Published online Feb 15, 2020. doi: 10.4251/wjgo.v12.i2.182
Peer-review started: October 11, 2019
First decision: November 11, 2019
Revised: December 19, 2019
Accepted: December 30, 2019
Article in press: December 30, 2019
Published online: February 15, 2020
Processing time: 127 Days and 3.1 Hours
FOLFIRINOX and gemcitabine plus nab-paclitaxel (Gem + nabPTX) were recently introduced for metastatic pancreatic cancer treatment. However, studies that compared these two regimens and studies in Asian populations are lacking.
To compare the treatment outcomes of FOLFIRINOX and Gem + nabPTX regimen for metastatic pancreatic cancer treatment in Korean population.
Patients with metastatic or recurrent pancreatic cancer treated with FOLFIRINOX (n = 86) or Gem + nabPTX (n = 81) as the first-line since January 2015 were identified using the Severance Hospital Pancreatic Cancer Cohort Registry. Treatment efficacy, treatment-related adverse events and economic aspects were compared.
Patients in the FOLFIRINOX group were significantly younger (54 vs 65 years; P < 0.001) and had better performance statuses at diagnosis. The median overall survival (10.7 vs 12.1 mo; P = 0.157), progression-free survival (8.0 vs 8.4 mo; P = 0.134), and objective response rates (33.7% vs 46.9%; P = 0.067) were not significantly different when compared with Gem + nabPTX group. Grade ≥ 3 neutropenia and gastrointestinal adverse events were more common in the FOLFIRINOX group. The drug costs of both regimens were similar.
Treatment efficacy and economic burdens were comparable between the two regimens. But, the details of adverse event were different. Gem + nabPTX regimen might be considered preferentially in certain conditions.
Core tip: Both FOLFIRINOX and gemcitabine plus nab-paclitaxel combination therapy are widely used as a treatment of choice in patients with metastatic pancreatic cancer. However, the treatment choice and sequence are not firmly established. In addition, researches on Asian populations in this regard are scarce. In the present study, we compared the treatment efficacy, safety, and economic aspects of FOLFIRINOX and gemcitabine plus nab-paclitaxel combination therapy. We believe that this study can help physicians and patients to select appropriate regimens while avoiding and preventing unnecessary complications.
- Citation: Cho IR, Kang H, Jo JH, Lee HS, Chung MJ, Park JY, Park SW, Song SY, An C, Park MS, Bang S. FOLFIRINOX vs gemcitabine/nab-paclitaxel for treatment of metastatic pancreatic cancer: Single-center cohort study. World J Gastrointest Oncol 2020; 12(2): 182-194
- URL: https://www.wjgnet.com/1948-5204/full/v12/i2/182.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i2.182
Pancreatic cancer demonstrates a very poor prognosis and is one of the main causes of cancer-related death worldwide[1,2]. While the treatment outcomes of other cancers have gradually improved, progress in the treatment outcomes of metastatic pancreatic cancer has remained stagnant[3]. Since the late 1990s, several efforts have been made to treat metastatic pancreatic cancer[4-6]. Recently, two effective regimens were introduced through large-scale clinical trials.
The FOLFIRINOX regimen, which consists of 5-fluorouracil (5-FU), leucovorin, oxaliplatin, and irinotecan, was introduced by the PRODIGE4/ACCORD11 trial[7]. In this clinical trial, FOLFIRINOX yielded superior survival rates when compared to gemcitabine monotherapy. Another randomized phase III trial, MPACT, showed that a combination of gemcitabine and nab-paclitaxel (Gem + nabPTX) yielded a statistically significant survival benefit and response rate when compared with gemcitabine monotherapy[8]. As a result, these two regimens are recommended as the first-line therapy for metastatic pancreatic cancer[9,10].
However, there are two possible impediments when treating patients in a clinical setting. The first is the treatment choice and sequence between the two standard regimens. There is a lack of data regarding a direct comparison of the two regimens in terms of the treatment outcome. In addition, reliable guidelines that help to select the appropriate regimen according to each patient are lacking. The second impediment concerns ethnic differences between western and east-Asian populations. Even though we reported the efficacy and adverse events of Gem + nabPTX in Korean population were similar to the western population, there are still lack of evidences for supporting the results of MPACT trial in Asian countries[11]. In terms of pharmacoethnicity, an understanding of the differences in treatment response and adverse events according to ethnicity helps to improve chemotherapeutic tolerability and effectiveness[12].
Therefore, the purpose of this study was to compare the efficacy, safety, and economic aspects of FOLFIRINOX and Gem + nabPTX in the treatment of metastatic pancreatic cancer in Korean population.
Patients with metastatic or recurrent pancreatic cancer who were treated with FOLFIRINOX or Gem + nabPTX since January 2015 were identified using the Severance Hospital Pancreatic Cancer Cohort Registry, which is a prospectively collected database of pancreatic cancer patients who received anticancer therapy at Severance Hospital since 2015. During the study period, a total of 924 patients were registered in the cohort registry.
The inclusion criteria were as follows: (1) ≥ 18 years of age; (2) Pathologically confirmed metastatic or recurred pancreatic adenocarcinoma; (3) At least one measurable or evaluable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1[13]; (4) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; (5) No prior anti-tumor treatment for metastatic or recurred pancreatic adenocarcinoma; and (6) Adequate organ function (absolute neutrophil count ≥ 1.5 × 109/L, serum creatinine < 1.5 mg/dL, or calculated creatinine clearance ≥ 60 mL/min per the Cockcroft and Gault formula) before chemotherapy.
Finally, 167 patients who met the enrolment criteria were identified as eligible patients. This study was approved by the Yonsei University Health System Institutional Review Board (Approval number: 4-2015-1058) and conducted in accordance with the principles set forth in the Declaration of Helsinki. Written informed consent was obtained from all patients.
In patients who received the FOLFIRINOX regimen, oxaliplatin (85 mg/m2), leucovorin (400 mg/m2), and irinotecan (180 mg/m2) were delivered via intravenous infusion, which was followed by 400 mg/m2 (bolus) and 2400 mg/m2 (continuous intravenous infusion over a 46-h period) of 5-FU administered every 2 wk. Patients treated with Gem + nabPTX received a slow (over 30–40 min) intravenous infusion of nab-paclitaxel (125 mg/m2) and gemcitabine (1000 mg/m2) on days 1, 8, and 15 of a 28-d cycle (every 4 wk). The dose of the chemotherapeutic agent was reduced and/or administration was delayed if serious treatment-related adverse events (AEs) occurred that made treatment intolerable. Chemotherapy was discontinued when life-threatening AEs or disease progression was identified.
At the beginning of treatment, the following tumor-related factors were examined and recorded: Patient demographics, patient body mass index (BMI), date of diagnosis, tumor size and location, location and number of metastases, and laboratory data including levels of carbohydrate antigen 19-9. To evaluate treatment efficacy, computed tomography, magnetic resonance imaging, or 18F-fluorodeoxyglucose-positron emission tomography was performed every 8 wk. All imaging studies were conducted and reviewed according to institutional standard protocols. Treatment responses according to the RECIST criteria were reported by designated radiologists and final disease assessments were independently performed by the responsible physicians.
To monitor for treatment-related AEs, the presence of an AE was carefully examined by physicians and registered nurses at each visit during chemotherapy. The category and severity grade of the AEs were precisely recorded in the patients’ medical records. Treatment-related AEs were assessed and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0[14].
The anticancer drug cost that was actually paid by the patient was calculated based on the median body surface area (1.61 m2). The total cost for 4 wk of treatment administration were compared between the two regimens, since each regimen had a different administration protocol per cycle. Then, 4 d of hospital costs per cycle were added to the cost of FOLFIRINOX because these patients were required to be hospitalized during the chemotherapy. When calculating the hospital cost, the cheapest room covered by the Korean National Health Insurance Service (NHIS) was used.
The primary endpoints were overall survival (OS) and progression-free survival (PFS). The secondary endpoints were the rate and severity of treatment-related AEs. OS was calculated as the date of diagnosis until the date of the most recent follow-up or death. PFS was computed from the date of diagnosis to disease progression (or the most recent follow-up or death). Object response was defined as complete response or partial response and disease control was defined as complete response + partial response and stable disease according to the RECIST criteria.
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, United States), SAS version 9.4 (SAS Institute, Cary, NC, United States), and R version 3.3.0 (The R Foundation for Statistical Computing, Vienna, Austria). Baseline patient characteristics, laboratory data, and the grade and frequency of AEs were used to calculate descriptive statistics. Student’s t-tests were used to compare continuous variables and chi-square or Fisher’s exact tests were used to compare categorical variables. Survival times and rates were estimated using the Kaplan-Meier method (with log-rank test). Estimated medians with 95% confidence intervals (CI) are reported. A Cox proportional-hazards model was used for the subgroup analysis to estimate the hazard ratios for OS and PFS.
The baseline characteristics of all patients are presented in Table 1. The patients who received FOLFIRINOX were significantly younger (54 vs 65 years; P < 0.001) and had better performance status scores at baseline (proportion of ECOG-PS score 0: 83.7% vs 70.4%; P = 0.040) than those who received Gem + nabPTX. The most common metastatic sites were the liver and peritoneum. Liver metastasis was more common in the FOLFIRINOX group (66.3% vs 49.4%; P = 0.027) and peritoneal carcinomatosis was more common in the Gem + nabPTX group (51.9% vs 40.7%; P = 0.148). There was no difference in the number of metastasis sites between the two groups. In terms of baseline laboratory data, a significantly higher neutrophil count was observed in the FOLFIRINOX group. Other laboratory data, including carbohydrate antigen 19-9, showed no differences between the two groups.
| Characteristics | FOLFIRINOX (n = 86) | Gem + Nab-paclitaxel (n = 81) | P value |
| Age (yr) | 54 (30-78) | 65 (42-79) | < 0.001 |
| Male sex | 49 (57.0) | 37 (45.7) | 0.144 |
| ECOG-PS | 0.040 | ||
| 0 | 72 (83.7) | 57 (70.4) | |
| 1 | 14 (16.3) | 24 (29.6) | |
| Body mass index | 22.13 (16.49-31.63) | 21.97 (16.11-29.59) | 0.432 |
| Tumor location1 | 0.398 | ||
| Head and neck | 40 (46.5) | 32 (39.5) | |
| Body and tail | 46 (53.5) | 48 (49.3) | |
| Metastasis site | |||
| Liver | 57 (66.3) | 40 (49.4) | 0.027 |
| Lung | 9 (10.5) | 12 (14.8) | 0.397 |
| Bone | 4 (4.7) | 6 (7.4) | 0.5262 |
| Peritoneum (carcinomatosis) | 35 (40.7) | 42 (51.9) | 0.148 |
| Distant LN | 33 (38.4) | 28 (34.6) | 0.610 |
| Othersite (e.g. adrenal gland) | 14 (16.3) | 20 (24.7) | 0.177 |
| No. of metastasis site | 0.726 | ||
| 1 site | 39 (45.3) | 38 (46.9) | |
| 2 sites | 30 (34.9) | 24 (29.6) | |
| 3 or more | 17 (19.8) | 19 (23.5) | |
| Laboratory data (at diagnosis) | |||
| WBC count (cells/μL) | 6765 (2830-21880) | 6240 (2580-12240) | 0.068 |
| Neutrophil count (cells/μL) | 4660 (1610-18930) | 4045 (1410-8540) | 0.035 |
| Prothrombin time (INR) | 1.01 (0.80-1.28) | 1.00 (0.83-1.16) | 0.176 |
| Total bilirubin (mg/dL) | 0.7 (0.2-13.5) | 0.6 (0.2-23.4) | 0.200 |
| AST (IU/L) | 24 (9-204) | 22 (9-765) | 0.286 |
| ALT (IU/L) | 27 (5-192) | 17 (5-717) | 0.117 |
| Alkaline phosphatase (IU/L) | 116 (43-957) | 92 (37-2080) | 0.798 |
| CA 19-9 at diagnosis (U/mL) | 585.3 (3.4-20000) | 305.2 (0.6-20000) | 0.678 |
The median follow-up period for all patients was 7.9 (range, 1.5–23.4) mo; during this period, 78 (46.7%) patients died and 101 (60.5%) patients experienced disease progression. The treatment data and efficacy of the two groups are presented in Table 2. The median number of chemotherapy cycles received by each patient in the FOLFIRINOX and Gem + nabPTX groups was 8 and 5, respectively. There was no statistically significant difference in the median duration of chemotherapy (FOLFIRINOX, 138 d vs Gem + nabPTX, 154 d; P = 0.249). The median relative dose intensities of gemcitabine and nab-paclitaxel were 93.3% and 86.2%, respectively. In the FOLFIRINOX group, 80% of the planned dose of 5-FU and 75% of oxaliplatin and irinotecan were administered to patients.
| FOLFIRINOX (n = 86) | Gem + nabPTX (n = 81) | P value | |
| Duration of chemotherapy | |||
| Cycles | 8 (2-24) | 5 (2-16) | |
| Duration, days | 138 (19-551) | 154 (32-554) | 0.249 |
| Accumulation dose, mg/m2 | |||
| Gemcitabine | 14000 (4000-40000) | ||
| Nab-paclitaxel | 1562.5 (375-4875) | ||
| 5-Fluorouracil | 16800 (5600-53200) | ||
| Oxaliplatin | 510 (170-1445) | ||
| Irinotecan | 1080 (360-3060) | ||
| Relative dose intensity, % | |||
| Gemcitabine | 93.3 (54.3-100) | ||
| Nab-paclitaxel | 86.2 (22.7-100) | ||
| 5-Fluorouracil | 80.0 (52.5-100.0) | ||
| Oxaliplatin | 75.0 (52.5-100.0) | ||
| Irinotecan | 75.0 (52.5-100.0) | ||
| Best response of chemotherapy | 0.067 | ||
| Complete response | 0 (0) | 0 (0) | |
| Partial response | 29 (33.7) | 38 (46.9) | |
| Stable disease | 31 (36.0) | 30 (37.0) | |
| Progression of disease | 26 (30.2) | 13 (16.0) | |
| Response rates | |||
| Objective response rate | 29 (33.7) | 38 (46.9) | 0.082 |
| Disease control rate | 60 (69.8) | 68 (84.0) | 0.03 |
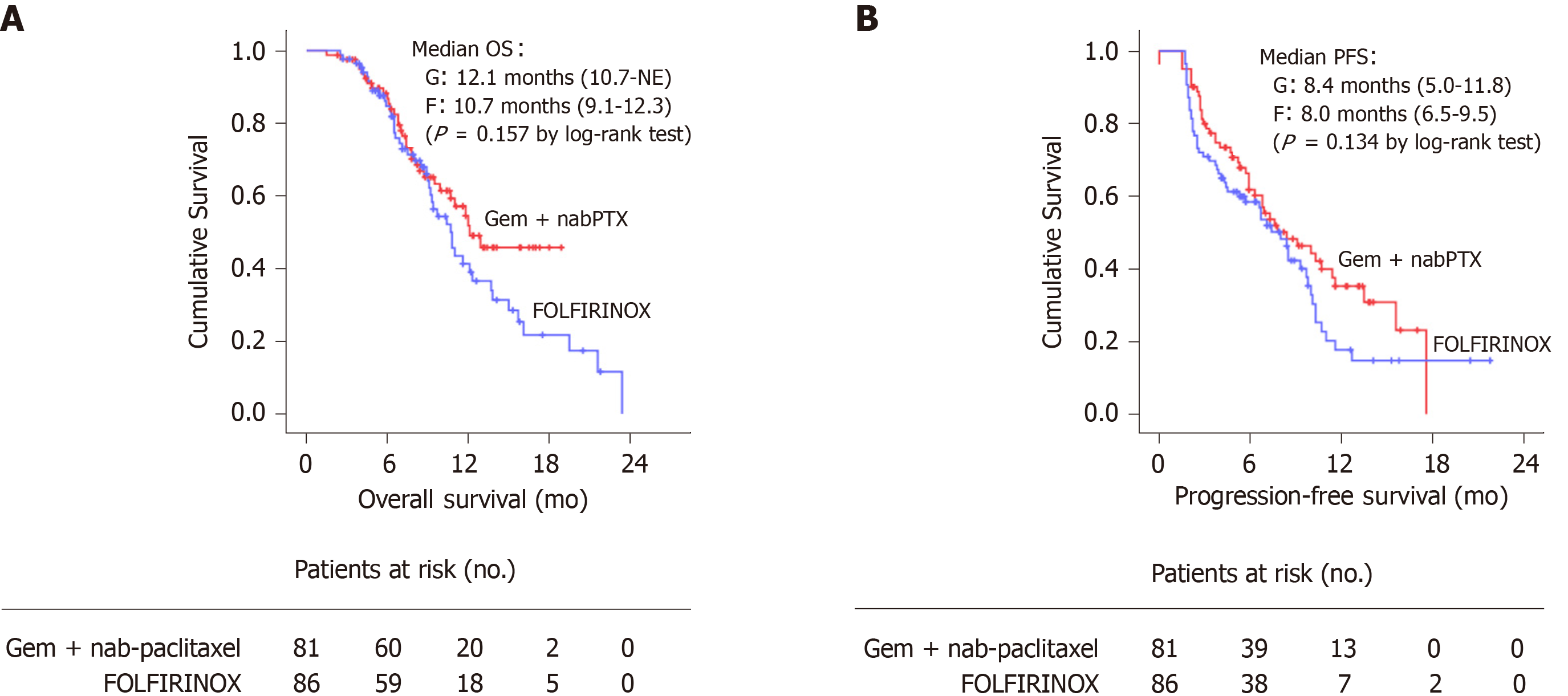
In aspect of efficacy, there was no statistically significant difference in the objective response rate between the two groups (P = 0.082). However, the Gem + nabPTX group showed a significantly higher disease control rate than the FOLFIRINOX group (84.0% vs 69.8%; P = 0.030). The median overall survival was 12.1 mo (95%CI, 10.7- not estimable) in the Gem + nabPTX group and 10.7 mo (95%CI, 9.1–12.3) in the FOLFIRINOX group (P = 0.157, Figure 1A). The median progression-free survival was 8.4 mo (95%CI, 5.0–11.8) in the Gem + nabPTX group and 8.0 mo (95%CI, 6.5–9.5) in the FOLFIRINOX group (P = 0.134, Figure 1B).
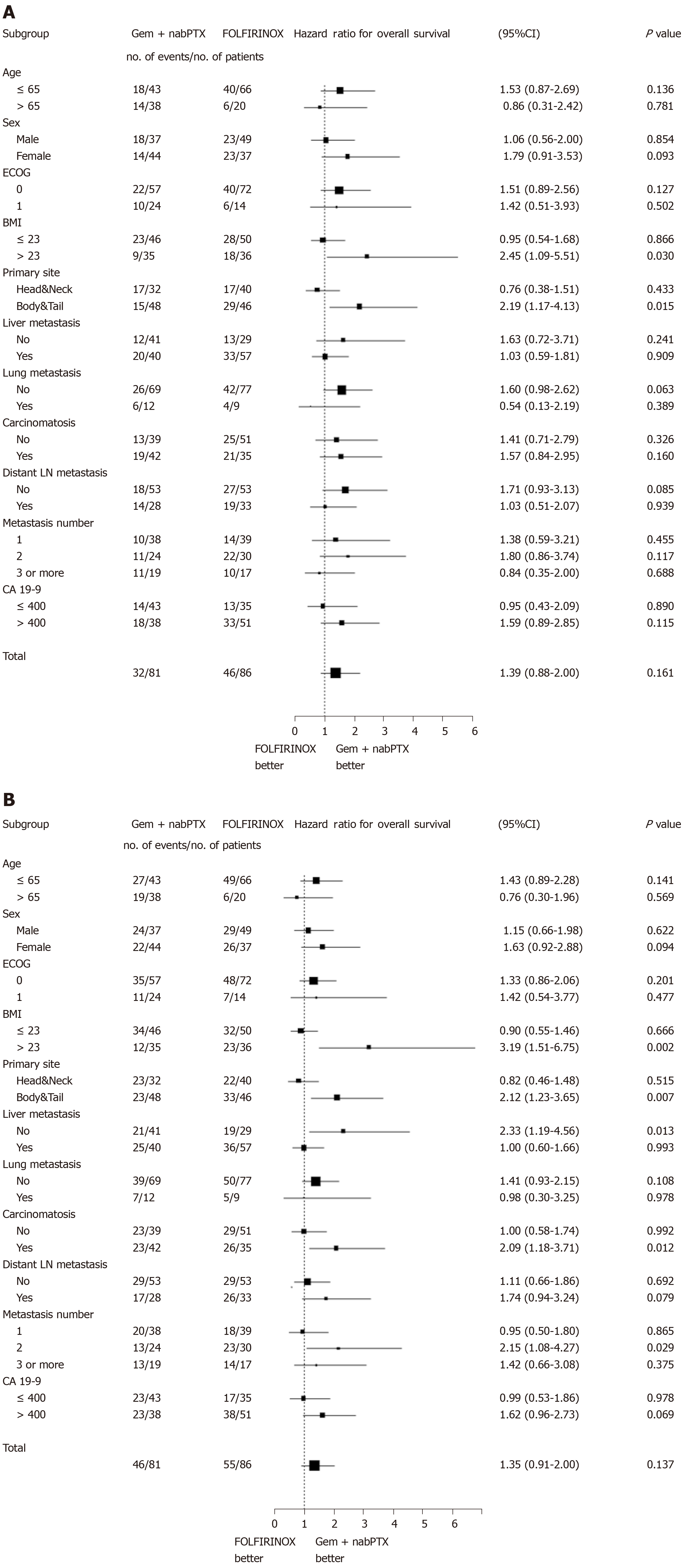
The treatment efficacy was consistently similar in both groups across the majority of subgroups (Figure 2). In patients who had pancreatic body/tail cancer and a BMI > 23, the risk of death significantly reduced with the Gem + nabPTX regimen. Similar trends were observed for PFS according to subgroup. In addition to primary cancer site and BMI, the presence of liver metastasis and carcinomatosis at diagnosis were associated with the hazard ratio of disease progression.
The treatment-related AEs observed in this study population are shown in Table 3. Notable AEs occurred in both groups. In terms of haematologic AEs, the incidence of severe (grade 3 or more) anemia and thrombocytopenia were similar between the two groups. Both groups demonstrated a notable incidence of severe neutropenia, but the FOLFIRINOX group showed a statistically significantly higher rate of severe neutropenia (74.4% vs 46.9%; P < 0.001). The granulocyte-colony stimulating factor (G-CSF) administration rate was also significantly higher in the FOLFIRINOX group.
| FOLFIRINOX (n = 86) | Gem + nabPTX (n = 81) | P value | |
| Hematologic adverse event | |||
| Grade ≥ 3 Anemia | 17 (19.8) | 12 (14.8) | 0.398 |
| Grade ≥ 3 Thrombocytopenia | 7 (8.1) | 5 (6.2) | 0.623 |
| Grade ≥ 3 Neutropenia | 64 (74.4) | 38 (46.9) | < 0.001 |
| Febrile neutropenia | 22 (25.6) | 13 (16.0) | 0.130 |
| Administration of G-CSF | 66 (76.7) | 15 (18.5) | < 0.001 |
| Neurologic adverse event | |||
| Peripheral neuropathy | 16 (18.6) | 46 (56.8) | < 0.001 |
| Grade ≥ 3 neuropathy | 3 (3.5) | 15 (18.5) | 0.002 |
| Median time to onset-days (range) | 120 (15-278) | 73.5 (17-284) | 0.051 |
| Gastrointestinal adverse event | |||
| Nausea/Vomiting | 43 (50.0) | 17 (21.0) | < 0.001 |
| Diarrhea | 15 (17.4) | 12 (14.8) | 0.645 |
| Grade ≥ 3 adverse events | 39 (45.3) | 16 (19.8) | < 0.001 |
| General weakness | 30 (34.9) | 40 (49.4) | 0.058 |
| Dermatologic adverse event | 12 (14.0) | 34 (42.0) | < 0.001 |
In the Gem + nabPTX group, more than half of the patients (46, 56.8%) showed peripheral neuropathy and 15 (18.5%) developed severe peripheral neuropathy after chemotherapy. On the other hand, the rate of neurologic AEs in the FOLFIRINOX group was significantly lower. The median time to onset of peripheral neuropathy was shorter in the Gem + nabPTX group, but not statistically significant (73.5 vs 120 d; P = 0.051). In terms of other non-hematologic AEs, the incidences of nausea/vomiting and severe gastrointestinal AEs were higher in the FOLFIRINOX group while the incidence of dermatologic AEs was higher in the Gem + nabPTX group.
Compared to those observed in previous phase-III trials (PRODIGE4/ACCORD11 and MPACT population), the proportions of patients who experienced severe neutropenia and febrile neutropenia were higher in the present study, regardless of the treatment regimen administered. In addition, a larger number of patients in the FOLFIRINOX group showed severe anemia and nausea/vomiting compared to that observed in previous trials, and the incidence of severe fatigue was more than 10% higher in the Gem + nabPTX group when compared with the MPACT population. (Supplemental Table 1).
Dose modification, treatment delay and cessation are shown in Table 4. Proportion of patients who experienced dose reduction of chemotherapeutic agent was significantly higher in FOLFIRINOX group patients than Gem + nabPTX group (88.4 % vs 60.5%; P < 0.001). In the FOLFIRINOX group, most patients experienced dose reduction prior to the 1st response evaluation (68 of 76 patients), whereas in the Gem + nabPTX group, many patients experienced dose reduction after the 1st response evaluation (30 of 49 patients).
| Variables | FOLFIRINOX (n = 86) | Gem + nabPTX (n = 81) | P value |
| Dose reduction | 76 (88.4) | 49 (60.5) | < 0.001 |
| At beginning | 43 (50) | 7 (8.6) | |
| Before 1st RE | 25 (29.1) | 12 (14.8) | |
| 1st RE-2nd RE | 5 (5.8) | 15 (18.5) | |
| After 2nd RE | 3 (3.5) | 15 (18.5) | |
| Delay of administration due to AE | 47 (54.7) | 51 (63.0) | 0.346 |
| Neurologic AE | 2 (2.4) | 14 (17.3) | |
| Hematologic AE | 30 (34.9) | 22 (27.2) | |
| Gastrointestinal AE | 3 (3.5) | 4 (4.9) | |
| General weakness | 8 (9.3) | 20 (24.7) | |
| Others | 6 (7.0) | 2 (2.5) | |
| Cessation of administration due to AE | 12 (14.0) | 17 (21.0) | 0.307 |
| Neurologic AE | 2 (2.4) | 3 (3.7) | |
| Hematologic AE | 1 (1.2) | 0 (0) | |
| Gastrointestinal AE | 1 (1.2) | 2 (2.5) | |
| General weakness | 8 (9.3) | 11 (13.6) | |
| Death | 0 (0) | 1 (1.2) |
Among the FOLFIRINOX group, 47 (54.7%) patients experienced delayed treatment and 12 (14.0%) patients discontinued chemotherapy due to adverse events. In the Gem + nabPTX group, 51 (63.0%) and 17 (21.0%) patients experienced treatment delay and discontinuation respectively. The proportion of treatment delay and cessation were not statistically different between both groups. The most common cause of delayed treatment was hematologic AE, and general weakness was the most common cause of early-termination of chemotherapy in both groups.
The anticancer drug cost was similar between the two groups. In patients treated with the FOLFIRINOX regimen, the cost of 1 cycle, which lasted 2 wk, was determined to be 52190 KRW. After adding the room charges, the total cost for 4 wk treatment of the FOLFIRINOX regimen was 138724 KRW. The cost for 4 wk of the Gem + nabPTX regimen was 168838 KRW. Drug costs of two regimens were not very different - only 30000 KRW (about 30 USD) per month.
In the present study, the oncologic outcomes of the FOLFIRINOX and Gem + nabPTX regimens were found to be similar. Although the disease control rate was higher in the Gem + nabPTX group, there was no significant difference in objective response rate, OS, or PFS. In the subgroup analysis, Gem + nabPTX regimen showed survival advantages in relation to the patients' baseline factors such as body/tail cancer, high BMI, presence of liver metastasis and peritoneal carcinomatosis. When comparing the two regimens in terms of safety, patients who received FOLFIRINOX were at higher risk for the development of high-grade neutropenia, while those who received Gem + nabPTX were at higher risk for neuropathy and fatigue.
Compared to previous clinical trial data, the treatment efficacy observed in this study population was favourable. Patients who received Gem + nabPTX showed improved OS (12.1 vs 8.5 mo) and PFS (8.4 vs 5.5 mo) than those in the MPACT. The FOLFIRINOX group patients also showed improved PFS (8.0 vs 6.4 mo) when compared to those in the PRODIGE 4/ACCORD 11 trial, with similar OS rates (10.8 vs 11.1 mo). On the basis of these data, we can consider that both the FOLFIRINOX and Gem + nabPTX regimens are very effective in Korean patients with metastatic pancreatic cancer.
However, treatment-related AEs were more common in this study population than in the previous clinical trials, especially hematologic AEs. Compared to the PRODIGE 4/ACCORD 11 trial[7] the FOLFIRINOX group patients in this study were younger (median age, 54 vs 61 years) and had better performance status scores at baseline (higher proportion of ECOG-PS score 0: 83.7% vs 37.4%). The median relative dose intensity of each agent was similar (5-FU, 80% vs 82%; oxaliplatin, 75% vs 78%; irinotecan, 75% vs 81%). However, the rate of hematologic AEs was remarkably high in this study population and similar to the proportion of hematologic AEs reported in a previous Japanese phase-II study[15]. Considering that the PRODIGE 4/ACCORD 11 trial was conducted at 48 French medical centers and that the rates of AEs in the Korean and Japanese population are similar, it can be assumed that there was an ethnic difference in the incidence of treatment-related AEs using the FOLFIRINOX regimen.
Ethnic differences in terms of drug efficacy or AEs are affected by local environment, dietary habits, genetic mutations, and genetic polymorphism[12]. Ethnic variations in polymorphisms can be an explanation for the racial differences in AEs. For example, the UGT1A1 polymorphism, which is related with the glucuronidation of SN-38 (an active metabolite of irinotecan) is associated with irinotecan-mediated diarrhea and neutropenia[16]. UGT1A1 No. 6 mutations, which are found predominantly in Asian populations, have been implicated in irinotecan toxicity[16-19]. Goetz et al[20] recommended UGT1A1 genotype-guided dosing of CAPIRINOX (capecitabine, oxaliplatin, and irinotecan) in their phase I study because the toxicity profile differed according to the presence of UGT1A1 polymorphisms. Defective cytochrome P450 3A4 (CYP3A4) variants are another example known to be related with paclitaxel-induced neuropathy[21,22]. Although direct associations with different pharmacokinetics according to ethnicity has not yet been established, ethnic differences in the frequency of polymorphisms of CYP3A4 have been reported[23].
Both regimens carry an unfavourable AE profile; therefore, dose modification strategies have been made. In terms of the FOLFIRINOX regimen, several studies used modified (reduced) doses through various methods to increase the patients’ tolerance. For example, Mahaseth et al[24] replaced the 5-FU bolus injection with haematopoietic growth factors. Stein et al[25] used a modified dose with a 25% reduction in both irinotecan and the 5-FU bolus. Li et al[26] used modified FOLFIRINOX (no 5-FU bolus, 85% oxaliplatin, and 75% irinotecan) in Chinese patients with metastatic pancreatic cancer. In their studies, the incidence of severe neutropenia, fatigue, and vomiting were reduced, without any compromise in treatment efficacy. Ahn et al[27] presented a modified biweekly Gem + nabPTX regimen that could reduce the incidence of severe neutropenia and neurotoxicity when compared with that reported in the MPACT data[27].
In our study population, 43 patients (50% of FOLFIRINOX group) started receiving the FOLFIRINOX regimen as a modified (reduced) dose. There was no significant difference in treatment duration and efficacy. Moreover, no significant difference was noted in the incidence of non-hematologic AEs. However, the incidences of severe (grade ≥ 3) neutropenia and febrile neutropenia were lower in patients who received the modified dose than in those who received the full dose (severe neutropenia, 62.8% vs 86.0%; P = 0.013, febrile neutropenia, 18.6% vs 32.6%; P = 0.138). It may be helpful to use a modified dose when initiating chemotherapy for toxicity-susceptible patients identified via early screening tests.
Second-line chemotherapy could be considered after first-line therapy failure if patients continue to demonstrate good performance status. Even after a failure of first-line treatment, effective second-line chemotherapy can prolong patients’ post-progression survival (PPS, overall survival from the notification of disease progression) and OS[28,29]. In this study population, 20 (24.7%) patients in the Gem + nabPTX group and 38 (44.2%) in the FOLFIRINOX group received second-line chemotherapy. XELOX (capecitabine plus oxaliplatin) was the most commonly prescribed second-line regimen in the Gem + nabPTX group and gemcitabine plus erlotinib was the most common second-line treatment administered in the FOLFIRINOX group. No difference in PPS was noted between the two groups (Gem + nabPTX group, 136 d [95%CI, 78.384–193.616]; FOLFIRINOX group, 148 d [95%CI, 120.576–175.424]; P = 0.762). Overall, patients who received second-line chemotherapy showed a significantly longer PPS than patients who did not (138 vs 39 d; P < 0.001) In particular, second-line chemotherapy was found to be more effective in patients who showed early progression within 3 mo after the first-line treatment (median PPS, 153 d). Recently, several studies have been conducted to assess the efficacy of second-line chemotherapy. Portal et al[30] showed that second-line Gem + nabPTX was effective after the failure of first-line FOLFIRINOX[30]. The NAPOLI-1 trial revealed that nanoliposomal irinotecan (nan-IRI) plus 5-FU was effective in patients previously treated with gemcitabine-based chemotherapy[31]. Another clinical trial showed that oxaliplatin, folinic acid, and fluorouracil (OFF regimen) was effective in gemcitabine-refractory pancreatic cancer patients[32]. Furthermore, there are ongoing studies testing FOLFIRINOX after Gem + nabPTX failure[33].
As more options for second-line chemotherapy are being introduced, questions surrounding treatment choice and sequence have arisen. The advantage of using FOLFIRINOX as a first-line regimen is that Gem + nabPTX, which has similar efficacy, can be used as the secondary drug. On the other hand, patients who receive Gem + nabPTX as the first-line drug can choose diverse 5-FU based regimens (i.e. OFF, nal-IRI + 5-FU, or FOLFIRINOX) as second-line treatment depending on their performance status. Although more favourable sequences need to be studied, the active use of FOLFIRINOX or Gem + nabPTX as a second-line regimen or appropriate use of new agents such as nal-IRI may help to improve the prognosis of patients who show early progression.
When we consider the economic aspects of anticancer treatment, we have to consider both anticancer drug costs and general management costs such as hospitalization or medication fees for AE control. Gemcitabine, nab-paclitaxel, oxaliplatin, and irinotecan are expensive drugs. Fortunately, in Korea, the NHIS provides economic benefits for cancer patients-the NHIS provides 95% of the drug cost. Therefore, when NHIS coverage for cancer patients is reflected, the costs of the two regimens (per month) are similar. However, in terms of potential cost burden, the FOLFIRINOX regimen seems to have some disadvantages. Patients must be hospitalized to receive the FOLFIRINOX regimen. During hospitalization, additional costs that are not covered by the NHIS, such as private rooms, can occur. In addition, due to the higher hematologic AE rates, the cost for prolonged hospitalization, G-CSF administration, and infection control (related to febrile neutropenia) are more likely to occur in patients receiving FOLFIRINOX. However, for a more accurate comparison, additional quantitative and comparative analysis is also needed in terms of the decreased labour productivity or quality of life due to admission or severe AEs such as peripheral neuropathy, fatigue, or alopecia[34,35].
This study had several limitations. First, it was a retrospective cohort study conducted only in a single center. A prospective randomized controlled trial is needed to confirm and validate the results of this study. And, to determine the ethnic differences in efficacy and safety more clearly, a larger scale nation-wide study will be helpful. Second, this study did not quantify the change in the quality of life. There was a lack of medical records and questionnaires that could help to more precisely analyse quality of life. Finally, we could not perform more advanced genetic analyses. If new technologies (e.g., next-generation sequencing) are actively used in clinical fields, it will be possible to collect and analyse genetic data more economically and easily.
The results of the present study suggest that the FOLFIRINOX and Gem + nabPTX regimens are similar in efficacy, but the type and rates of the AE are somewhat different: neurologic AEs were more common in the Gem + nabPTX group and hematologic AEs were more common in the FOLFIRINOX group. Given the subgroup analysis of this study, Gem + nabPTX regimen could be considered as a priority in patients with specific baseline conditions.
FOLFIRINOX regimen and combination of gemcitabine and nab-paclitaxel (Gem + nabPTX) are recommended as the first-line therapy for metastatic pancreatic cancer. However, there is a lack of data regarding a direct comparison of the two regimens in efficacy and safety.
When treating metastatic pancreatic cancer patients, physicians would like to select appropriate chemotherapeutic regimens while avoiding and preventing unnecessary complications and economic burdens. By comparing the efficacy and safety of two regimens, this study can help physicians’ decision of treatment choice and sequence.
The purpose of this study is to compare the efficacy, safety, and economic aspects of FOLFIRINOX and Gem + nabPTX in the treatment of metastatic pancreatic cancer in Korean population.
Patients with metastatic or recurrent pancreatic cancer treated with FOLFIRINOX (n = 86) or Gem + nabPTX (n = 81) as the first-line since January 2015 were identified using the Severance Hospital Pancreatic Cancer Cohort Registry. Treatment efficacy, treatment-related adverse events and economic aspects were compared.
The median overall survival (FOLFIRINOX 10.7 vs Gem + nabPTX 12.1 mo; P = 0.157), progression-free survival (FOLFIRINOX 8.0 vs Gem + nabPTX 8.4 mo; P = 0.134), and objective response rates (FOLFIRINOX 33.7% vs Gem + nabPTX 46.9%; P = 0.067) were not significantly different between two regimens. Neurologic adverse events were more common in the Gem + nabPTX group and Grade ≥ 3 neutropenia and gastrointestinal adverse events were more common in the FOLFIRINOX group. The drug costs of both regimens were similar.
Treatment efficacy and economic burdens were comparable between the two regimens. But, the type and rates of the adverse events were somewhat different. Given the subgroup analysis of this study, Gem + nabPTX regimen might be considered preferentially in patients with specific baseline conditions.
This study will help clinicians choose an appropriate chemotherapeutic regimen for metastatic pancreatic cancer. To confirm and validate the results of this study, a larger scale prospective study would be helpful.
The authors thanks to Jun Tae Kim for his dedication in handling, managing and auditing of cohort data.
| 1. | Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1397] [Cited by in RCA: 1791] [Article Influence: 179.1] [Reference Citation Analysis (1)] |
| 2. | Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Prediction of Cancer Incidence and Mortality in Korea, 2017. Cancer Res Treat. 2017;49:306-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 84] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 3. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12135] [Cited by in RCA: 13047] [Article Influence: 1304.7] [Reference Citation Analysis (3)] |
| 4. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4351] [Cited by in RCA: 4223] [Article Influence: 145.6] [Reference Citation Analysis (0)] |
| 5. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2794] [Article Influence: 147.1] [Reference Citation Analysis (0)] |
| 6. | Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schüller J, Saletti P, Bauer J, Figer A, Pestalozzi B, Köhne CH, Mingrone W, Stemmer SM, Tàmas K, Kornek GV, Koeberle D, Cina S, Bernhard J, Dietrich D, Scheithauer W; Swiss Group for Clinical Cancer Research; Central European Cooperative Oncology Group. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25:2212-2217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 438] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 7. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5916] [Article Influence: 394.4] [Reference Citation Analysis (24)] |
| 8. | Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, Harris M, Reni M, Dowden S, Laheru D, Bahary N, Ramanathan RK, Tabernero J, Hidalgo M, Goldstein D, Van Cutsem E, Wei X, Iglesias J, Renschler MF. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4035] [Cited by in RCA: 5157] [Article Influence: 396.7] [Reference Citation Analysis (12)] |
| 9. | Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A, Ruggiero JT, Copur MS, Lau M, Urba S, Laheru D. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34:2784-2796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 248] [Cited by in RCA: 241] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 10. | National Comprehensive Cancer Network. Pancreatic adenocarcinoma. In: NCCN Clinical Practice Guidelines in Oncology (NCCN guidelines) 2018. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx. |
| 11. | Cho IR, Kang H, Jo JH, Lee HS, Chung MJ, Park JY, Park SW, Song SY, Chung JB, An C, Park MS, Jung SY, Bang S. Efficacy and treatment-related adverse events of gemcitabine plus nab-paclitaxel for treatment of metastatic pancreatic cancer "in a Korean" population: A single-center cohort study. Semin Oncol. 2017;44:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | O'Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res. 2009;15:4806-4814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 13. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 22868] [Article Influence: 1345.2] [Reference Citation Analysis (1)] |
| 14. | National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE). 4th ed, 2009. Available from: http://www.meddramsso.com. |
| 15. | Okusaka T, Ikeda M, Fukutomi A, Ioka T, Furuse J, Ohkawa S, Isayama H, Boku N. Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci. 2014;105:1321-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Sai K, Saito Y, Sakamoto H, Shirao K, Kurose K, Saeki M, Ozawa S, Kaniwa N, Hirohashi S, Saijo N, Sawada J, Yoshida T. Importance of UDP-glucuronosyltransferase 1A1*6 for irinotecan toxicities in Japanese cancer patients. Cancer Lett. 2008;261:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Gagné JF, Montminy V, Belanger P, Journault K, Gaucher G, Guillemette C. Common human UGT1A polymorphisms and the altered metabolism of irinotecan active metabolite 7-ethyl-10-hydroxycamptothecin (SN-38). Mol Pharmacol. 2002;62:608-617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 267] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Jinno H, Tanaka-Kagawa T, Hanioka N, Saeki M, Ishida S, Nishimura T, Ando M, Saito Y, Ozawa S, Sawada J. Glucuronidation of 7-ethyl-10-hydroxycamptothecin (SN-38), an active metabolite of irinotecan (CPT-11), by human UGT1A1 variants, G71R, P229Q, and Y486D. Drug Metab Dispos. 2003;31:108-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 19. | Han JY, Lim HS, Shin ES, Yoo YK, Park YH, Lee JE, Jang IJ, Lee DH, Lee JS. Comprehensive analysis of UGT1A polymorphisms predictive for pharmacokinetics and treatment outcome in patients with non-small-cell lung cancer treated with irinotecan and cisplatin. J Clin Oncol. 2006;24:2237-2244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 240] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Goetz MP, McKean HA, Reid JM, Mandrekar SJ, Tan AD, Kuffel MA, Safgren SL, McGovern RM, Goldberg RM, Grothey AA, McWilliams R, Erlichman C, Ames MM. UGT1A1 genotype-guided phase I study of irinotecan, oxaliplatin, and capecitabine. Invest New Drugs. 2013;31:1559-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 21. | de Graan AJ, Elens L, Sprowl JA, Sparreboom A, Friberg LE, van der Holt B, de Raaf PJ, de Bruijn P, Engels FK, Eskens FA, Wiemer EA, Verweij J, Mathijssen RH, van Schaik RH. CYP3A4*22 genotype and systemic exposure affect paclitaxel-induced neurotoxicity. Clin Cancer Res. 2013;19:3316-3324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 22. | Apellániz-Ruiz M, Lee MY, Sánchez-Barroso L, Gutiérrez-Gutiérrez G, Calvo I, García-Estévez L, Sereno M, García-Donás J, Castelo B, Guerra E, Leandro-García LJ, Cascón A, Johansson I, Robledo M, Ingelman-Sundberg M, Rodríguez-Antona C. Whole-exome sequencing reveals defective CYP3A4 variants predictive of paclitaxel dose-limiting neuropathy. Clin Cancer Res. 2015;21:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Phan VH, Tan C, Rittau A, Xu H, McLachlan AJ, Clarke SJ. An update on ethnic differences in drug metabolism and toxicity from anti-cancer drugs. Expert Opin Drug Metab Toxicol. 2011;7:1395-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Mahaseth H, Brutcher E, Kauh J, Hawk N, Kim S, Chen Z, Kooby DA, Maithel SK, Landry J, El-Rayes BF. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas. 2013;42:1311-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, Staugaard C, Indukala D, Boustani AM, Patel V, Cha CH, Salem RR, Chang B, Hochster HS, Lacy J. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer. 2016;114:737-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 167] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 26. | Li X, Ma T, Zhang Q, Chen YG, Guo CX, Shen YN, Sun PW, Li GG, Gao SL, Que RS, Lou JY, Yu RS, Yuan Y, Wei QC, Wei SM, Zhang Y, Zheng L, Bai XL, Liang TB. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer Lett. 2017;406:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 27. | Ahn DH, Krishna K, Blazer M, Reardon J, Wei L, Wu C, Ciombor KK, Noonan AM, Mikhail S, Bekaii-Saab T. A modified regimen of biweekly gemcitabine and nab-paclitaxel in patients with metastatic pancreatic cancer is both tolerable and effective: a retrospective analysis. Ther Adv Med Oncol. 2017;9:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, Dogan Y, Gebauer B, Schumacher G, Reichardt P. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer--a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011;47:2306-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 447] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 29. | Pelzer U, Schwaner I, Stieler J, Adler M, Seraphin J, Dörken B, Riess H, Oettle H. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur J Cancer. 2011;47:1676-1681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 252] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 30. | Portal A, Pernot S, Tougeron D, Arbaud C, Bidault AT, de la Fouchardière C, Hammel P, Lecomte T, Dréanic J, Coriat R, Bachet JB, Dubreuil O, Marthey L, Dahan L, Tchoundjeu B, Locher C, Lepère C, Bonnetain F, Taieb J. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br J Cancer. 2015;113:989-995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 31. | Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, Hubner RA, Chiu CF, Schwartsmann G, Siveke JT, Braiteh F, Moyo V, Belanger B, Dhindsa N, Bayever E, Von Hoff DD, Chen LT; NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 884] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 32. | Oettle H, Riess H, Stieler JM, Heil G, Schwaner I, Seraphin J, Görner M, Mölle M, Greten TF, Lakner V, Bischoff S, Sinn M, Dörken B, Pelzer U. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423-2429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 347] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 33. | Taieb J, Pointet AL, Van Laethem JL, Laquente B, Pernot S, Lordick F, Reni M. What treatment in 2017 for inoperable pancreatic cancers? Ann Oncol. 2017;28:1473-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Luengo-Fernandez R, Leal J, Gray A, Sullivan R. Economic burden of cancer across the European Union: a population-based cost analysis. Lancet Oncol. 2013;14:1165-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 549] [Cited by in RCA: 683] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 35. | Tingstedt B, Andersson E, Flink A, Bolin K, Lindgren B, Andersson R. Pancreatic cancer, healthcare cost, and loss of productivity: a register-based approach. World J Surg. 2011;35:2298-2305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country of origin: South Korea
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aosasa S, Tang Y S-Editor: Zhang L L-Editor: A E-Editor: Qi LL