©The Author(s) 2026.
World J Gastrointest Oncol. Feb 15, 2026; 18(2): 115689
Published online Feb 15, 2026. doi: 10.4251/wjgo.v18.i2.115689
Published online Feb 15, 2026. doi: 10.4251/wjgo.v18.i2.115689
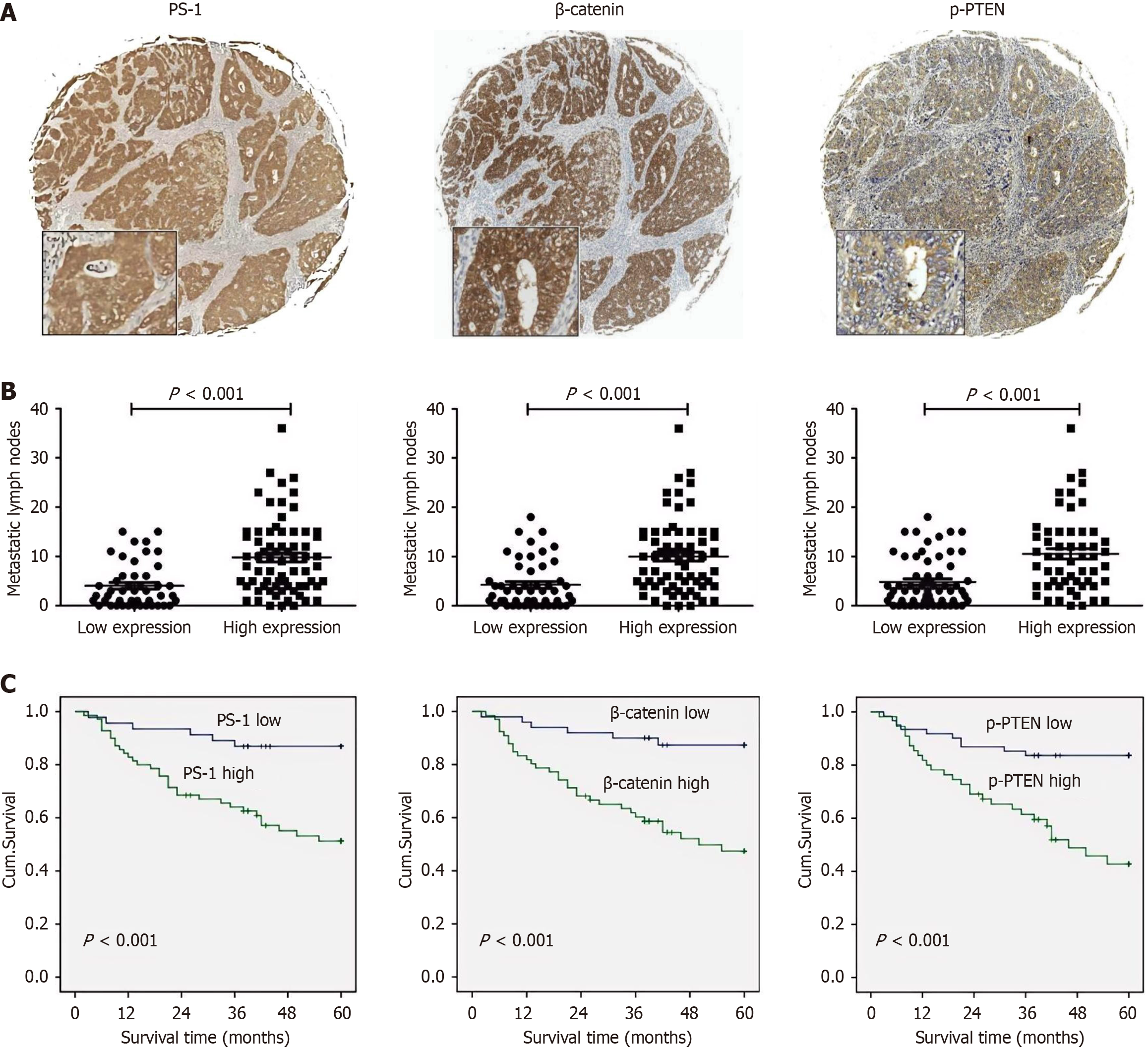
Figure 1 Prognostic values of presenilin-1, β-catenin, and phosphorylation of tensin homolog deleted on chromosome ten in gastric cancer tissues.
A: Representative immunohistochemical staining of presenilin-1 (PS-1), β-catenin, and phosphorylation of tensin homolog deleted on chromosome ten (p-PTEN) in continuous tissue sections from gastric tumor tissues. Original magnification, × 40; B: Significant differences of metastatic lymph nodes between low and high expression of PS-1 (left), β-catenin (middle), and p-PTEN (right); C: The overall survival rate of patients with high PS-1 (left), β-catenin (middle), and p-PTEN (right) expression were significantly lower than that of patients with low protein expression. PS-1: Presenilin-1; p-PTEN: Phosphorylation of tensin homolog deleted on chromosome ten.
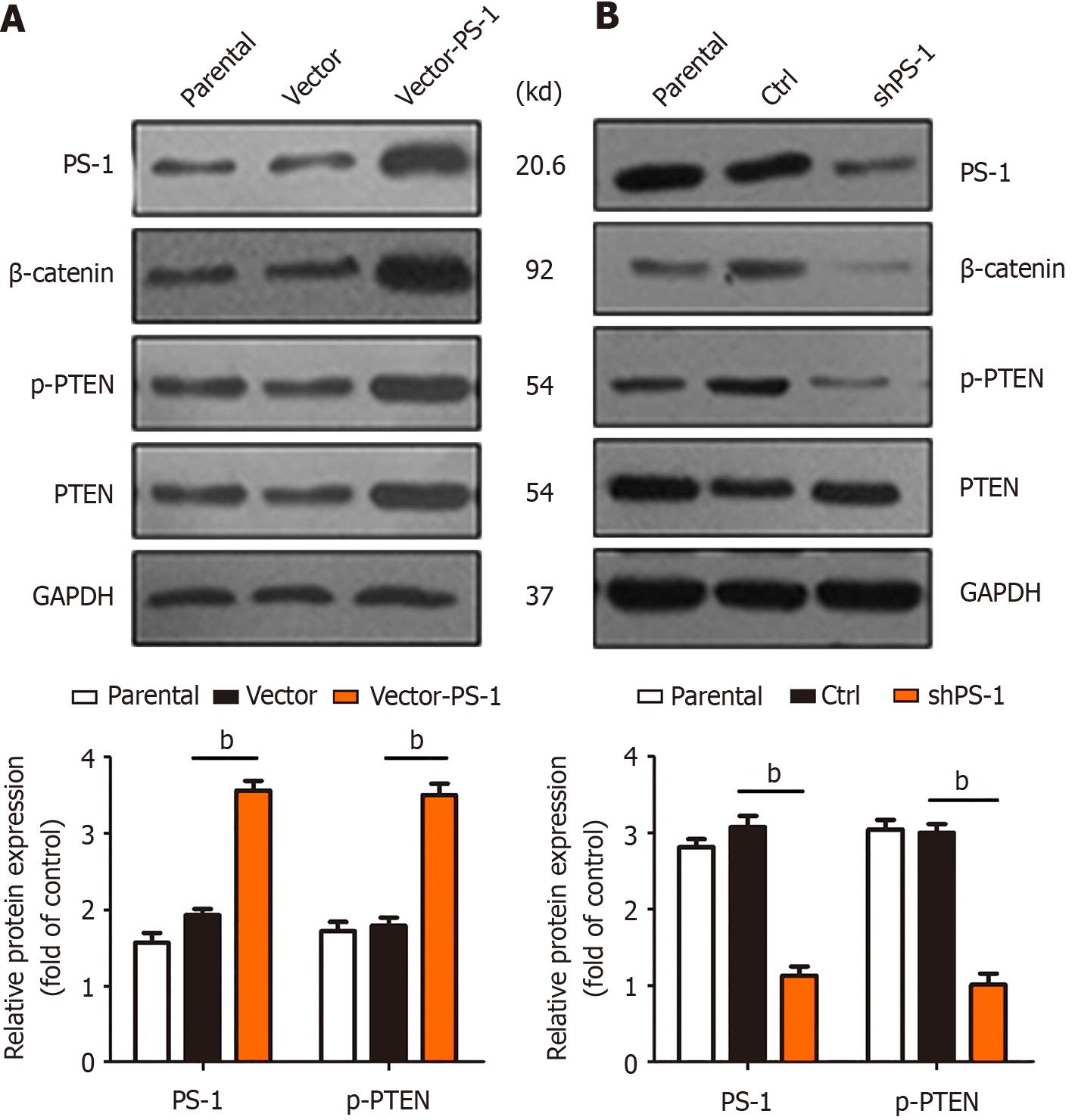
Figure 2 Presenilin-1 enhanced the expression of phosphorylation of tensin homolog deleted on chromosome ten in gastric cancer cells.
A and B: Western blot analysis testing the expression of presenilin-1 (PS-1), β-catenin, phosphorylation of tensin homolog deleted on chromosome ten (PTEN), and PTEN by upregulating and downregulating PS-1. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control. The quantified analysis of the expression of PS-1 and phosphorylation of PTEN is presented. The values were shown as mean ± SD of three independent experiments. bP < 0.01. PS-1: Presenilin-1; p-PTEN: Phosphorylation of tensin homolog deleted on chromosome ten; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; Ctrl: Control; PTEN: Phosphorylation of tensin homolog deleted on chromosome ten.
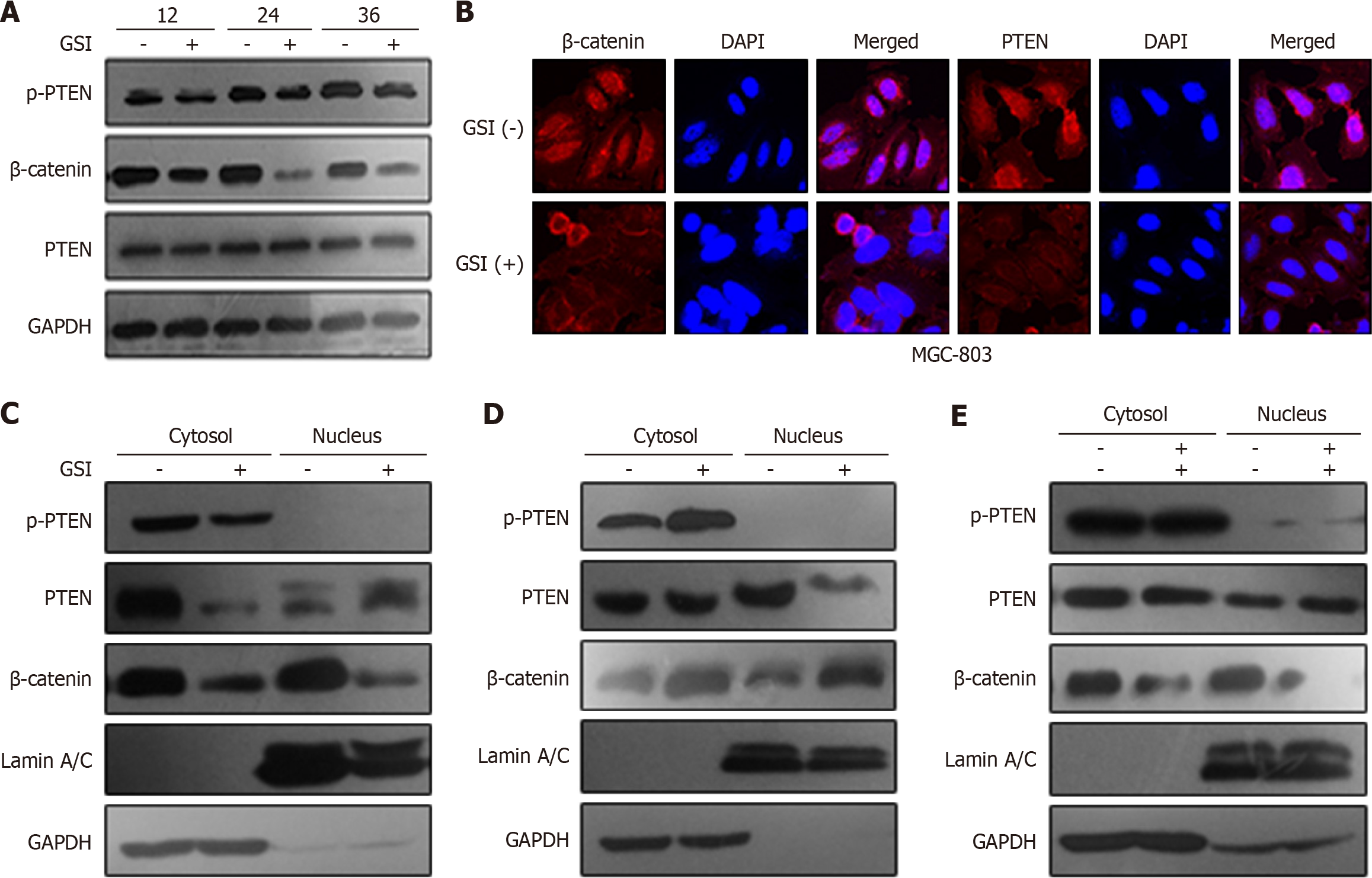
Figure 3 Presenilin-1 regulated tensin homolog deleted on chromosome ten phosphorylation and subcellular localization through activating β-catenin.
A: MGC-803 cells were treated with 1 μM γ-secretase inhibitor (GSI) for 12 h, 24 h, and 36 h. Time-dependent protein expressions of β-catenin, phosphorylation of tensin homolog deleted on chromosome ten (PTEN), and PTEN with or without GSI treatment were detected via western blot analysis. Glyceraldehyde-3-phosphate dehydrogenase was used as a loading control; B: Localization of β-catenin and PTEN after GSI treatment. Cells were treated with 1 μM GSI for 24 h, and immunocytochemical analysis was performed with different antibodies. Nuclei were detected by DAPI staining; C-E: Subcellular localization of phosphorylation of PTEN, PTEN, and β-catenin after treatment of GSI, lentivirus presenilin-1, and both lentivirus presenilin-1 and β-catenin small interfering RNA. Cytoplasmic and nuclear fractions were isolated via the subcellular fraction assay. Glyceraldehyde-3-phosphate dehydrogenase was used as a control in the cytoplasm and lamin A/C in the nucleus. GSI: Γ-secretase inhibitor; p-PTEN: Phosphorylation of tensin homolog deleted on chromosome ten; PTEN: Tensin homolog deleted on chromosome ten; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase.
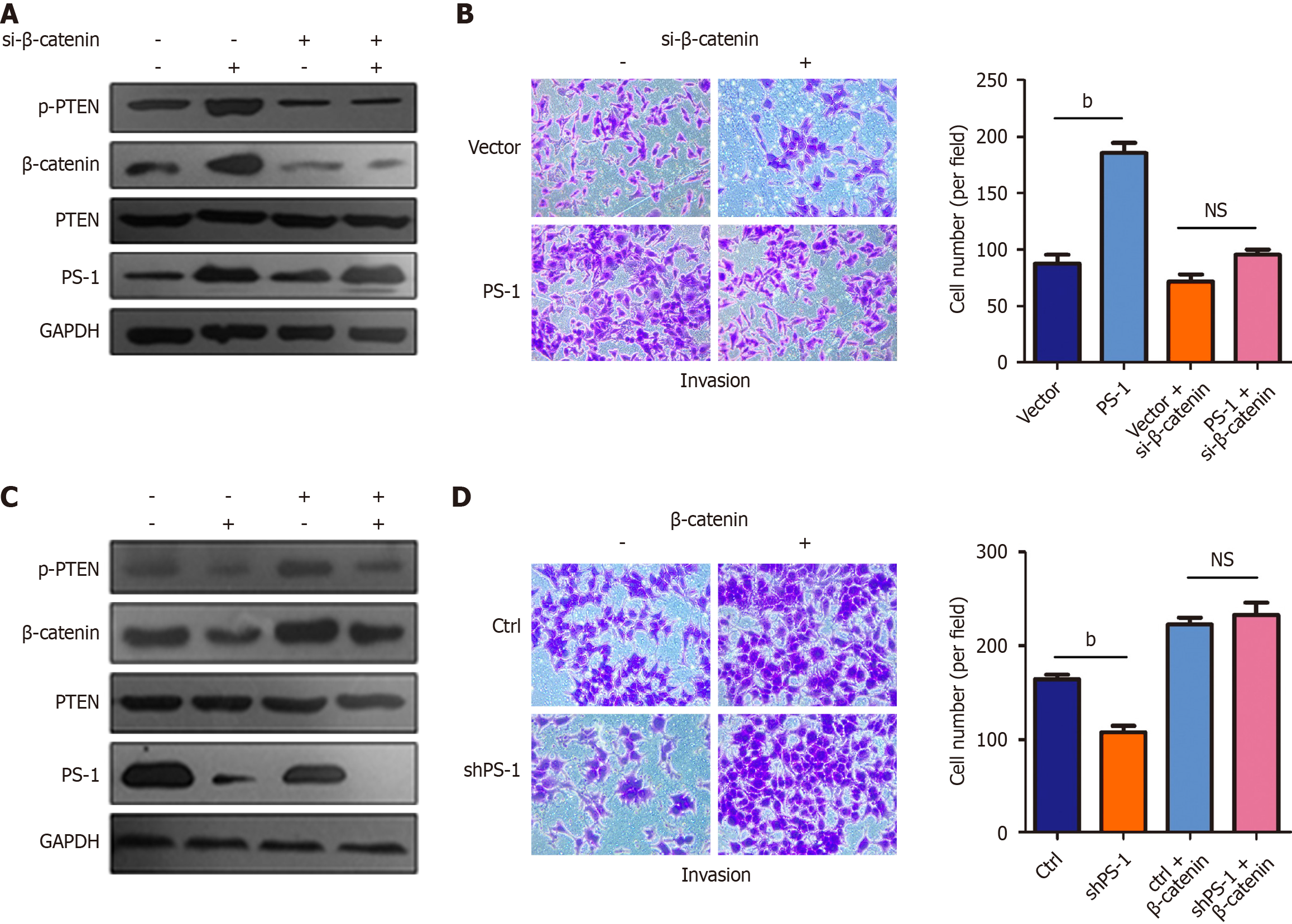
Figure 4 Presenilin-1 promoted gastric cancer cell invasion via β-catenin.
A: The expression of total phosphorylation of tensin homolog deleted on chromosome ten, tensin homolog deleted on chromosome ten, and β-catenin were measured by western blot after transfection with β-catenin small interfering RNA and lentiviral presenilin-1 (PS-1); B: Decreasing expression of β-catenin reversed the enhanced invasion induced by upregulating PS-1. The quantifications were presented as mean ± SD on the right; C: The above proteins were measured after overexpression of β-catenin in stable knockdown PS-1 cell line MGC-803; D: Β-catenin increased the invasion ability of MGC-803 cells when PS-1 was downregulated. The quantifications were presented as mean ± SD on the right. bP < 0.01. NS: No significance; PS-1: Presenilin-1; p-PTEN: Phosphorylation of tensin homolog deleted on chromosome ten; GAPDH: Glyceraldehyde-3-phosphate dehydrogenase; Ctrl: Control; PTEN: Phosphorylation of tensin homolog deleted on chromosome ten.
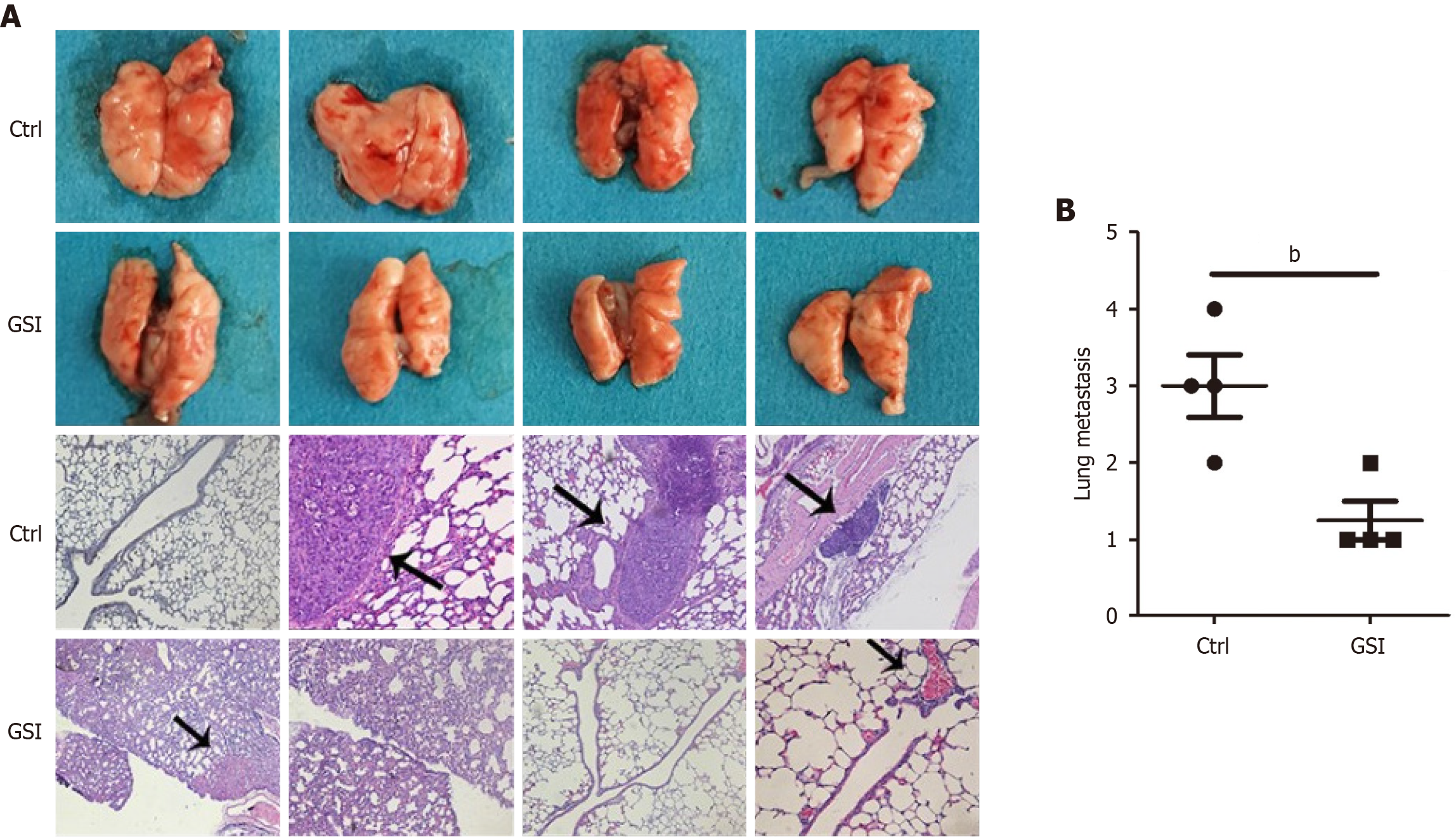
Figure 5 γ-secretase inhibitor inhibited gastric cancer cell metastases in nude mice.
A: Metastases (black arrows) in the lungs at 40 days after inoculation; B: The corresponding quantifications were presented as mean ± SD. Metastases were seldom detected in the γ-secretase inhibitor treatment group. bP < 0.01. GSI: γ-secretase inhibitor; Ctrl: Control.
- Citation: Lin X, Lin GF, Gu FT, Li YL. Increasing expression of presenilin 1, β-catenin, and p-PTEN and its regulatory roles on cell invasion in gastric cancer. World J Gastrointest Oncol 2026; 18(2): 115689
- URL: https://www.wjgnet.com/1948-5204/full/v18/i2/115689.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i2.115689