combined with late systemic treatment also seems to have better clinical results. We believe that for highly selected patients, under the guarantee of salvage surgery and complete postoperative follow-up, the treatment strategy of LE with patient participation in decision-making after NCRT can not only obtain organ preservation but also achieve good long-term survival outcomes.
Our results show that LE is feasible and effective for rectal cancer with significant tumor regression after NCRT. LE was successfully performed in all patients with a median postoperative hospital stay of 4 days. The median operation time was 57.0 minutes, the median intraoperative blood loss was about 10 mL, and no intraoperative adverse events occurred. These results are better than those of conventional TME surgery, which is consistent with the results reported in the meta-analysis[18]. Four patients underwent salvage APR surgery, and organ preservation was achieved in all 29 patients. Previous studies have found that the incidence of complications of LE after NCRT is not low, mainly manifested as incision dehiscence, bleeding, and pain[15-17,27]. Gascon et al[28] reported the results of local full-thickness excision for 404 patients with rectal adenoma or rectal cancer, with a complication rate of 12.6%, among which postoperative bleeding was the most common early complication, with an incidence rate of 8%, but the patients in this study did not receive radio chemotherapy before surgery. Geubels et al[29] reported LE in patients with regeneration after watch and wait (WW) and the authors believed that LE after radiotherapy had higher surgical complications compared with patients who did not receive radiotherapy. In our study, all patients received preoperative IMRT, which caused intestinal edema, fibrosis changes, and relatively poor intestinal quality, which could explain the high overall complication rate of 21.2% (7/33). The most common complication was chronic perineal pain (71.4%, 5/7), followed by bleeding (43%, 3/7), stenosis (14.3%, 1/7), and fecal incontinence (14.3%, 1/7). However, all complications were grade I-II and improved after the drug or conservative treatment, and no serious postoperative adverse events occurred. Therefore, we believe that LE has good safety for rectal cancer with a good response after NCRT. There is no unified standard for LE after chemoradiotherapy. The experience of our center is as follows: For residual tumors or scars after chemoradiotherapy, it is recommended to perform localized scar and full-thickness bowel wall resection followed by full-thickness suture. Because the defect of the intestinal wall after tumor resection, especially the significant retraction of the mucosa, will make the tension of the primary suture high. So the problems of incision dehiscence, dead space, and infection caused by large-scale mucosal resection and simple mucosal suture should be avoided. For the mass of the anterior wall, attention should be paid to the protection of the prostate or vagina with a rich blood supply during the suture, to avoid intraoperative or postoperative bleeding.
As a surgical treatment method that ignores the whole mesentery, LE breaks the long-standing principle of TME, but local recurrence is its Achilles heel, and patients with different T stages have different recurrence rates[30]. In a multicenter prospective study of LE in T1 patients and LE plus adjuvant chemoradiotherapy in T2 patients, the 7-year local recurrence rate was 8% in T1 patients and 18% in T2 patients[23]. A systematic review including 20 studies and 1068 patients showed that the local recurrence rate of ypT0 patients was 4%, and that of ypT1-3 patients was more than 21.9%[31]. In our study, the local recurrence rate was 0% (0/17) in patients with ypT0 and 7.1% (1/14) in patients with ypT1-3. No recurrence or metastasis events occurred in the 2 patients with ypT3. One of the patients with ypT3 underwent salvage surgery, and the other patient refused salvage surgery and took an observation strategy. Local recurrence occurred in only 1 (ycT2/ypT2) of 31 patients with cM0 rectal cancer and did not result in uncontrolled regional disease. Our study showed a very high local control rate, and the overall 5-year LRFS rate was 96.7%. The 5-year DFS rate was 88.4%, which is similar to CARTS Study[9], but our study had more clinical stage III patients and a better local recurrence rate. Although 3 patients (9.7%, 3/31) had disease progression (recurrence and/or metastasis), the OS of the patients after systemic therapy was up to 76.5 months, and the 5-year OS rate was 92.9%, showing a good survival result. Similar to our findings, ACOSOG Z6041 also showed favorable oncological outcomes. When the authors performed NCRT plus LE surgery in T2N0 patients, distant recurrence occurred in 6% of patients and local recurrence occurred in 4% of patients, resulting in 3-year disease-free and OS rates of 88% and 95%, respectively[15]. We believe that this better prognosis result was due to patient screening. In our study, after intensive NCRT, most of the patients were the ypT0-2 stage, and only 2 were ypT3 stage. On the other hand, salvage surgery based on postoperative pathology ensures a good prognosis for patients. The 2 initial M1 patients were both alive at the last follow-up, and the OS was 27.5 months and 42.0 months, respectively, which achieved a good prognosis but needed more data support. We believe that for highly selected patients, under the guarantee of salvage surgery and complete postoperative follow-up, the treatment strategy of LE after NCRT can not only obtain organ preservation but also achieve good long-term survival outcomes.
Our study did not involve the analysis of predictive factors for tumor recurrence and metastasis. However, the KM survival curve showed that the LRFS, DFS, and OS curves of patients with earlier ycT and ypT stages (ycT ≤ T1 and ypT ≤ T1) were better than those of other patients. Previous reports have shown that the pT stage, sm stage, tumor grading, histological risk status, and local surgical resection technique are independent risk factors for local recurrence[6,32]. Other studies have also shown a large difference in the recurrence rate of different T stages. It has been reported that the 5-year LRFS after local resection is 28%, which is 18% in T1 patients and 47% in T2 patients[30]. In our study, the 5-year LRFS rates of patients with ycT0-1 and ypT0-1 were both 100%, so we should strengthen the follow-up of patients with ypT2 and more after LE.
Our study had some limitations. First, it was a retrospective study. High-quality prospective randomized controlled studies may be needed to verify our better results. Second, the number of patients is not large, and this limited our statistical analyses, and we may need to expand the sample size for further verification.
For rectal cancer patients with near-cCR after NCRT, selective implementation of LE is a safe and effective treatment strategy. For patients judged as cCR or possible pCR, the non-surgical strategy of the WW strategy can be selected[11]. Even in the WW process, early local regeneration followed by LE is a safe and effective treatment strategy. So, we emphasize the importance of predicting tumor response to NCRT, which is also described in our other article. Minimally invasive or nonsurgical treatment with patient participation in decision-making can be performed for highly selected patients.
LE for rectal cancer with significant tumor regression after NCRT can obtain better safety, efficiency, and oncological prognosis. Minimally invasive or nonsurgical treatment with patient participation in decision-making can be performed for highly selected patients. More studies are needed to verify this result.
| 1. | National Health Commission of the People's Republic of China. National guidelines for diagnosis and treatment of colorectal cancer 2020 in China (English version). Chin J Cancer Res. 2020;32:415-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Zaborowski A, Stakelum A, Winter DC. Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer. Br J Surg. 2019;106:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 3. | Lindgren R, Hallböök O, Rutegård J, Sjödahl R, Matthiessen P. What is the risk for a permanent stoma after low anterior resection of the rectum for cancer? A six-year follow-up of a multicenter trial. Dis Colon Rectum. 2011;54:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 4. | Bulens PP, Smets L, Debucquoy A, Joye I, D'Hoore A, Wolthuis A, Debrun L, Dekervel J, Van Cutsem E, Dresen R, Vandecaveye V, Deroose CM, Sagaert X, Haustermans K. Nonoperative versus operative approach according to the response to neoadjuvant chemoradiotherapy for rectal cancer: A prospective cohort study. Clin Transl Radiat Oncol. 2022;36:113-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Morson BC, Bussey HJ, Samoorian S. Policy of local excision for early cancer of the colorectum. Gut. 1977;18:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 195] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Dekkers N, Dang H, van der Kraan J, le Cessie S, Oldenburg PP, Schoones JW, Langers AMJ, van Leerdam ME, van Hooft JE, Backes Y, Levic K, Meining A, Saracco GM, Holman FA, Peeters KCMJ, Moons LMG, Doornebosch PG, Hardwick JCH, Boonstra JJ. Risk of recurrence after local resection of T1 rectal cancer: a meta-analysis with meta-regression. Surg Endosc. 2022;36:9156-9168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Lee L, Kelly J, Nassif GJ, Atallah SB, Albert MR, Shridhar R, Monson JRT. Chemoradiation and Local Excision for T2N0 Rectal Cancer Offers Equivalent Overall Survival Compared to Standard Resection: a National Cancer Database Analysis. J Gastrointest Surg. 2017;21:1666-1674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Halverson AL, Morris AM, Cleary RK, Chang GJ. For Patients with Early Rectal Cancer, Does Local Excision Have an Impact on Recurrence, Survival, and Quality of Life Relative to Radical Resection? Ann Surg Oncol. 2019;26:2497-2506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens JJME, van Meerten E, Tanis PJ, de Hingh IHJT, van der Schelling GP, Acherman Y, Leijtens JWA, Bremers AJA, Beets GL, Hoff C, Verhoef C, Marijnen CAM, de Wilt JHW; CARTS Study Group. Long-term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg. 2019;154:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 179] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 10. | Landmann RG, Wong WD, Hoepfl J, Shia J, Guillem JG, Temple LK, Paty PB, Weiser MR. Limitations of early rectal cancer nodal staging may explain failure after local excision. Dis Colon Rectum. 2007;50:1520-1525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Wang L, Zhang XY, Zhao YM, Li SJ, Li ZW, Sun YS, Wang WH, Wu AW; Rectal Cancer Cooperative Group of Peking University Cancer Hospital. Intentional Watch and Wait or Organ Preservation Surgery Following Neoadjuvant Chemoradiotherapy Plus Consolidation CAPEOX for MRI-defined Low-risk Rectal Cancer: Findings From a Prospective Phase 2 Trial (PKUCH-R01 Trial, NCT02860234). Ann Surg. 2023;277:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 12. | Cerdán-Santacruz C, Vailati BB, São Julião GP, Habr-Gama A, Perez RO. Watch and wait: Why, to whom and how. Surg Oncol. 2022;43:101774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 13. | Smith FM, Ahad A, Perez RO, Marks J, Bujko K, Heald RJ. Local Excision Techniques for Rectal Cancer After Neoadjuvant Chemoradiotherapy: What Are We Doing? Dis Colon Rectum. 2017;60:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Madoff RD. Total mesorectal neglect in the age of total mesorectal excision. J Clin Oncol. 2013;31:4273-4275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, Thomas CR Jr, Chan E, Cataldo PA, Marcet JE, Medich DS, Johnson CS, Oommen SC, Wolff BG, Pigazzi A, McNevin SM, Pons RK, Bleday R. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16:1537-1546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 312] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 16. | Perez RO, Habr-Gama A, Lynn PB, São Julião GP, Bianchi R, Proscurshim I, Gama-Rodrigues J. Transanal endoscopic microsurgery for residual rectal cancer (ypT0-2) following neoadjuvant chemoradiation therapy: another word of caution. Dis Colon Rectum. 2013;56:6-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 17. | Rullier E, Rouanet P, Tuech JJ, Valverde A, Lelong B, Rivoire M, Faucheron JL, Jafari M, Portier G, Meunier B, Sileznieff I, Prudhomme M, Marchal F, Pocard M, Pezet D, Rullier A, Vendrely V, Denost Q, Asselineau J, Doussau A. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (1)] |
| 18. | Ahmad NZ, Abbas MH, Abunada MH, Parvaiz A. A Meta-analysis of Transanal Endoscopic Microsurgery versus Total Mesorectal Excision in the Treatment of Rectal Cancer. Surg J (N Y). 2021;7:e241-e250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Wang L, Li ZY, Li ZW, Li YH, Sun YS, Ji JF, Gu J, Cai Y. Efficacy and safety of neoadjuvant intensity-modulated radiotherapy with concurrent capecitabine for locally advanced rectal cancer. Dis Colon Rectum. 2015;58:186-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Li C, Guan Z, Zhao Y, Sun T, Li Z, Wang W, Li Z, Wang L, Wu A. Predictors of pathologic complete response in patients with residual flat mucosal lesions after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Chin J Cancer Res. 2022;34:383-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Leijtens JWA, Smits LJH, Koedam TWA, Orsini RG, van Aalten SM, Verseveld M, Doornebosch PG, de Graaf EJR, Tuynman JB. Long-term oncological outcomes after local excision of T1 rectal cancer. Tech Coloproctol. 2023;27:23-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (1)] |
| 22. | Ung L, Chua TC, Engel AF. A systematic review of local excision combined with chemoradiotherapy for early rectal cancer. Colorectal Dis. 2014;16:502-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Greenberg JA, Shibata D, Herndon JE 2nd, Steele GD Jr, Mayer R, Bleday R. Local excision of distal rectal cancer: an update of cancer and leukemia group B 8984. Dis Colon Rectum. 2008;51:1185-91; discussion 1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Guerrieri M, Gesuita R, Ghiselli R, Lezoche G, Budassi A, Baldarelli M. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol. 2014;20:9556-9563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Peltrini R, Imperatore N, Di Nuzzo MM, Pellino G. Towards personalized treatment of T2N0 rectal cancer: A systematic review of long-term oncological outcomes of neoadjuvant therapy followed by local excision. J Gastroenterol Hepatol. 2022;37:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Peltrini R, Sacco M, Luglio G, Bucci L. Local excision following chemoradiotherapy in T2-T3 rectal cancer: current status and critical appraisal. Updates Surg. 2020;72:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 27. | Arezzo A, Arolfo S, Allaix ME, Munoz F, Cassoni P, Monagheddu C, Ricardi U, Ciccone G, Morino M. Results of neoadjuvant short-course radiation therapy followed by transanal endoscopic microsurgery for t1-t2 n0 extraperitoneal rectal cancer. Int J Radiat Oncol Biol Phys. 2015;92:299-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Gascon MA, Aguilella V, Martinez T, Antinolfi L, Valencia J, Ramírez JM. Local full-thickness excision for sessile adenoma and cT1-2 rectal cancer: long-term oncological outcome. Langenbecks Arch Surg. 2022;407:2431-2439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 29. | Geubels BM, Meyer VM, van Westreenen HL, Beets GL, Grotenhuis BA; On Behalf Of The Dutch Watch And Wait Consortium. Role of Local Excision for Suspected Regrowth in a Watch and Wait Strategy for Rectal Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 30. | Mellgren A, Sirivongs P, Rothenberger DA, Madoff RD, García-Aguilar J. Is local excision adequate therapy for early rectal cancer? Dis Colon Rectum. 2000;43:1064-71; discussion 1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 332] [Cited by in RCA: 289] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 31. | Hallam S, Messenger DE, Thomas MG. A Systematic Review of Local Excision After Neoadjuvant Therapy for Rectal Cancer: Are ypT0 Tumors the Limit? Dis Colon Rectum. 2016;59:984-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Morino M, Allaix ME, Caldart M, Scozzari G, Arezzo A. Risk factors for recurrence after transanal endoscopic microsurgery for rectal malignant neoplasm. Surg Endosc. 2011;25:3683-3690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/