Published online Aug 15, 2023. doi: 10.4251/wjgo.v15.i8.1475
Peer-review started: June 20, 2023
First decision: July 7, 2023
Revised: July 17, 2023
Accepted: July 27, 2023
Article in press: July 27, 2023
Published online: August 15, 2023
Processing time: 51 Days and 6.7 Hours
Gastric cancer (GC) is a common malignant tumor of the digestive system with a high degree of malignancy. It usually develops insidiously without any specific symptoms in the early stages. As one of the diseases caused by abnormal gene changes, GC has abnormal expression of various oncogenes and products during its development. Tumor markers such as carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA199) and carbohydrate antigen 724 (CA724) are not expressed or lowly expressed in normal people, but significantly increased after carcinogenesis. Monitoring the changes in the levels of tumor markers such as CEA, CA199 and CA724 is conducive to early diagnosis and evaluation of the occurrence of some solid tumors.
To investigate the expression of CEA, CA199 and CA724 in GC and their correlation with clinical features, hoping to provide more effective markers for the early preventive diagnosis of GC.
Of 87 patients with GC admitted to our hospital from September 2020 to December 2021 were included in the GC group, and another 80 healthy people who came to our hospital for physical examination with normal results during the same period were selected as the control group. The serum CEA, CA199, and CA724 levels were compared between the two groups, and the serum CEA, CA199, and CA724 levels were compared in patients with GC at different TNM stages, and the differences in the positive rates of CEA, CA199, and CA724 alone and in combination in detecting TNM stages of GC and GC were compared. In addition, the relationship between the levels of tumor markers CEA, CA199 and CA724 and the clinicopathological characteristics of GC patients was also analyzed. The relationship between the serum levels of CEA, CA199 and CA724 and the survival period of GC patients was analyzed by Pearson.
The serum levels of CEA, CA199 and CA724 in GC group were significantly higher than those in control group (P < 0.05). With the increase of TNM stage, the serum CEA, CA199 and CA724 expression levels in GC patients increased significantly, and the differences between groups were statistically significant (P < 0.05). The positive rate of the CA724 single test was higher than that of CEA and CA199 single test (P < 0.05). The positive rate of the three combined tests was 95.40% (83/87), which was higher than that of CEA, CA199 and CA724 single tests. The difference was statistically significant (P < 0.05). The combined detection positive rates of CEA, CA199, and CA724 in stages I, II, III, and IV of GC were 89.66%, 93.10%, 98.85%, and 100.00% respectively, all of which were higher than the individual detection rates of CEA, CA199, and CA724. The differences were statistically significant (P < 0.05). There was no significant difference in serum CEA, CA199 and CA724 levels between GC patients with different genders, smoking history and alcohol history (P > 0.05). However, the serum CEA, CA199 and CA724 levels were significantly higher in GC patients aged ≥ 45 years, TNM stage III-IV, with lymph node metastasis and tumor diameter ≥ 5 cm than in GC patients aged < 45 years, TNM stage I-II, without lymph node metastasis and tumor diameter < 5 cm (P < 0.05).
The expression levels of serum tumor markers CEA, CA199 and CA724 in patients with GC are high and rise with the increase of TNM stage. The levels of CEA, CA199 and CA724 are related to age, TNM stage, lymph node metastasis and tumor diameter. The combined detection of CEA, CA199 and CA724 is helpful to improve the diagnostic accuracy of GC with high clinical guidance value.
Core Tip: In this retrospective analysis, the expression levels of tumor markers carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA199) and carbohydrate antigen 724 (CA724) in gastric cancer (GC) patients and normal people were detected. In addition, the serum CEA, CA199 and CA724 levels in GC patients with different TNM stages, and the differences in the positive rates of TNM stages of GC and GC detected by CEA, CA199 and CA724 alone and in combination were compared. The relationship between tumor markers CEA, CA199 and CA724 levels and clinicopathological characteristics of GC patients was analyzed.
- Citation: Wang R, Zuo CL, Zhang R, Zhu LM. Carcinoembryonic antigen, carbohydrate antigen 199 and carbohydrate antigen 724 in gastric cancer and their relationship with clinical prognosis. World J Gastrointest Oncol 2023; 15(8): 1475-1485
- URL: https://www.wjgnet.com/1948-5204/full/v15/i8/1475.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i8.1475
Gastric cancer (GC) is a common malignant tumor of the digestive system originating from gastric epithelial cells, which can occur at any age, especially in the middle-aged and elderly population aged 40 to 60 years, and is more common in males than in females[1], with greater clinical harm, more than 1 million new cases each year worldwide, advanced GC has a high mortality rate, and the prognosis is unsatisfactory, seriously threatening the life safety and quality of life of patients[2,3]. According to authoritative literature, GC as a disease with high molecular and phenotypic consistency, the etiological factors are diverse, of which age, smoking, Helicobacter pylori infection, low fruit and vegetable intake and high salt intake are all high risk factors for the incidence of GC[4,5]. Timely adjustment of diet, smoking cessation and appropriate exercise are expected to be effective means to prevent the incidence of GC, while for patients who have suffered from GC, early examination of tumor markers is more beneficial to early diagnosis of the disease, which is of great significance for improving the survival rate and prognosis of patients[6].
Carcinoembryonic antigen (CEA), first extracted from colon cancer and embryonic tissues, is an acidic glycoprotein with human embryonic antigen characteristics, and can also exist in the form of membrane structural proteins on the surface of cancer cells and surrounding body fluids[7]. It is a common marker of solid tumors such as colon cancer, lung cancer, breast cancer and GC, and the change of its expression level can be used as an effective indicator to evaluate the therapeutic effect and prognosis of malignant tumor diseases[8,9]. Carbohydrate antigen 199 (CA199), an oligosaccharide tumor-associated antigen, is a high-molecular weight glycoprotein mixture, a glycolipid substance on the cell membrane. It can be secreted by the human colon, stomach, pancreas and other epithelia, and significantly increased expression in digestive tract tumors. It can be used as a monitoring index to predict the treatment, follow-up and judgment of recurrence of digestive tract tumors[10]. Carbohydrate antigen 724 (CA724) is a specific glycoprotein of tumor cells. As a high molecular glycoprotein CEA, CA724 is an important indicator for staging tumor and judging digestive tract tumors. Previous studies have also suggested that patients with GC often show elevated expression of CA724. Monitoring changes in CA724 levels has a high guiding value for the screening and treatment of GC[11]. Although a large number of studies have found that CEA, CA199 and CA724 have the potential to be used as markers to predict the development of digestive tract tumors[12,13], there are few reports on the expression of CEA, CA199 and CA724 in GC and their correlation with clinical features. Based on this, this study analyzed the clinical data of patients with GC admitted to our hospital retrospectively in order to investigate the expression levels of tumor markers such as CEA, CA199 and CA724 in patients with GC and their correlation with clinical parameters. It is expected to provide more data to support the early prevention, screening and treatment of GC.
Of 87 patients with GC admitted to our hospital from September 2020 to December 2021 were selected as the study subjects and included in the GC group, including 46 males and 41 females; aged 35 to 80 years, mean age (59.11 ± 6.02) years; 52 patients with smoking history, 35 patients without smoking history; 56 patients with alcohol history, 31 patients without alcohol history; TNM stage[14] was 20 patients in stage I, 23 patients in stage II, 28 patients in stage III, and 16 patients in stage IV. Another 80 healthy people who came to the hospital for physical examination during the same period and had normal results were selected as the control group, including 42 males and 38 females; aged 35-79 years, mean age (58.94 ± 5.76) years; 43 cases with smoking history, 37 cases without smoking history; 45 cases with alcohol history, 35 cases without alcohol history. There was no significant difference in gender, age, smoking and alcohol history between the two groups (P > 0.05), and the balance was comparable, as shown in Table 1.
| Group | n | Gender (n) | Age (yr) | Smoking history (case) | Alcohol history (n) | |||
| Male | Female | Yes | No | Yes | No | |||
| Gastric cancer group | 87 | 46 | 41 | 59.11 ± 6.02 | 52 | 35 | 56 | 31 |
| Control group | 80 | 41 | 39 | 58.94 ± 5.76 | 43 | 37 | 45 | 35 |
| χ2 | 0.044 | 0.186 | 0.616 | 1.149 | ||||
| P value | 0.834 | 0.853 | 0.433 | 0.284 | ||||
Inclusion criteria: All patients in the GC group were pathologically confirmed as GC; no radiotherapy, chemotherapy, immunotherapy and other anti-tumor treatment were received before admission; the control group was normal physical examination results and healthy people; aged ≥ 18 years; clinical history and data were complete.
Exclusion criteria: Patients with heart, liver, kidney and other vital organ dysfunction; combined with other malignant tumor diseases; combined autoimmune diseases, blood diseases; combined cognitive dysfunction or mental illness; pregnant, lactating women.
Of 5 mL of fasting cubital venous blood was collected from patients in the GC group in the morning on the next day after admission and from the control group in the morning on the day of physical examination. The whole blood was centrifuged at 3000 r/min using a high-speed centrifuge for 10 min to obtain the upper layer of serum, which was stored at -80 °C thereafter for future use. Serum CEA, CA199 and CA724 levels were measured by automatic electrochemiluminescence immunoassay analyzer (manufacturer: Roche Biotechnology, Switzerland, model: cobas e601) in the two groups of subjects, respectively. All kits were purchased from Shanghai Enzyme Linked Immunology Co., Ltd. All experimental procedures were performed in strict accordance with the kit instructions. The criteria for positive detection were as follows: CEA > 3.4 ng/mL, CA199 > 39 U/mL, CA724 > 9.8 U/mL, and if one of the indicators in the combined detection was positive, it was judged as positive.
SPSS 22.0 software was used to process and analyze all data in this study. Measurement data were expressed as (mean ± SD). One-way analysis of variance was used to compare multiple groups. t test was used to compare two groups. Enumeration data were expressed as percentage and χ2 test was used. Pearson analysis was performed to analyze the relationship between CEA, CA199 and CA724 levels and the survival period of GC patients. P < 0.05 was considered statistically significant.
This study has obtained approval from the Ethics Committee of The First People’s Hospital of Lianyungang, and has been conducted in accordance with the principles outlined in the Helsinki Declaration and Good Clinical Practice guidelines. All patients information are strictly confidential, so the informed consent was waived by the Ethics Committee.
The expression levels of serum tumor markers CEA, CA199 and CA724 in the two groups were compared. The results showed that the serum CEA, CA199 and CA724 levels in the GC group were significantly higher than those in the control group, and the differences were statistically significant (P < 0.05), as shown in Table 2.
| Group | n | CEA (ng/mL) | CA199 (U/mL) | CA724 (U/mL) |
| Gastric cancer group | 87 | 23.69 ± 4.83 | 52.75 ± 8.52 | 41.32 ± 6.53 |
| Control group | 80 | 2.58 ± 0.79 | 4.62 ± 1.49 | 5.10 ± 1.27 |
| t value | 38.611 | 49.819 | 48.759 | |
| P value | < 0.001 | < 0.001 | < 0.001 |
The differences in the expression levels of serum tumor markers CEA, CA199 and CA724 between patients with different TNM stages of GC were compared, and the results showed that the expression levels of serum CEA, CA199 and CA724 in patients with GC increased significantly with TNM stage, and the differences between groups were statistically significant (P < 0.05), as shown in Table 3.
| Group | n | CEA (ng/mL) | CA199 (U/mL) | CA724 (U/mL) |
| Gastric cancer stage I group | 20 | 18.05 ± 4.16 | 46.31 ± 6.78 | 34.29 ± 5.46 |
| Gastric cancer stage II group | 23 | 21.43 ± 4.22a | 50.25 ± 5.27a | 39.41 ± 5.89a |
| Gastric cancer stage III group | 28 | 25.58 ± 5.37a,b | 54.33 ± 7.64a,b | 43.68 ± 6.03a,b |
| Gastric cancer stage IV group | 16 | 30.68 ± 4.83a,b,c | 61.63 ± 8.11a,b,c | 48.72 ± 6.95a,b,c |
| F value | 29.482 | 21.709 | 25.139 | |
| P value | < 0.001 | < 0.001 | < 0.001 |
The differences in the positive rates of GC detected by serum tumor markers CEA, CA199 and CA724 alone and in combination were compared. The results showed that the positive rate of CA724 single detection was higher than that of CEA and CA199 single detection (P < 0.05); the positive rate of the three combined detection was 95.40% (83/87), higher than that of CEA, CA199 and CA724 single detection, and the differences were statistically significant (P < 0.05), as shown in Table 4.
The positive rates of CEA, CA199 and CA724 in detecting different TNM stages of GC were compared. The results showed that the positive rates of CEA, CA199 and CA724 in detecting I, II, III and IV stages of GC were 89.66%, 93.10%, 98.85% and 100.00%, respectively, which were higher than those in CEA, CA199 and CA724. The differences were statistically significant (P < 0.05) in Table 5.
| Indicators | Gastric cancer stage I | Gastric cancer stage II | Gastric cancer stage III | Gastric cancer stage IV |
| CEA | 45 (51.72) | 44 (50.57) | 47 (54.02) | 48 (55.17) |
| CA199 | 42 (48.28) | 48 (55.17) | 50 (57.47) | 52 (59.77) |
| CA724 | 59 (67.82) | 60 (68.97) | 64 (73.56) | 65 (74.71) |
| Combined | 78 (89.66)a,b,c | 81 (93.10)a,b,c | 86 (98.85)a,b,c | 87 (100.00)a,b,c |
The differences in serum CEA, CA199 and CA724 levels between GC patients with different genders, ages, smoking history, alcohol history, TNM stage of tumor, lymph node metastasis and tumor diameter were compared, and the correlation between the changes in their levels and clinicopathological characteristics was analyzed. The results showed that there was no significant difference in serum CEA, CA199 and CA724 levels between GC patients with different genders, smoking history and alcohol history (P > 0.05), while the serum CEA, CA199 and CA724 Levels in GC patients with age ≥ 45 years, TNM stage III-IV, lymph node metastasis and tumor diameter ≥ 5 cm were significantly higher than those in GC patients with age < 45 years, TNM stage I-II, no lymph node metastasis and tumor diameter < 5 cm. The differences were statistically significant (P < 0.05), as shown in Table 6.
| Clinical pathology | n | CEA (ng/mL) | CA199 (U/mL) | CA724 (U/mL) | |
| Gender | Male | 46 | 23.42 ± 4.65 | 52.49 ± 7.83 | 41.29 ± 6.18 |
| Female | 41 | 23.99 ± 5.12 | 53.04 ± 7.67 | 41.35 ± 6.22 | |
| Age | < 45 years old | 60 | 20.47 ± 8.53 | 49.18 ± 9.13 | 39.02 ± 6.45 |
| ≥ 45 years old | 27 | 30.85 ± 8.77a | 60.68 ± 8.85a | 46.43 ± 6.76a | |
| Smoking history | Yes | 52 | 23.51 ± 4.43 | 52.66 ± 8.41 | 41.57 ± 6.17 |
| No | 35 | 23.96 ± 4.50 | 52.88 ± 8.35 | 40.95 ± 6.03 | |
| Alcohol history | Yes | 56 | 23.49 ± 4.81 | 52.69 ± 7.41 | 41.30 ± 5.94 |
| No | 31 | 24.05 ± 4.87 | 52.86 ± 9.03 | 41.36 ± 6.12 | |
| TNM staging | Phase I-II | 43 | 21.02 ± 4.65 | 48.63 ± 8.75 | 37.25 ± 6.88 |
| Phase III-IV | 44 | 26.30 ± 4.72b | 56.78 ± 9.24b | 45.30 ± 7.13b | |
| lymphatic metastasis | No | 45 | 20.83 ± 4.23 | 46.16 ± 7.36 | 38.11 ± 6.59 |
| Yes | 42 | 26.75 ± 4.88c | 59.81 ± 8.48c | 44.76 ± 6.13c | |
| Tumor diameter | < 5 cm | 49 | 19.61 ± 4.67 | 47.02 ± 8.55 | 35.26 ± 6.62 |
| ≥ 5 cm | 38 | 28.95 ± 5.01d | 60.14 ± 9.31d | 49.13 ± 6.89d |
In order to investigate the relationship between serum CEA, CA199 and CA724 levels and prognosis in patients with GC, we measured serum CEA, CA199 and CA724 levels in 87 patients with GC before and 3 mo after radical gastrectomy. The results showed that the serum levels of CEA, CA199 and CA724 decreased in patients 3 mo after surgery compared with those before surgery, and the differences were statistically significant (P < 0.05) in Table 7.
| Group | n | CEA (ng/mL) | CA199 (U/mL) | CA724 (U/mL) |
| Before procedure | 87 | 33.11 ± 3.63 | 57.72 ± 6.64 | 31.23 ± 3.45 |
| After procedure | 87 | 7.24 ± 1.81 | 14.23 ± 2.37 | 4.12 ± 1.12 |
| t value | 59.488 | 57.536 | 69.712 | |
| P value | < 0.001 | < 0.001 | < 0.001 |
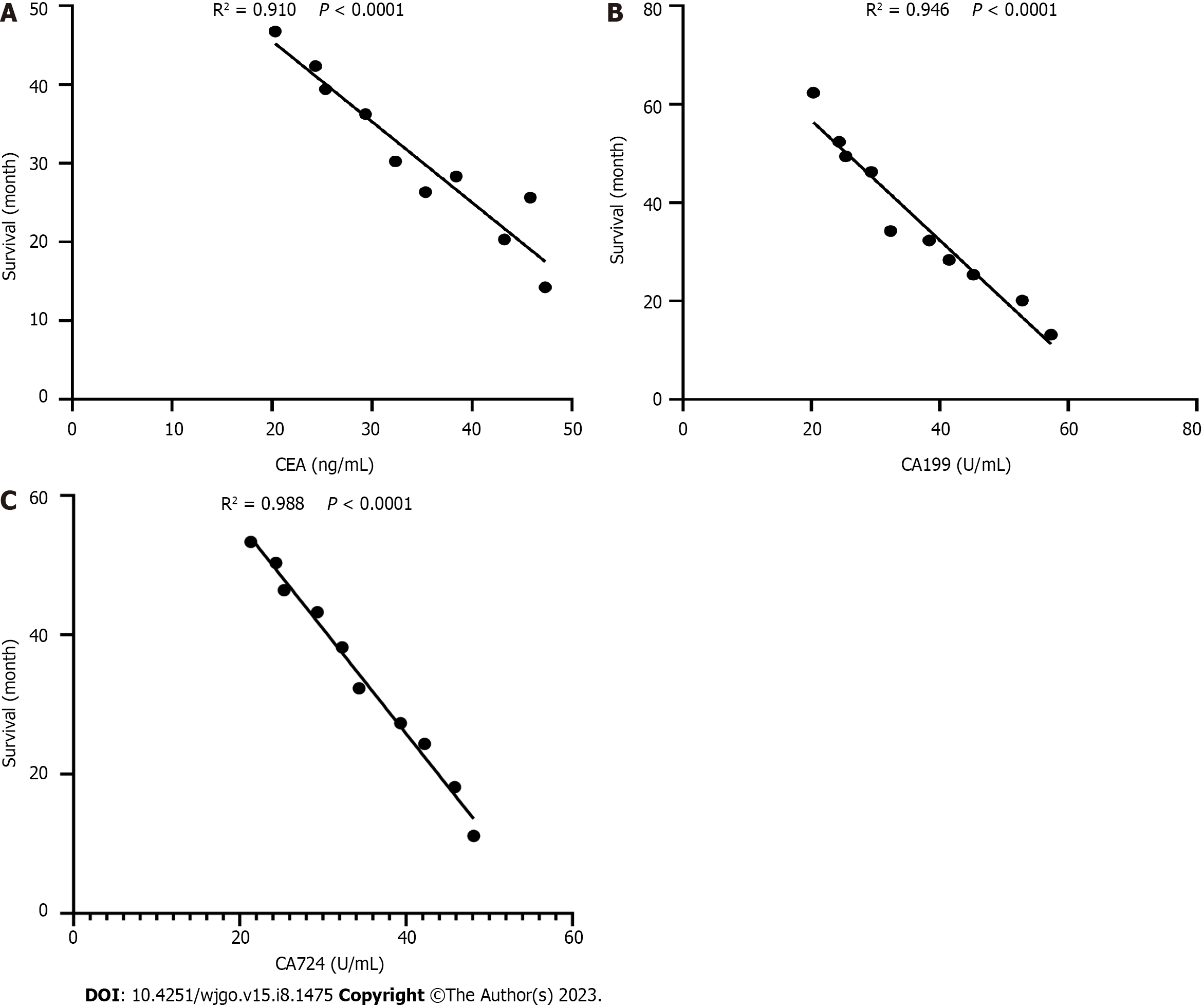
Of 10 patients were selected from GC patients, and the relationship between the serum levels of CEA, CA199, and CA724 in GC and the survival rate of GC patients was analyzed using Pearson. The results showed that the levels of CEA, CA199 and CA724 were negatively correlated with the survival period of GC patients (Figures 1A-C).
GC is one of the malignant tumors of the digestive tract originating from the epithelial cells of the surface mucosa of the gastric wall, mostly occurring in the antrum, pylorus, cardia and other parts[15,16]. The advanced GC has a very high degree of malignancy and is a great threat to the patient's survival cycle and quality of life[17,18]. Early GC has insignificant symptoms and low diagnostic yield. Most patients are often diagnosed in the late stage, missing the optimal surgical period, and the therapeutic effect is greatly reduced[19]. At present, clinical treatment of advanced GC is mostly performed by adjuvant chemoradiotherapy, molecular targeted therapy and immunotherapy, but because patients with advanced GC are mostly accompanied by distant metastasis and chemotherapy resistance[20-22], the therapeutic effect of chemoradiotherapy is poor. Thus, it is of great significance to find new molecular targets for early diagnosis and treatment of GC.
With the continuous exploration of GC, more and more scholars have found that a variety of highly expressed tumor markers, such as CEA, CA199, CA724, etc. can be detected in tumor tissues and serum samples of patients with GC. The abnormal changes of these tumor markers can not only be used as an effective indicator for the diagnosis and evaluation of GC progression, but also predict the prognosis of patients to a certain extent[23-25]. Ma et al[26] found that CEA is an independent prognostic factor in patients with GC, and the establishment of a CNLR prognostic scoring system combined with CEA and neutrophil-lymphocyte ratio can accurately predict the 3-year and 5-year survival rates of patients with GC. It has also been pointed out that the incidence of GC is significantly correlated with gender, age, CEA, alpha-fetoprotein (AFP), CA125, CA199 and CA242 positive levels, and AFP, CEA, CA125, CA199 and CA242 positive levels are significantly higher in M1 GC patients than in M0 patients[27]. Wang et al[28] compared the serum CA724 levels between patients with benign gastric lesions and patients with GC, and the results showed that the expression of CA724 in the serum of patients with GC was significantly increased, and the area under the curve of serum CA724 in the diagnosis of GC was 0.849, with high sensitivity and specificity in differentiating GC, which is an important indicator for early screening and auxiliary diagnosis of GC. The above studies suggest that tumor markers such as CEA, CA199, and CA724 have the potential to be used as targets for the diagnosis and treatment of GC, and based on this, this study investigated the expression of serum CEA, CA199, and CA724 in GC with their correlation with clinicopathological features in order to prevent and control the incidence of GC early and improve the survival rate and quality of life of patients with GC.
In this study, serum CEA, CA199 and CA724 levels were detected and compared between 87 patients with GC and 80 healthy subjects, and serum CEA, CA199 and CA724 levels were compared between patients with different TNM stages of GC. The results showed that serum CEA, CA199 and CA724 levels in patients with GC were significantly higher than those in the control group, and with the increase of TNM stage, serum CEA, CA199 and CA724 expression levels in patients with GC were significantly increased, and the differences between the groups were statistically significant (P < 0.05). In addition, the positive rate of CA724 single detection was higher than that of CEA and CA199 single detection, and the positive rate of the three combined detection was higher than that of CEA, CA199 and CA724 single detection. The positive rates of CEA, CA199 and CA724 in stage I, II, III and IV of GC were higher than those in CEA, CA199 and CA724 (P < 0.05). This suggests that tumor markers CEA, CA199 and CA724 are significantly highly expressed in patients with GC and are closely related to the severity of the disease. Monitoring CEA, CA199 and CA724 levels is helpful for clinical differentiation of the incidence and progression of GC. The combined use of the three tumor markers in GC has a higher detection rate and has a higher clinical application value. CEA is one of the most widely used tumor markers in clinical practice, which was first extracted and isolated from colon cancer metastases by Canadian scholars gold and Freedman[29] and first applied in the diagnosis of colorectal cancer. With the continuous development of molecular diagnosis, CEA has also been gradually applied in the clinical differentiation of breast cancer, lung cancer, extrahepatic cholangiocarcinoma and GC[30-33]. Similar to CEA, CA199 was first discovered in colon cancer cells in 1979, and its main active components are salivary glycolipids and salivary glycoproteins, which are not or rarely expressed in normal tissues[34,35], while they are significantly highly expressed in tumor tissues such as breast cancer and gastrointestinal adenocarcinoma and have the potential to diagnose gastrointestinal tumor diseases such as GC[36]. CA724 is one of the novel tumor markers, which was first discovered in 1981 and is widely used in the diagnosis of breast cancer and gastro
In addition, in order to further investigate the relationship between tumor markers CEA, CA199 and CA724 and clinicopathological characteristics, we collected basic data such as age, gender, smoking history, alcohol history, TNM stage, lymph node metastasis and tumor diameter of GC patients to compare the differences in serum CEA, CA199 and CA724 expression in GC patients with different characteristics. The result shows that there was no significant statistical difference in serum CEA, CA199 and CA724 levels between GC patients with different genders, smoking history and alcohol history (P > 0.05), while serum CEA, CA199 and CA724 levels in GC patients aged ≥ 45 years, TNM stage III-IV, with lymph node metastasis, and tumor diameter ≥ 5 cm were significantly higher than those in GC patients aged < 45 years, TNM stage I-II, without lymph node metastasis, and tumor diameter < 5 cm (P < 0.05). This indicates that the expression of tumor markers CEA, CA199 and CA724 is closely related to the age, TNM stage, lymph node metastasis and tumor diameter of patients with GC, and rises with the increase of patient age, TNM stage and deterioration of the disease. It suggests that the expression levels of tumor markers CEA, CA199 and CA724 can be closely detected in clinical work, so as to effectively diagnose GC, and evaluate the severity of the patient’s disease, which is conducive to guiding the clinical development of effective treatment options. However, this study also has some limitations, such as failure to deeply investigate the effect of tumor markers CEA, CA199 and CA724 expression on the prognosis of patients with GC due to enrollment time constraints, which will be further supplemented subsequently.
On the other hand, we also assessed the prognostic value of tumor markers in GC. The data showed that serum CEA, CA199, and CA724 levels decreased significantly after 3 mo of radical gastrectomy for GC. This has been shown in the study by Jing et al[39]. In addition, Pearson analysis showed a negative correlation between serum levels of CEA, CA199, and CA724 and survival in GC patients. These markers may be of value in predicting secondary progression. However, due to the small number of patients in this study, CEA, CA199, and CA724 data all need to be treated with caution.
In summary, the expression levels of serum tumor markers CEA, CA199 and CA724 in patients with GC were significantly higher than those in the normal population, and the serum levels of CEA, CA199 and CA724 rised with the increase of TNM stage. In addition, the serum levels of CEA, CA199 and CA724 in patients with GC of different ages, TNM stages, lymph node metastases and tumor diameters were different, and the serum tumor markers were significantly highly expressed in patients with older age, more advanced TNM stages, lymph node metastases and larger tumor diameters. The combined detection of the three items helped to improve the diagnostic accuracy of GC, and could predict the progression of patients to a certain extent, providing a new molecular target and ideas for the clinical treatment and prognosis evaluation of GC, which is worthy of clinical promotion and reference.
Tumor markers carcinoembryonic antigen (CEA), carbohydrate antigen 199 (CA199) and carbohydrate antigen 724 (CA724) are rarely expressed or not expressed in normal tissues, but significantly increased in solid cancers such as gastric cancer (GC), and monitoring the levels of tumor markers such as CEA, CA199 and CA724 is of some value for early screening and treatment of GC.
Tumor markers CEA, CA199, and CA724 are highly expressed in GC and are associated with clinicopathology.
This study aims to investigate the expression levels of serum CEA, CA199 and CA724 in patients with GC and analyze their correlation with clinical practice.
The differences of serum CEA, CA199 and CA724 between patients with GC and normal people and the differences of each index between patients with GC at different TNM stages were compared to determine the positive rates of tumor markers alone and in combination in the diagnosis of GC and GC stages, and the correlation between CEA, CA199 and CA724 and the clinicopathology of patients with GC was analyzed.
The serum levels of CEA, CA199 and CA724 in patients with GC were significantly higher than those in the control group, and the expression levels of various indicators raised significantly with the increase of TNM stage. The positive rate of CA724 single test was higher than that of CEA and CA199 single test, and the positive rate of three combined tests was higher than that of CEA, CA199 and CA724 single test. The positive rates of CEA, CA199 and CA724 in stage I, II, III and IV of GC were higher than those in CEA, CA199 and CA724. The levels of serum CEA, CA199, and CA724 were significantly higher in GC patients aged ≥ 45 years, TNM stage III to IV, with lymph node metastasis, and tumor diameter ≥ 5 cm than GC patients aged < 45 years, TNM stage I to II, without lymph node metastasis, and tumor diameter < 5 cm.
The expression levels of CEA, CA199 and CA724 in serum of patients with GC were high and increased with the increase of TNM stage. The expression levels of tumor markers were related to age, TNM stage, lymph node metastasis and tumor diameter. The combined detection of the three items was helpful to improve the diagnostic accuracy of GC.
The number of enrolled patients should be further expanded, and prognostic data should be collected to investigate the effect of tumor marker CEA, CA199 and CA724 expression levels on the prognosis, which is more valuable and comprehensive guidance for clinical treatment and disease evaluation.
| 1. | Milano AF. 20-Year Comparative Survival and Mortality of Cancer of the Stomach by Age, Sex, Race, Stage, Grade, Cohort Entry Time-Period, Disease Duration & Selected ICD-O-3 Oncologic Phenotypes: A Systematic Review of 157,258 Cases for Diagnosis Years 1973-2014: (SEER*Stat 8.3.4). J Insur Med. 2019;48:5-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Sexton RE, Al Hallak MN, Diab M, Azmi AS. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39:1179-1203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 530] [Article Influence: 88.3] [Reference Citation Analysis (1)] |
| 3. | Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354-362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1193] [Cited by in RCA: 1261] [Article Influence: 63.1] [Reference Citation Analysis (15)] |
| 4. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 3283] [Article Influence: 547.2] [Reference Citation Analysis (6)] |
| 5. | Petryszyn P, Chapelle N, Matysiak-Budnik T. Gastric Cancer: Where Are We Heading? Dig Dis. 2020;38:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (1)] |
| 6. | Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26-38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 770] [Article Influence: 96.3] [Reference Citation Analysis (1)] |
| 7. | Konishi T, Shimada Y, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, Yaeger R, Saltz L, Smith JJ, Nash GM, Guillem JG, Paty PB, Garcia-Aguilar J, Gonen M, Weiser MR. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol. 2018;4:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 8. | Mueller R, Yasmin-Karim S, DeCosmo K, Vazquez-Pagan A, Sridhar S, Kozono D, Hesser J, Ngwa W. Increased carcinoembryonic antigen expression on the surface of lung cancer cells using gold nanoparticles during radiotherapy. Phys Med. 2020;76:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (14)] |
| 9. | Roșu MC, Mihnea PD, Ardelean A, Moldovan SD, Popețiu RO, Totolici BD. Clinical significance of tumor necrosis factor-alpha and carcinoembryonic antigen in gastric cancer. J Med Life. 2022;15:4-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (2)] |
| 10. | Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong Y, Qian Y, Huang Q, Ni Q, Liu C, Yu X. Roles of CA19-9 in pancreatic cancer: Biomarker, predictor and promoter. Biochim Biophys Acta Rev Cancer. 2021;1875:188409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 246] [Article Influence: 49.2] [Reference Citation Analysis (1)] |
| 11. | Xu Y, Zhang P, Zhang K, Huang C. The application of CA72-4 in the diagnosis, prognosis, and treatment of gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Lin Z, Bian H, Chen C, Chen W, Li Q. Application of serum pepsinogen and carbohydrate antigen 72-4 (CA72-4) combined with gastrin-17 (G-17) detection in the screening, diagnosis, and evaluation of early gastric cancer. J Gastrointest Oncol. 2021;12:1042-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Kotzev AI, Draganov PV. Carbohydrate Antigen 19-9, Carcinoembryonic Antigen, and Carbohydrate Antigen 72-4 in Gastric Cancer: Is the Old Band Still Playing? Gastrointest Tumors. 2018;5:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Kumagai K, Sano T. Revised points and disputed matters in the eighth edition of the TNM staging system for gastric cancer. Jpn J Clin Oncol. 2021;51:1024-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin. 2021;71:264-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 1180] [Article Influence: 236.0] [Reference Citation Analysis (9)] |
| 16. | Janjigian YY, Kawazoe A, Yañez P, Li N, Lonardi S, Kolesnik O, Barajas O, Bai Y, Shen L, Tang Y, Wyrwicz LS, Xu J, Shitara K, Qin S, Van Cutsem E, Tabernero J, Li L, Shah S, Bhagia P, Chung HC. The KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature. 2021;600:727-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 520] [Article Influence: 104.0] [Reference Citation Analysis (1)] |
| 17. | Li WQ, Zhang JY, Ma JL, Li ZX, Zhang L, Zhang Y, Guo Y, Zhou T, Li JY, Shen L, Liu WD, Han ZX, Blot WJ, Gail MH, Pan KF, You WC. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. BMJ. 2019;366:l5016. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 18. | Yu J, Huang C, Sun Y, Su X, Cao H, Hu J, Wang K, Suo J, Tao K, He X, Wei H, Ying M, Hu W, Du X, Hu Y, Liu H, Zheng C, Li P, Xie J, Liu F, Li Z, Zhao G, Yang K, Liu C, Li H, Chen P, Ji J, Li G; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Effect of Laparoscopic vs Open Distal Gastrectomy on 3-Year Disease-Free Survival in Patients With Locally Advanced Gastric Cancer: The CLASS-01 Randomized Clinical Trial. JAMA. 2019;321:1983-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 560] [Article Influence: 80.0] [Reference Citation Analysis (1)] |
| 19. | Machlowska J, Baj J, Sitarz M, Maciejewski R, Sitarz R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 964] [Article Influence: 160.7] [Reference Citation Analysis (0)] |
| 20. | Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 672] [Article Influence: 74.7] [Reference Citation Analysis (0)] |
| 21. | Fukuoka S, Hara H, Takahashi N, Kojima T, Kawazoe A, Asayama M, Yoshii T, Kotani D, Tamura H, Mikamoto Y, Hirano N, Wakabayashi M, Nomura S, Sato A, Kuwata T, Togashi Y, Nishikawa H, Shitara K. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J Clin Oncol. 2020;38:2053-2061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 584] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 22. | Park SH, Lim DH, Sohn TS, Lee J, Zang DY, Kim ST, Kang JH, Oh SY, Hwang IG, Ji JH, Shin DB, Yu JI, Kim KM, An JY, Choi MG, Lee JH, Kim S, Hong JY, Park JO, Park YS, Lim HY, Bae JM, Kang WK; ARTIST 2 investigators. A randomized phase III trial comparing adjuvant single-agent S1, S-1 with oxaliplatin, and postoperative chemoradiation with S-1 and oxaliplatin in patients with node-positive gastric cancer after D2 resection: the ARTIST 2 trial(☆). Ann Oncol. 2021;32:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 202] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 23. | Vuijk FA, Hilling DE, Mieog JSD, Vahrmeijer AL. Fluorescent-guided surgery for sentinel lymph node detection in gastric cancer and carcinoembryonic antigen targeted fluorescent-guided surgery in colorectal and pancreatic cancer. J Surg Oncol. 2018;118:315-323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Zhu Y, Zhao W, Mao G. Perioperative lymphocyte-to-monocyte ratio changes plus CA199 in predicting the prognosis of patients with gastric cancer. J Gastrointest Oncol. 2022;13:1007-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 25. | Tong Y, Zhao Y, Shan Z, Zhang J. CA724 predicts overall survival in locally advanced gastric cancer patients with neoadjuvant chemotherapy. BMC Cancer. 2021;21:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 26. | Ma Y, Lin J, Hou J, Xiao Q, Yu F, Ma Z, Li P, Tu R, Xie J, Zheng C, Yan S, Huang C. A novel prognosis marker based on combined preoperative carcinoembryonic antigen and systemic inflammatory response for resectable gastric cancer. J Cancer. 2021;12:927-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Li X, Li S, Zhang Z, Huang D. Association of multiple tumor markers with newly diagnosed gastric cancer patients: a retrospective study. PeerJ. 2022;10:e13488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Wang Y, Cui D, Li D, Wang H, Wu Y. Clinical Value on Combined Detection of Serum CA724, DKK1, and TK1 in Diagnosis of Gastric Cancer. J Oncol. 2022;2022:6941748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 29. | Sandberg ML, Wang X, Martin AD, Nampe DP, Gabrelow GB, Li CZ, McElvain ME, Lee WH, Shafaattalab S, Martire S, Fisher FA, Ando Y, Liu E, Ju D, Wong LM, Xu H, Kamb A. A carcinoembryonic antigen-specific cell therapy selectively targets tumor cells with HLA loss of heterozygosity in vitro and in vivo. Sci Transl Med. 2022;14:eabm0306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 30. | Liu JX, Zhang Q, Bai JS, Wang JM, Fu AS, Liu RX, Zhou XY, Gao S, Chen QC, Zhang JB, Ge YL. Carcinoembryonic Antigen Elevation in Broncholithiasis Patients Initially Misdiagnosed as Lung Cancer: a Case Report. Clin Lab. 2022;68. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 31. | Kim HS, Han Y, Kang JS, Kang YH, Lee M, Sohn HJ, Kim H, Kwon W, Jang JY. Serum carcinoembryonic antigen and carbohydrate antigen 19-9 as preoperative diagnostic biomarkers of extrahepatic bile duct cancer. BJS Open. 2021;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Hao C, Zhang G, Zhang L. Serum CEA levels in 49 different types of cancer and noncancer diseases. Prog Mol Biol Transl Sci. 2019;162:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 33. | Fujita K, Omori T, Hara H, Shinno N, Yamamoto M, Aoyama Y, Sugimura K, Kanemura T, Takeoka T, Yasui M, Matsuda C, Takahashi H, Wada H, Nishimura J, Haraguchi N, Hasegawa S, Nakai N, Asukai K, Mukai Y, Miyata H, Ohue M, Sakon M. Clinical importance of carcinoembryonic antigen messenger RNA level in peritoneal lavage fluids measured by transcription-reverse transcription concerted reaction for advanced gastric cancer in laparoscopic surgery. Surg Endosc. 2022;36:2514-2523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Jiao X, Peng T, Liang Z, Hu Y, Meng B, Zhao Y, Xie J, Gong X, Jiang Y, Fang X, Yu X, Dai X. Lateral Flow Immunoassay Based on Time-Resolved Fluorescence Microspheres for Rapid and Quantitative Screening CA199 in Human Serum. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 16] [Reference Citation Analysis (0)] |
| 35. | Zeng P, Li H, Chen Y, Pei H, Zhang L. Serum CA199 levels are significantly increased in patients suffering from liver, lung, and other diseases. Prog Mol Biol Transl Sci. 2019;162:253-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Zhu N, Xing X, Cao L, Zhang Y, Zhang T, Li Z, Zou F, Li Q. Study on the Diagnosis of Gastric Cancer by Magnetic Beads Extraction and Mass Spectrometry. Biomed Res Int. 2020;2020:2743060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 37. | Ding J, Zhang H, Wu Z. Study on the Differential Value of Tumor Marker CA724 on Primary Gastric Cancer. J Oncol. 2021;2021:2929233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Tong Y, Zhu Y, Zhao Y, Jiang C, Wang W, Shan Z, Sun F, Liu D, Zhang J. CA724 Predicts Tumor Regression Grade in Locally Advanced Gastric Cancer Patients with Neoadjuvant Chemotherapy. J Cancer. 2021;12:6465-6472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 39. | Jing R, Cui M, Ju S, Pan S. The Changes and Clinical Significance of Preoperative and Postoperative Serum CEA and CA19-9 in Gastric Cancer. Clin Lab. 2020;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hoshino I, Japan; Shen L, China S-Editor: Wang JJ L-Editor: A P-Editor: Zhang XD