Published online Nov 15, 2020. doi: 10.4251/wjgo.v12.i11.1288
Peer-review started: July 7, 2020
First decision: September 17, 2020
Revised: September 27, 2020
Accepted: October 19, 2020
Article in press: October 19, 2020
Published online: November 15, 2020
Processing time: 127 Days and 17.2 Hours
Microangiopathic hemolytic anemia (MAHA) with thrombocytopenia and organ failure caused by tumor-associated thrombotic microangiopathy (TMA) is a life-threatening oncological emergency. Rapid diagnosis and precise distinction from other forms of TMA is crucial for appropriate therapy, which aims at treating the underlying malignancy. However, the prognosis of patients with cancer-related (CR)-MAHA is limited. To date, less than 50 patients with gastric cancer and CR-MAHA have been reported, mainly as single case reports, and detailed information on treatment strategies and outcome are scarce. We analyzed the characteristics and outcomes data of CR-MAHA patients with gastric cancer treated at our center between 2012 and 2019.
To gain knowledge about CR-MAHA and the course of disease.
We retrospectively analyzed patients using an institutional prospectively maintained database. Patients who had CR-MAHA but other cancer types or cancer of unknown primary were excluded. The basic requirements for inclusion were: Histologically proven gastric adenocarcinoma; and clinical diagnosis of hemolytic anemia with schistocytes with or without thrombocytopenia. The observation period for each patient started with the first day of documented symptoms. The follow-up period for this analysis ended on February 1, 2020.
We identified eight patients with a median age of 54 years. Histologically, all patients had (partial) diffuse subtypes of gastric adenocarcinoma with partial or complete signet cell morphology. All patients had metastatic disease and one patient had a microsatellite instability-high (MSI-H) tumor. In three patients, clinical signs of MAHA preceded the diagnosis of cancer, and in two patients, CR-MAHA indicated recurrent disease. All patients had severe hemolytic anemia and thrombocytopenia. Six patients experienced severe bone pain, and five patients had dyspnea. Systemic, 5-fluorouracil-based combination chemotherapy was initiated in six patients, which resulted in rapid initial response with significant improvement of clinical symptoms and blood values. Progression-free survival (PFS) of the whole cohort was 1.9 wk and median overall survival (OS) was 1.9 wk. For patients with chemotherapy, PFS was 9.0 wk and OS was 10.3 wk. The patient with the MSI-H tumor has been undergoing immunotherapy for more than 3 years.
The benefit of chemotherapy in CR-MAHA patients is limited. Immunotherapy for patients with MSI-H tumors may lead to long-term tumor control even in CR-MAHA patients.
Core Tip: Microangiopathic hemolytic anemia (MAHA) with thrombopenia and organ failure caused by tumor-associated thrombotic microangiopathy is a rare and life-threatening oncological emergency. The only proven therapy is the treatment of the underlying malignancy. In our retrospective series, 6 of 8 cancer-related (CR)-MAHA patients with advanced gastric cancer received combination chemotherapy with an overall survival (OS) of 10.3 wk. For the whole cohort, OS was only 1.8 wk. Second-line treatment did not show any benefit. One patient with microsatellite instability-high tumor has been undergoing immunotherapy for more than 3 years, which to the best of our knowledge is the first reported case of long-term survival in a CR-MAHA patient with advanced disease.
- Citation: Berger AK, Allgäuer M, Apostolidis L, Schulze-Schleithoff AE, Merle U, Jaeger D, Haag GM. Cancer-related microangiopathic hemolytic anemia in patients with advanced gastric cancer: A retrospective single-center analysis. World J Gastrointest Oncol 2020; 12(11): 1288-1295
- URL: https://www.wjgnet.com/1948-5204/full/v12/i11/1288.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i11.1288
Tumor-associated thrombotic microangiopathy (TMA) caused by systemic microvascular metastases and bone marrow involvement is a life-threatening oncological emergency leading to microangiopathic hemolytic anemia (MAHA) with thrombocytopenia and organ failure of variable severity[1]. This alarming and often fatal clinical constellation is described as a rare event in several solid tumors entities and may sometimes reveal occult disseminated malignancy[2,3]. It has to be clearly distinguished from drug-induced TMA in cancer patients[4] and hereditary or acquired primary TMA syndromes such as ADAMTS13 deficiency or Shiga toxin-mediated TMA[5]. Those forms are also clinically characterized by MAHA, thrombocytopenia and organ injury, but based on diverging underlying pathomechanisms, immediate specific therapeutic measures like plasma exchange treatment or application of eculizumab[6,7] can be crucial for forms of primary TMA. For cancer patients with drug-induced TMA, removal of the causative drug is often sufficient[1]. In contrast, for patients with cancer-related (CR)-MAHA, the only beneficial therapy is the treatment of the underlying malignancy, highlighting the need for rapid diagnosis to avoid ineffective therapeutic interventions such as plasmapheresis. Still, the prognosis of this subgroup of cancer patients is very poor[8].
To date, the most comprehensive clinical data for CR-MAHA patients come from two heterogeneous thrombotic thrombocytopenic purpura (TTP)-registry series that included 10 and 20 patients with different tumor types[2,9]. Gastric cancer was identified in 13.3% (4/30) of those patients. A large retrospective literature review covering a time period of 33 years reported on 168 CR-MAHA patients and identified another 40 patients with gastric cancer, reported mainly as single case reports[8]. Here, we add our experience with 8 patients presenting with CR-MAHA and gastric cancer treated at our university hospital center between 2012 and 2019. We present the patients’ clinical and laboratory findings and outcomes, and report our experience with oncological treatment for this rare condition. To the best of our knowledge, this is the largest cohort of gastric cancer patients with CR-MAHA reported to date.
We identified patients who were diagnosed with CR-MAHA and gastric adenocarcinoma at our center between January 2012 and January 2020. Patients who had CR-MAHA but other cancer types or cancer of unknown primary were excluded from this analysis. The data were obtained from an institutional prospectively maintained database. The basic requirements for inclusion were histologically proven diagnosis of gastric adenocarcinoma; and clinical diagnosis of hemolytic anemia with schistocytes with or without thrombocytopenia. The observation period for each patient started with the first day of documented symptoms. The follow-up period for this analysis ended on February 1, 2020.
If systemic treatment was initiated, the choice of treatment was up to the attending oncologist considering the individual patient’s condition. When antitumor treatment was withheld, this was a shared decision considering the patient’s choice, his condition, and the physician’s advice. Clinical data were routinely collected and documented by the attending oncologists and medical staff via an electronic medical record. Information included: Time of first CR-MAHA-associated symptoms, time of diagnosis of MAHA from or to diagnosis of gastric cancer (prior, concurrent, after), site and histologic subtype of carcinoma, laboratory features, clinical signs of organ failure, histologic analysis of the bone marrow, start and stop date of antitumor treatment, response to therapy, and date of progression and date of death.
Overall survival (OS) was defined as the time from documented CR-MAHA diagnosis to death. Progression-free survival (PFS) was defined as the time from CR-MAHA diagnosis to documented tumor progression or death, whichever occurred first. Residual survival (RS) among patients with salvage therapy was defined as the time from start of second-line therapy to death. Diagnosis of CR-MAHA was defined as the timepoint when the term was documented the first time. Onset of CR-MAHA symptoms was defined as the first documented medical contact (as inpatient or outpatient) the patient had for associated symptoms (e.g., dyspnea, pain, bleeding) or laboratory findings (anemia, thrombocytopenia, coagulopathy).
The study was approved by the local Ethics Committee University of Heidelberg, No. S-335/2014.
We identified 8 patients meeting the inclusion criteria. An overview of the patients’ characteristics is given in Table 1. The median age was 50 years (range 28-76) for diagnosis of gastric cancer and 54 years (range 28-76) for diagnosis of CR-MAHA. Four patients were female. The gastric carcinoma was located in the stomach in 7 patients (87.5%), and at the gastroesophageal junction in 1 patient (12.5%). Histologically, 4 patients (50%) had adenocarcinomas of the diffuse subtype, and 4 patients (50%) showed mixed intestinal/diffuse differentiation. In all cases, the tumor was of partial or complete signet ring cell morphology. Five patients (62.5%) were human epidermal growth factor receptor 2 (HER2)-negative, and HER2 status was unknown in 3 patients (37.5%). One patient (12.5%) had microsatellite instability-high (MSI-H) tumor, and MSI status is unknown in all other patients. All patients had metastatic disease. Five patients (62.5%) had synchronous metastases, and 3 patients (37.5%) had secondary metastatic disease after definitive surgery that occurred 0.5, 2, and 10 years, respectively, after first treatment. For all patients, survival data were available. At time of database lock, 7 patients (87.5%) had died of their disease.
| Patient | Sex | Age1 | Site of tumor | CR-MAHA2 | Metastases | Initial symptoms | Onset symptoms to diagnosis in d | Chemotherapy for CR- MAHA |
| 1 | F | 47 | Stomach | Prior | OSS, LN, OVA | BP | 1 | Yes |
| 2 | M | 68 | Stomach | After | LR, PUL | DYS, BP | 4 | No |
| 3 | M | 53 | Stomach | Prior | OSS, LN | DYS, BP | 22 | Yes |
| 4 | F | 36 | GE-junction | After | OSS, HEP | DYS, BP | 0 | Yes |
| 5 | F | 61 | Stomach | Concurrent | OSS, PC, PUL, LN | DYS | 0 | Yes |
| 6 | M | 54 | Stomach | After | OSS, PUL | DYS, BP | 6 | Yes |
| 7 | F | 28 | Stomach | Concurrent | HEP | DYS, APH | 10 | Yes |
| 8 | M | 76 | Stomach | Prior | OSS, PUL | No | 5 | No |
In 3 patients (37.5%), clinical signs of MAHA preceded the diagnosis of cancer. Before admission to our hospital, these patients were inpatients or outpatients of external medical facilities. One patient was admitted to our hospital with severe backpain, anemia and thrombocytopenia, suspected to have “acute leukemia” only days after the patient sought medical attention for the first time. The second patient was transferred after 5 days with “unclear coagulopathy.” The third patient had been under diagnostic evaluations for backpain and progressing anemia and thrombocytopenia for 3 wk. In 2 patients (25%), CR-MAHA indicated recurrent disease after 0.5 and 10 years, respectively. The patient with recurrent metastatic disease after 10 years was treated at an external hospital for severe backpain and thrombocytopenia for 6 d, and the patient with secondary metastases after 0.5 years presented in our emergency department with new back pain, fever and dyspnea. In 2 patients (25%), onset of CR-MAHA was concurrent with first cancer diagnosis, and 1 patient (12.5%) was under palliative chemotherapy (paclitaxel-ramucirumab) when CR-MAHA occurred.
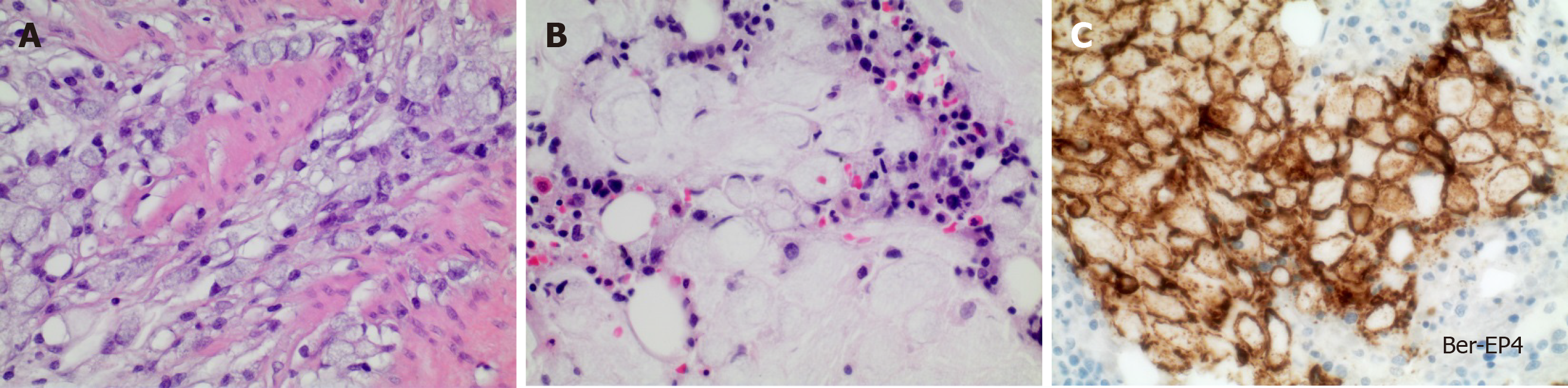
In all patients, laboratory analysis revealed hemolytic anemia with a median hemoglobin concentration of 7.7 g/dL (range 5.4-8.4 g/dL, reference 12-16 g/dL), median schistocytes of 33% (range 15-97%, reference < 5%) and decreased haptoglobin (< 0.1 g/L, reference 0.3-2.0 g/L). The median platelet count was 40/nL (range 8-168/nL, reference 150-440/nL). Median lactate dehydrogenase level was 853 U/L (range 415-4765 U/L, reference < 249 U/L). At time of CR-MAHA, 1 patient (12.5%) had intermittent neurological symptoms (aphasia), 6 (75%) suffered from dyspnea and 5 (62.5%) complained about severe backpain, requiring analgesia with opioids. In 5 of 8 patients (62.5%), bone marrow biopsy was performed, of which 3 showed infiltration by adenocarcinoma (Figure 1). In 1 patient there were no signs of infiltration and 1 patient had a dry tap.
After diagnosis of CR-MAHA, systemic chemotherapy was administered in 6 patients (75%). Median time between CR-MAHA diagnosis at our center and start of chemotherapy was 1 d (range 0-2). Median time between first symptoms and start of chemotherapy was 5 d (range 0-22). All patients received 5-fluorouracil (5-FU)-based combinations. Three (37.5%) patients were treated with a triple combination according to the FLOT regimen[10], 3 patients (37.5%) received doublet combinations (5-FU plus oxaliplatin in 2 cases, 5-FU plus irinotecan and ramucirumab in 1 case). Four patients (66.7% of patients starting treatment) showed rapid initial response to chemotherapy with significant improvement of clinical symptoms and blood values already before the second cycle at day 14 (Table 2). Two patients (25%) deteriorated rapidly despite chemotherapy and died only 1 and 5 d afterwards. Three patients (37.5%) received second-line chemotherapies (FOLFIRI, FOLFIRI-ramucirumab and paclitaxel, respectively). In one patient (patient 4), further analysis revealed a MSI-H tumor. Thus, after showing disease progression during treatment with FOLFIRI/ ramucirumab, a checkpoint inhibitor therapy was initiated. A detailed summary of patients and treatment responses is given in Table 2.
| Patient number | 1st chemo therapy | 2nd chemo therapy | MAHA baseline | MAHA day 14 | PFS in wk | RS in wk | OS in wk | ||||
| Pts | Hb | Sch | Plt | Hb | Sch | ||||||
| 1 | FLO | FOLFIRI | 8 | 5.9 | 31 | 84 | 9.6 | 12 | 9 | 1.1 | 10.3 |
| 2 | No | NA | 32 | 7.7 | 40 | NA | NA | NA | 0.1 | NA | 0.1 |
| 3 | FLO | Paclitaxel | 34 | 8.0 | 15 | 65 | 10.7 | - | 25.7 | 0.1 | 27.1 |
| 4 | FOLFIRI-Ram1 | NA | 168 | 5.4 | 40 | 204 | 11.6 | - | 32.1 | NA | NA |
| 5 | FLOT | No | 130 | 7.8 | 35 | NA | NA | NA | 1.0 | NA | 1.0 |
| 6 | FLOT | FOLFIRI-Ram | 44 | 8.4 | 25 | 208 | 8.8 | 25 | 25.4 | 2.3 | 28.0 |
| 7 | FLOT | No | 46 | 6.9 | 97 | NA | NA | NA | 0.3 | NA | 0.3 |
| 8 | No | NA | 36 | 8.2 | 17 | NA | NA | NA | 1.86 | NA | 1.86 |
At time of analysis, 7 patients had died of their disease. The median PFS of the whole cohort was 1.9 wk (95% confidence interval [CI]: 0.0-12.9), median OS was 1.9 wk (95%CI: 0.0-14.7). For the 6 patients starting chemotherapy, PFS was 9.0 wk (95%CI: 0.0-38.3) and OS was 10.3 wk (95%CI: 0.0-41.7). For patients who received second-line chemotherapy after progression, median RS was 1.1 wk (95%CI: 0.0-3.2). The patient with the MSI-H tumor has been undergoing immunotherapy for more than 3 years.
CR-MAHA was first described in 1970 by Brain et al[11] in 12 patients with metastatic mucin-secreting adenocarcinomas of which 6 were of gastric origin. Less than 50 patients with gastric cancer and CR-MAHA have been described, mainly as single case reports[11]. The pathogenesis of CR-MAHA is not completely understood. Microvascular obstruction with tumor emboli causing red cell fragmentation and platelet consumption especially in the bone marrow and the lung are supposed to be the underlying pathological mechanism[1,8], which is in part supported by available autopsy data[12]. Of note, all of our patients showed, at least partial, signet ring cell morphology of their tumors. Brain et al[11] presumed that mucin-forming tumor cells may especially promote CR-MAHA. The incidence of CR-MAHA is estimated to be 0.25-0.45 persons per million per year[8] but data on CR-MAHA in overall cancer cases or for different cancer entities are lacking. At our center, between 2012 and 2020, approximately 4% of patients newly diagnosed with metastatic gastric cancer receiving palliative treatment developed CR-MAHA. This rather high rate might be explained by the fact that the awareness of our specialized oncologic center for CR-MAHA is high and that predominantly patients with high tumor burden and/or critical clinical symptoms are sent to our center.
Clinically, a rapid and precise distinction between CR-MAHA and other MAHA forms is crucial, since misdiagnosis, e.g., as a primary TMA syndrome will result in inadequate and resource-consuming treatments, including therapeutic plasma exchange[13]. Given the observed often dramatic clinical deterioration of patients within days and the dismal survival times of 3 d reported by others[2], it is evident that a prolonged diagnostic process can close the narrow time-window for application of systemic antitumor treatment. We had the experience that cross-specialty awareness for CR-MAHA leads to marked improvements in timely diagnosis of this condition. Helpful distinguishing clinical features of patients with CR-MAHA were published by Morton et al[1]. In line with these, dyspnea as well as the occurrence of severe back or bone pain, exceeding the extent of radiologically apparent metastatic disease, were recurrent clinical findings in our cohort. One of our patients developed CR-MAHA while under palliative chemotherapy with paclitaxel and ramucirumab. Drug-induced MAHA was considered unlikely using the Morton criteria, and a later bone marrow biopsy revealed metastatic infiltration by signet ring- cells. Distinguishing CR-MAHA from CR-disseminated intravascular coagulation (DIC) can be challenging. However, the vast majority (87.5%) of our patients had no signs of consumption coagulopathy or fibrinolysis when MAHA was diagnosed. In patients with a prolonged clinical history of MAHA-suspect symptoms, coagulation parameters might be altered since CR-MAHA itself can cause a secondary DIC due to organ damage. In addition, more severe microangiopathic changes on the blood smear can be observed in patients with CR-MAHA in comparison to DIC patients. In our patient with slight laboratory signs of coagulopathy, prolonged CR-MAHA was considered as the more appropriate clinical diagnosis in line with his significantly increased schistocytes and the severely decreased hemoglobin levels.
Our data show, that with prompt application of systemic chemotherapy a subset of patients can achieve tumor control and a relieve of symptoms for a limited time period (mostly few months). The small patient number does not allow for comparison of different chemotherapeutic regimen in this setting. Whether primary immunotherapy in MSI-H CR-MAHA patients would be more beneficial remains unclear, but the required prompt clinical response rather demands for combined chemotherapy to rapidly reduce tumor burden. However, the OS of CR-MAHA patients is clearly reduced compared to unaffected patients with metastatic gastric carcinoma where the median survival is currently in the range of 10-12 mo[14]. Of interest, among all of our patients undergoing second-line therapy, disease progression during first-line treatment manifested with recurrent onset of bone pain and deterioration of blood test results. Thus, laboratory analysis should be performed diligently and could probably be used for early identification of insufficient tumor control. However, the RS after start of second-line treatment was dismal with only a few days. Therefore, the use of chemotherapy beyond the first-line situation seems questionable and should be discussed individually with the patient on a case-by-case basis.
To the best of our knowledge, this is the first report of a patient with CR-MAHA and a MSI-H tumor. Of note, this patient achieved long-term tumor control with the best long-term survival of all CR-MAHA patients reported to date. Thus, in patients with CR-MAHA and successful initial disease stabilization, further testing for MSI seems reasonable and may offer the chance for long-term survival.
CR-MAHA is a rare event in patients with gastric cancer that can occur at every time point during the course of disease. Rapid diagnosis of CR-MAHA may allow for application of systemic chemotherapy, which is the only causative treatment. Chemotherapy can lead to a disease stabilization and relief of symptoms for a limited time period. The results of second-line approaches are disappointing. CR-MAHA patients with MSI-H tumors may benefit enormously from checkpoint inhibition including long-time tumor control. Thus, MSI testing is strongly recommended.
Cancer-related microangiopathic hemolytic anemia (CR-MAHA) is an infrequent but alarming oncological emergency in patients with solid tumors. Advanced gastric cancer seems among the tumor types with the highest association with CR-MAHA. Data on appropriate treatment and patients' outcome are scarce.
To obtain knowledge about CR-MAHA and the course of disease to help guide treatment decisions in future patients with CR-MAHA and gastric cancer.
Frequency, patient and tumor characteristics, symptom load, treatment efficacy and patient outcomes.
We analyzed a prospectively maintained database for patients with CR-MAHA and gastric cancer at our high-volume university cancer center between 2012 and 2019.
We identified 8 patients of whom 6 started polychemotherapy. Four of six showed initial response to treatment, but the survival was poor. Patients under chemotherapy had an overall survival (OS) of 10.3 wk. For the whole cohort, OS was 1.9 wk. One patient with microsatellite instability-high (MSI-H) tumor responded extremely well to immunotherapy with long-time survival exceeding 3 years.
CR-MAHA in gastric cancer patients is a condition with an overall limited prognosis. Some patients respond to first-line treatment for several months. Second-line treatment does not seem beneficial. Testing for MSI status is recommended.
First-line chemotherapy should be discussed with patients with CR-MAHA and gastric cancer, but the limited prognosis should be addressed by the attending oncologists. We do not encourage for second-line approaches. MSI-H tumors seem to act differently, even in fatal conditions such as CR-MAHA. It remains unclear, if combined chemo-immunotherapy in those patients would be beneficial.
| 1. | Morton JM, George JN. Microangiopathic Hemolytic Anemia and Thrombocytopenia in Patients With Cancer. J Oncol Pract. 2016;12:523-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 2. | Francis KK, Kalyanam N, Terrell DR, Vesely SK, George JN. Disseminated malignancy misdiagnosed as thrombotic thrombocytopenic purpura: A report of 10 patients and a systematic review of published cases. Oncologist. 2007;12:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 3. | Lin YC, Chang HK, Sun CF, Shih LY. Microangiopathic hemolytic anemia as an initial presentation of metastatic cancer of unknown primary origin. South Med J. 1995;88:683-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Hausberg M, Felten H, Pfeffer S. Treatment of Chemotherapy-Induced Thrombotic Microangiopathy with Eculizumab in a Patient with Metastatic Breast Cancer. Case Rep Oncol. 2019;12:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:654-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 746] [Cited by in RCA: 817] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 6. | Bendapudi PK, Hurwitz S, Fry A, Marques MB, Waldo SW, Li A, Sun L, Upadhyay V, Hamdan A, Brunner AM, Gansner JM, Viswanathan S, Kaufman RM, Uhl L, Stowell CP, Dzik WH, Makar RS. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4:e157-e164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 349] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 7. | Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nürnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1155] [Article Influence: 88.8] [Reference Citation Analysis (0)] |
| 8. | Lechner K, Obermeier HL. Cancer-related microangiopathic hemolytic anemia: clinical and laboratory features in 168 reported cases. Medicine (Baltimore). 2012;91:195-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 9. | Oberic L, Buffet M, Schwarzinger M, Veyradier A, Clabault K, Malot S, Schleinitz N, Valla D, Galicier L, Bengrine-Lefèvre L, Gorin NC, Coppo P; Reference Center for the Management of Thrombotic Microangiopathies. Cancer awareness in atypical thrombotic microangiopathies. Oncologist. 2009;14:769-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, Stoehlmacher J, Clemens MR, Mahlberg R, Fritz M, Seipelt G, Sievert M, Pauligk C, Atmaca A, Jäger E. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19:1882-1887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 11. | Brain MC, Azzopardi JG, Baker LR, Pineo GF, Roberts PD, Dacie JV. Microangiopathic haemolytic anaemia and mucin-forming adenocarcinoma. Br J Haematol. 1970;18:183-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 100] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Francis KK, Kojouri K and George JN. Occult systemic carcinoma masquerading as thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Commun Oncol. 2005;2:339-343. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Choy B, Alvarez Argote J, Treml A. Cancer-related microangiopathic hemolytic anemia. Transfusion. 2016;56:1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Wagner AD, Syn NL, Moehler M, Grothe W, Yong WP, Tai BC, Ho J, Unverzagt S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Germany
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen L, Nishida T, Paydas S S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li JH