Published online Feb 15, 2020. doi: 10.4251/wjgo.v12.i2.195
Peer-review started: September 8, 2019
First decision: November 11, 2019
Revised: November 14, 2019
Accepted: November 28, 2019
Article in press: November 28, 2019
Published online: February 15, 2020
Processing time: 160 Days and 9.1 Hours
Brain metastasis (BM) from colorectal cancer (CRC) is rarely encountered clinically, and its prognosis has not been fully evaluated.
To construct a scoring system and accurately predict the survival of patients with synchronous BM at diagnosis of CRC.
A retrospective study of 371 patients with synchronous BM from CRC was performed, using the data from 2010 to 2014 from the Surveillance, Epidemiology, and End Results database. Survival time and prognostic factors were statistically analyzed by the Kaplan-Meier method and Cox proportional hazards models, respectively. A scoring system was developed using the independent prognostic factors, and was used to measure the survival difference among different patients.
For the 371 patients, the median overall survival was 5 mo, survival rates were 27% at 1 year and 11.2% at 2 years. Prognostic analysis showed that age, carcinoembryonic antigen level and extracranial metastasis to the liver, lung or bone were independent prognostic factors. A scoring system based on these three prognostic factors classified the patients into three prognostic subgroups (scores of 0-1, 2-3, and 4). The median survival of patients with scores of 0-1, 2-3 and 4 was 14, 5 and 2 mo, respectively (P < 0.001). Subgroup analysis showed that there were significant differences in prognosis among the groups. Score 2-3 vs 0-1: hazard ratio (HR) = 2.050, 95%CI: 1.363-3.083; P = 0.001; score 4 vs 0-1: HR = 3.721, 95%CI: 2.225-6.225; P < 0.001; score 2-3 vs 4: HR = 0.551, 95%CI: 0.374-0.812; P = 0.003.
The scoring system effectively distinguishes long-term and short-term survivors with synchronous BM from CRC. These results are helpful in providing a reference for guiding therapy.
Core tip: There is no prognostic scoring system specifically for synchronous brain metastasis (BM) from colorectal cancer (CRC). This is believed to be the first study to construct such a system. We found that the scoring system accurately distinguished survival differences among patients, which contributed to the individual management of patients with BM from CRC.
- Citation: Quan JC, Guan X, Ma CX, Liu Z, Yang M, Zhao ZX, Sun P, Zhuang M, Wang S, Jiang Z, Wang XS. Prognostic scoring system for synchronous brain metastasis at diagnosis of colorectal cancer: A population-based study. World J Gastrointest Oncol 2020; 12(2): 195-204
- URL: https://www.wjgnet.com/1948-5204/full/v12/i2/195.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i2.195
According to the latest cancer statistics, the incidence and mortality of colorectal cancer (CRC) rank fourth and second, respectively[1], and CRC is a severe threat to human health. Metastasis is the leading reason for treatment failure and cancer-associated death[2]. Around 25% of CRC patients develop metastases at the time of diagnosis[3]. Metastatic sites include the liver, lung, bone, and brain, but compared with lung and liver metastases, CRC brain metastasis (BM) is uncommon, with an incidence of only 0.3%-9%[4]. Despite being uncommon, however, once BM occurs, patients have a poor prognosis, with a median survival of 2-9.6 mo[5-10]. In addition to poor prognosis, BM is often accompanied by neurological symptoms such as headache, nausea, and hemiparesis, which often lead to poor quality of life. In view of the poor prognosis and quality of life, more attention should be paid to BM from CRC.
Reasonable treatment is helpful in improving the prognosis of patients, and accurate prognostic evaluation is also important to guide therapy, and the two complement each other. However, the survival of some patients with BM is different in clinical practice. Thus, establishing a scoring system to accurately distinguish the survival differences and then choose individualized treatment is crucial. At present, although there have been some studies on the prognostic analysis of BM from CRC, these studies are mostly limited to single institution-based data[11-13], and contradictory views still exist[14-20].
BM from CRC includes synchronous and metachronous BM. Previous studies have shown that synchronous BM account for only 3.4%-43% of total BM[6,21]. Therefore, compared to overall BM, synchronous BM is rarer, and the inadequate number of cases also limits in-depth research. At present, only a few studies have focused on the analysis of synchronous BM. To date, there is no prognostic scoring system specifically for synchronous BM from CRC; therefore, the disease is not adequately understood.
In the present study, we aimed to comprehensively evaluate the prognostic factors of synchronous BM at diagnosis of CRC by means of the Surveillance, Epidemiology, and End Results (SEER) database. On this basis, we constructed a scoring system to accurately predict survival.
We performed a retrospective study using the SEER database. The study included patients with synchronous BM from CRC between 2010 and 2014. In the present study, synchronous BM was defined as BM at the time of CRC diagnosis. Patients were excluded if the absence or presence of BM was unknown. In addition, patients who died from other causes, or were alive with no survival time were also excluded, as were those with appendix malignancies and no evidence of primary tumor. In total, 371 patients were evaluated.
The following clinicopathological variables were included: Age (< 60, 60-74, ≥ 75 years); race (white, black, other); gender; primary tumor site (colon or rectosigmoid/rectum); tumor grade (well/moderately differentiated, or poorly differentiated/undifferentiated); histological type (adenocarcinoma, mucinous carcinoma, signet ring-cell carcinoma, or other); carcinoembryonic antigen (CEA) level (negative or positive); T stage (T1/T2 or T3/T4); N stage (N0 or N1/N2); and survival time. Data for liver, lung and bone metastases were obtained from the SEER database. To clarify the relationship between liver, lung and bone metastases and BM, the status of extracranial metastasis to liver, lung or bone was also analyzed as a variable.
To stratify the prognosis of patients with synchronous BM, we developed a scoring system based on the independent prognostic factors, and 0, 1 or 2 points were assigned to each significant variable. The scoring system was formed by summing the points of each prognostic factor, and the scoring system finally classified patients into different prognostic subgroups. This study was approved by the Ethics Committee of Cancer Hospital, Chinese Academy of Medical Sciences, Beijing, China.
Survival curves were evaluated using the Kaplan-Meier method and compared with the log-rank test. Cox proportional hazards models were used to determine the prognostic factors of patients with BM. Factors that were significant in univariate analysis were included in the multivariate analysis for determination of the final independent prognostic factors. In further analysis, a scoring system was used to stratify the prognosis of patients with BM into different subgroups, and the median survival of different subgroups was compared, hazard ratio (HR) and 95%CI were calculated. P < 0.05 was deemed to be significant. All analyses were performed by SPSS version 20.0 (IBM, Armonk, NY, USA).
The present study included 371 patients with synchronous BM from CRC. Two hundred and seventy patients had concomitant liver (n = 199), lung (n = 177) or bone (n = 81) metastases. The probability of concomitant liver, lung or bone metastases was 53.6%, 47.7% and 21.8%, respectively. The detailed patient characteristics are shown in Table 1.
| Variable | No. of patients |
| Age (yr) | |
| < 60 | 144 (38.8) |
| 60-74 | 157 (42.3) |
| ≥ 75 | 70 (18.9) |
| Race | |
| White | 295 (79.5) |
| Black | 45 (12.1) |
| Other | 31 (8.4) |
| Gender | |
| Male | 199 (53.6) |
| Female | 172 (46.4) |
| Primary tumor site | |
| Colon | 215 (58.0) |
| Rectosigmoid/rectum | 112 (30.2) |
| Unknown | 44 (11.9) |
| Tumor grade | |
| Well/moderately differentiated | 152 (41.0) |
| Poorly/undifferentiated | 101 (27.2) |
| Unknown | 118 (31.8) |
| Histology types | |
| Adenocarcinoma | 320 (86.3) |
| Mucinous carcinoma | 13 (3.5) |
| Signet ring-cell carcinoma | 10 (2.7) |
| Other | 20 (5.4) |
| Unknown | 8 (2.2) |
| CEA level | |
| Negative | 46 (12.4) |
| Positive | 206 (55.5) |
| Unknown | 119 (32.1) |
| T stage | |
| T1/T2 | 57 (15.4) |
| T3/T4 | 164 (44.2) |
| Unknown | 150 (40.4) |
| N stage | |
| N0 | 119 (32.1) |
| N1/N2 | 172 (46.4) |
| Unknown | 80 (21.6) |
| Liver metastasis | |
| No | 166 (44.7) |
| Yes | 199 (53.6) |
| Unknown | 6 (1.6) |
| Lung metastasis | |
| No | 184 (49.6) |
| Yes | 177 (47.7) |
| Unknown | 10 (2.7) |
| Bone metastasis | |
| No | 276 (74.4) |
| Yes | 81 (21.8) |
| Unknown | 14 (3.8) |
| Extracranial metastasis to liver, lung or bone | |
| No | 97 (26.1) |
| Yes | 270 (72.8) |
| Unknown | 4 (1.1) |
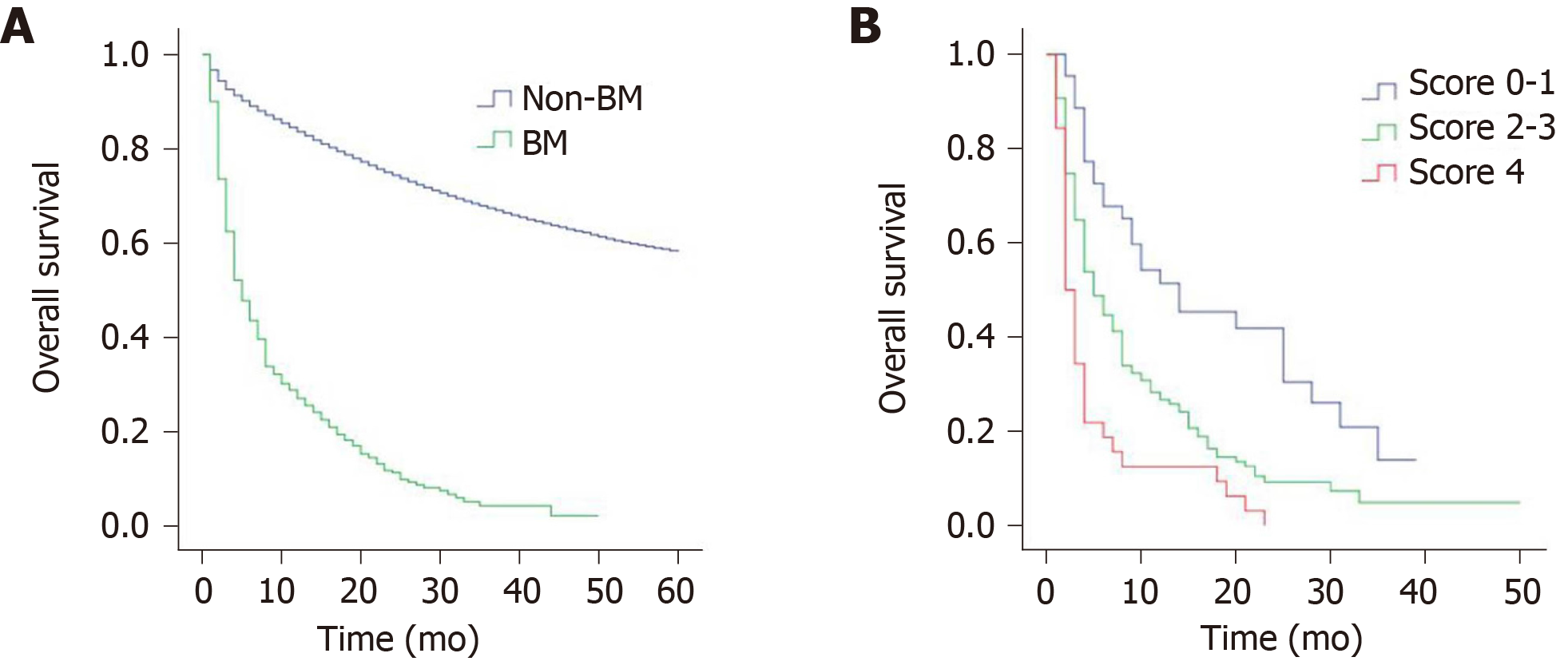
The median overall survival of patients with BM was 5 mo, with a 1-year survival rate of 27.0% and 2-year survival rate of 11.2%. Figure 1A shows the survival curves for patients with BM and non-BM (data from non-BM were not shown in this study). The results of univariate analysis revealed that age, CEA level, and extracranial metastasis to liver, lung or bone are significant factors affecting the survival of patients with BM (Table 2). However, no significant differences were found in terms of race, gender, primary tumor site, tumor grade, histological types, T stage and N stage. When the above significant variables were included in the multivariate analysis, age, CEA level, and extracranial metastasis to liver, lung or bone were independent prognostic factors (Table 3). Patients aged 60-74 years (P = 0.012), and ≥ 75 years (P < 0.001) had shorter survival compared with those aged < 60 years. Survival of CEA-positive patients was shorter than that of CEA-negative patients (P = 0.020). Similarly, patients with concomitant liver, lung or bone metastases were significantly associated with poorer prognosis (P = 0.023).
| Variable | Median survival (mo) | HR | 95%CI | P value |
| Age (yr) | ||||
| < 60 | 8 | 1 | - | - |
| 60-74 | 4 | 1.398 | 1.082-1.807 | 0.010 |
| ≥ 75 | 3 | 2.434 | 1.787-3.315 | < 0.001 |
| Race | ||||
| White | 5 | 1 | - | - |
| Black | 6 | 0.990 | 0.703-1.395 | 0.955 |
| Other | 4 | 1.309 | 0.889-1.926 | 0.172 |
| Gender | ||||
| Male | 4 | 1 | - | - |
| Female | 6 | 0.932 | 0.743-1.169 | 0.543 |
| Primary tumor site | ||||
| Colon | 5 | 1 | - | - |
| Rectosigmoid/Rectum | 6 | 0.849 | 0.656-1.098 | 0.211 |
| Unknown | 3 | 1.451 | 1.025-2.053 | 0.036 |
| Tumor grade | ||||
| Well/moderately differentiated | 8 | 1 | - | - |
| Poorly/undifferentiated | 5 | 1.185 | 0.895-1.569 | 0.236 |
| Unknown | 4 | 1.527 | 1.170-1.994 | 0.002 |
| Histology types | ||||
| Adenocarcinoma | 5 | 1 | - | - |
| Mucinous carcinoma | 8 | 0.761 | 0.416-1.394 | 0.377 |
| Signet ring-cell carcinoma | 4 | 0.923 | 0.451-1.888 | 0.826 |
| Other | 4 | 1.314 | 0.814-2.122 | 0.264 |
| Unknown | 3 | 2.416 | 1.133-5.149 | 0.022 |
| CEA level | ||||
| Negative | 8 | 1 | - | - |
| Positive | 5 | 1.964 | 1.331-2.896 | 0.001 |
| Unknown | 4 | 2.149 | 1.431-3.225 | < 0.001 |
| T stage | ||||
| T1/T2 | 4 | 1 | - | - |
| T3/T4 | 6 | 0.942 | 0.669-1.326 | 0.731 |
| Unknown | 4 | 1.125 | 0.798-1.585 | 0.502 |
| N stage | ||||
| N0 | 5 | 1 | - | - |
| N1/N2 | 6 | 0.952 | 0.733-1.238 | 0.715 |
| Unknown | 4 | 1.239 | 0.907-1.691 | 0.178 |
| Extracranial metastasis to liver, lung or bone | ||||
| No | 6 | 1 | - | - |
| Yes | 4 | 1.400 | 1.071-1.831 | 0.014 |
| Unknown | 7 | 1.544 | 0.566-4.269 | 0.392 |
| Variable | HR | 95%CI | P value |
| Age (yr) | |||
| < 60 | 1 | - | - |
| 60–74 | 1.395 | 1.075-1.809 | 0.012 |
| ≥ 75 | 2.497 | 1.818-3.430 | < 0.001 |
| CEA level | |||
| Negative | 1 | - | - |
| Positive | 1.613 | 1.078-2.413 | 0.020 |
| Unknown | 1.865 | 1.229-2.829 | 0.003 |
| Extracranial metastasis to liver, lung or bone | |||
| No | 1 | - | - |
| Yes | 1.383 | 1.045-1.831 | 0.023 |
| Unknown | 1.280 | 0.461-3.551 | 0.636 |
To accurately stratify the survival of different patients with BM, we constructed a scoring system of 0-4 (Table 4). For example, patients aged < 60 years (0 point), CEA-negative patients (0 point), and absence of extracranial metastasis to liver, lung or bone (0 point) scored 0. Patients aged ≥ 75 years (2 points), CEA-positive patients (1 point), and presence of extracranial metastasis to liver, lung or bone (1 point) scored 4. According to the scoring system, the patients were divided into three prognosis subgroups: group I (score 0-1), group II (score 2-3), group III (score 4). The median survival was 14 mo for group I, 5 mo for group II, 2 mo for group III, and the differences were statistically significant (P < 0.001). The survival curves for the three subgroups are shown in Figure 1B. Compared with group I patients, group II-III patients had significantly poorer survival (group II, P = 0.001; group III, P < 0.001), similarly, the prognosis of group II patients was significantly better than that of group III (P = 0.003) (Table 5).
| Variable | Point |
| Age (yr) | |
| < 60 | 0 |
| 60-74 | 1 |
| ≥ 75 | 2 |
| CEA level | |
| Negative | 0 |
| Positive | 1 |
| Extracranial metastasis to liver, lung or bone | |
| No | 0 |
| Yes | 1 |
Understanding the prognostic factors of BM is crucial for assessing survival and guiding treatment. However, the current prognostic factors for BM from CRC have not reached consensus[14-20]. The reason for the above contradictions may be due to the differences in variables and sample sizes in different studies. Little is known about synchronous BM from CRC, which is mainly because of the small number of cases. Compared with the single-center, small-sample studies, population-based research can make up for the above limitations and may better reflect the state of the disease. Therefore, in order to understand the disease better, we performed a population-based retrospective analysis. We analyzed the prognosis of 371 patients with synchronous BM and consequently constructed a prognostic scoring system. The system was based on three independent prognostic factors: Age, CEA level, and extracranial metastasis to liver, lung or bone. Our results confirmed that the prognosis of different patients was significantly different, and the scoring system accurately classified patient survival. To our knowledge, we are the first group to construct a scoring system specifically for synchronous BM from CRC.
BM from CRC occurs in the late stage of CRC, and is often associated with extracranial metastases such as liver, lung and bone at diagnosis. This is also one of the factors leading to poor prognosis[10,12]. In this study, 72.8% of patients had concomitant liver, lung or bone metastases, which was significantly associated with poorer prognosis. This result confirmed the findings of Gu et al[10], who analyzed 93 patients with BM. Median survival in the presence and absence of extracranial metastasis was 7 and 13 mo, respectively. The prognosis of patients with extracranial metastasis was worse than those without extracranial metastasis. Matsunaga et al[12] reached the same conclusion that the presence of extracranial metastasis worsened prognosis. These results suggest that extracranial metastases are an important prognostic factor in patients with BM from CRC.
CEA is a common tumor marker and is often used for CRC diagnosis and postoperative follow-up monitoring. Moreover, the prognostic value of CEA in BM from CRC has been verified by other researchers[6,22]. Consistent with previous studies, our study showed that CEA is an independent prognostic factor, and median survival was 5 and 8 mo for patients with CEA-positive and CEA-negative disease, respectively. The survival of CEA-positive patients was significantly shorter than that of CEA-negative patients.
Age is another important prognostic factor. In our study, patients were divided into three age groups: < 60, 60-74 and ≥ 75 years. We found that patients aged < 60 years had the best prognosis, followed by those aged 60-74 years, and those aged ≥ 75 years had the worst prognosis. Consistent with our findings, Yang et al[23] classified patients into five age groups: < 40, 40-49, 50-59, 60-69 and ≥ 70 years; they confirmed that the prognosis of patients aged ≥ 70 years was significantly worse than in those aged < 40 years. Similarly, Farnell et al[14] also emphasized the value of age in predicting the prognosis of BM. These studies suggested that older patients tend to have poorer prognosis than young patients with BM from CRC. Therefore, with the increase in the aging population, it is necessary to pay more attention to elderly patients.
As reported in previous studies[24], patients with cancer at the same stage often have different prognoses. Similarly, CRC patients with BM also face the same problem. Kim et al[22] conducted a single-center study of 107 CRC patients with BM. They developed a graded prognostic assessment and divided the patients into three prognostic subgroups with a median survival of 2.3, 4.3 and 12.7 mo. Their results showed that the prognosis of patients with different grades differed significantly. However, in their study, there was no clear distinction between synchronous and metachronous BM. Therefore, the prognosis of synchronous BM is not clear. Unlike that study, our study specifically focused on synchronous BM, and our scoring system divided the patients into three subgroups with scores ranging from 0-1 to 4, with a median survival of 14, 5 and 2 mo, respectively. We found that the higher the score, the worse the prognosis. Patients with scores of 0-1 had the best prognosis, with a median survival of up to 14 mo. However, the prognosis was worst in patients with a score of 4, and their median survival was only 2 mo. Therefore, in clinical practice, the prognosis of patients with BM should not be generalized, and individualized survival evaluation should be made based on the patient’s own situation.
Our study had some limitations. Firstly, the SEER database only provides information on the presence or absence of BM at initial diagnosis; thus, our study only assessed patients with BM at initial presentation of CRC. Patients who developed BM later in the disease course could not be commented upon in our analysis. Secondly, information regarding the Karnofsky performance status, number of BM, detailed treatment of BM and molecular markers was not provided in the SEER database; therefore, these factors were not included in our study. In the future, a large multicenter study is needed to confirm the value of these variables in synchronous BM.
In conclusion, this study confirmed the prognostic factors of synchronous BM from CRC and constructed a prognostic scoring system. The scoring system more accurately distinguished the prognostic differences among different patients and can be used as an effective prognostic predictive tool to help clinicians quickly and conveniently predict survival.
Synchronous brain metastasis (BM) from colorectal cancer (CRC) is rare, and the prognosis is poor. However, only a few studies have focused on the analysis of synchronous BM, and there is no prognostic scoring system specifically for synchronous BM from CRC to date. Therefore, more studies on synchronous BM from CRC are needed.
We comprehensively evaluated the prognostic factors of synchronous BM, and further constructed a scoring system to accurately predict survival.
This study was designed to confirm the clinical value of the prognostic scoring system for synchronous BM at diagnosis of CRC.
We retrospectively studied patients with synchronous BM from CRC using the Surveillance, Epidemiology, and End Results database. The Kaplan-Meier method was used to assess the median survival time, and Cox proportional hazards models were used to determine the independent prognostic factors. A scoring system was constructed to stratify the patients into different subgroups, and the survival differences among different subgroups were compared.
The results showed that age, carcinoembryonic antigen level and extracranial metastasis to liver, lung or bone were independent prognostic factors. A scoring system based on the three independent prognostic factors classified the patients into three prognostic subgroups: group I (score 0-1), group II (score 2-3), and group III (score 4). The median survival was 14 mo for group I, 5 mo for group II, and 2 mo for group III, and there were significant differences in prognosis among the groups (P < 0.001).
This study is the first to construct a scoring system specifically for synchronous BM from CRC, and we confirm that the scoring system accurately distinguishes the survival differences among different patients.
The scoring system can be used as an effective prognostic predictive tool to help clinicians quickly and conveniently predict survival.
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56705] [Article Influence: 7088.1] [Reference Citation Analysis (135)] |
| 2. | Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3059] [Cited by in RCA: 3724] [Article Influence: 248.3] [Reference Citation Analysis (0)] |
| 3. | Van Cutsem E, Oliveira J; ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2009;20 Suppl 4:61-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 123] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 4. | Kruser TJ, Chao ST, Elson P, Barnett GH, Vogelbaum MA, Angelov L, Weil RJ, Pelley R, Suh JH. Multidisciplinary management of colorectal brain metastases: a retrospective study. Cancer. 2008;113:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Michl M, Thurmaier J, Schubert-Fritschle G, Wiedemann M, Laubender RP, Nüssler NC, Ruppert R, Kleeff J, Schepp W, Reuter C, Löhe F, Karthaus M, Neumann J, Kirchner T, Engel J, Heinemann V. Brain Metastasis in Colorectal Cancer Patients: Survival and Analysis of Prognostic Factors. Clin Colorectal Cancer. 2015;14:281-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Noura S, Ohue M, Shingai T, Fujiwara A, Imada S, Sueda T, Yamada T, Fujiwara Y, Ohigashi H, Yano M, Ishikawa O. Brain metastasis from colorectal cancer: prognostic factors and survival. J Surg Oncol. 2012;106:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Tapia Rico G, Price TJ, Karapetis C, Piantadosi C, Padbury R, Roy A, Maddern G, Moore J, Carruthers S, Roder D, Townsend AR. Brain metastasis in advanced colorectal cancer: results from the South Australian metastatic colorectal cancer (SAmCRC) registry. Cancer Biol Med. 2017;14:371-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Fokas E, Henzel M, Hamm K, Surber G, Kleinert G, Engenhart-Cabillic R. Multidisciplinary treatment of brain metastases derived from colorectal cancer incorporating stereotactic radiosurgery: analysis of 78 patients. Clin Colorectal Cancer. 2011;10:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Wroński M, Arbit E. Resection of brain metastases from colorectal carcinoma in 73 patients. Cancer. 1999;85:1677-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 10. | Gu XD, Cai YT, Zhou YM, Li ZY, Xiang JB, Chen ZY. Prognostic factors and multidisciplinary treatment modalities for brain metastases from colorectal cancer: analysis of 93 patients. BMC Cancer. 2015;15:902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Kim DY, Ryu CG, Jung EJ, Paik JH, Hwang DY. Brain metastasis from colorectal cancer: a single center experience. Ann Surg Treat Res. 2018;94:13-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Matsunaga S, Shuto T, Kawahara N, Suenaga J, Inomori S, Fujino H. Gamma Knife surgery for brain metastases from colorectal cancer. Clinical article. J Neurosurg. 2011;114:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Tokoro T, Okuno K, Hida JC, Ueda K, Yoshifuji T, Daito K, Sugiura F. Prognostic factors for patients with advanced colorectal cancer and symptomatic brain metastases. Clin Colorectal Cancer. 2014;13:226-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Farnell GF, Buckner JC, Cascino TL, O'Connell MJ, Schomberg PJ, Suman V. Brain metastases from colorectal carcinoma. The long term survivors. Cancer. 1996;78:711-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 15. | Suzuki Y, Yamaguchi T, Matsumoto H, Nakano D, Honda G, Shinoura N, Karasawa K, Takahashi K. Prognostic factors and treatment effects in patients with curatively resected brain metastasis from colorectal cancer. Dis Colon Rectum. 2014;57:56-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Kye BH, Kim HJ, Kang WK, Cho HM, Hong YK, Oh ST. Brain metastases from colorectal cancer: the role of surgical resection in selected patients. Colorectal Dis. 2012;14:e378-e385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Pietrantonio F, Aprile G, Rimassa L, Franco P, Lonardi S, Cremolini C, Biondani P, Sbicego EL, Pasqualetti F, Tomasello G, Niger M, Casagrande M, Ghidini M, Muni R, Montrone S, Bergamo F, Berenato R, Fontanella C, Bozzarelli S, Moretto R, Battaglin F, Di Bartolomeo M, de Braud F, Miceli R. A new nomogram for estimating survival in patients with brain metastases secondary to colorectal cancer. Radiother Oncol. 2015;117:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Nieder C, Hintz M, Grosu AL. Predicted survival in patients with brain metastases from colorectal cancer: Is a current nomogram helpful? Clin Neurol Neurosurg. 2016;143:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Nieder C, Hintz M, Grosu AL. Colorectal cancer metastatic to the brain: analysis of prognostic factors and impact of KRAS mutations on presentation and outcome. Clin Transl Oncol. 2016;18:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Schoeggl A, Kitz K, Reddy M, Zauner C. Stereotactic radiosurgery for brain metastases from colorectal cancer. Int J Colorectal Dis. 2002;17:150-155. [PubMed] |
| 21. | Mege D, Ouaissi M, Fuks D, Metellus P, Peltier J, Dufour H, Regimbeau JM, Dahan L, Sielezneff I, Sastre B. Patients with brain metastases from colorectal cancer are not condemned. Anticancer Res. 2013;33:5645-5648. [PubMed] |
| 22. | Kim BH, Park HJ, Kim K, Han SW, Kim TY, Jeong SY, Park KJ, Chie EK. Novel graded prognostic assessment for colorectal cancer patients with brain metastases. Int J Clin Oncol. 2018;23:1112-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Yang L, He W, Xie Q, Liu S, Kong P, Jiang C, Zhang B, Xia L. Brain metastases in newly diagnosed colorectal cancer: a population-based study. Cancer Manag Res. 2018;10:5649-5658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Huang B, Feng Y, Zhu L, Xu T, Huang L, Cai G. Smaller tumor size is associated with poor survival in stage II colon cancer: An analysis of 7,719 patients in the SEER database. Int J Surg. 2016;33 Pt A:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kmietowicz Z, Tsikas D S-Editor: Wang JL L-Editor: Webster JR E-Editor: Qi LL