©The Author(s) 2026.
World J Gastrointest Oncol. Jan 15, 2026; 18(1): 114021
Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.114021
Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.114021
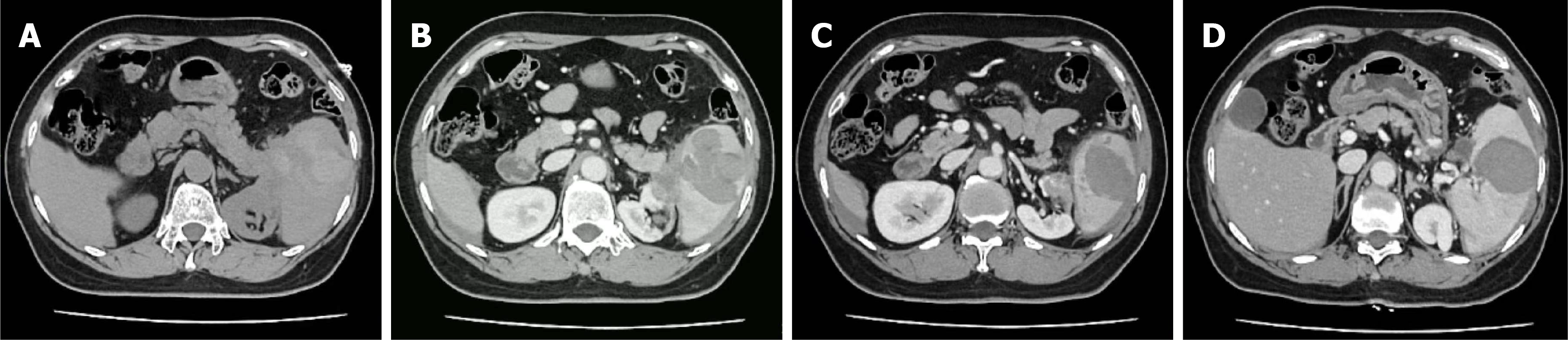
Figure 1 Emergency abdominal computed tomography scan revealed splenic rupture with perisplenic and intraperitoneal hemorrhage.
A: Extensive perisplenic hemorrhage with obscuration of the normal anatomic interface between the pancreatic tail, spleen, and kidney; B and C: Disruption of the renal parenchyma observed on different computed tomography planes; D: A hypodense fluid-filled area within the spleen.
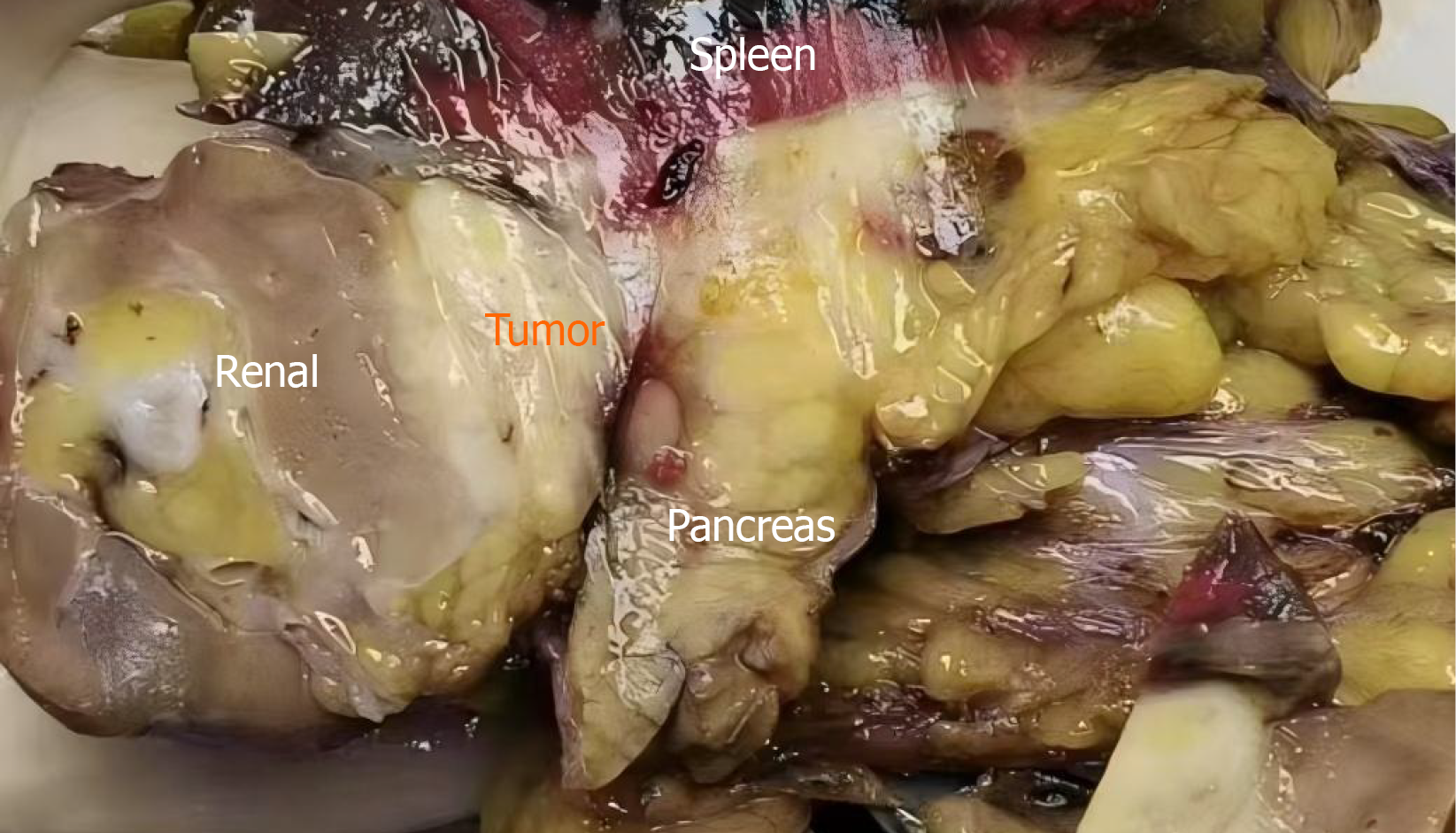
Figure 2
Gross specimen showing a ruptured splenic mass with dense adhesions to the pancreatic tail and left kidney, necessitating en bloc resection of the spleen, distal pancreas, and left kidney.
Figure 3 Representative hematoxylin and eosin staining.
A: Low-power view showing a nodular lesion with adjacent spleen and pancreas. The spleen is located in the upper part of the image, while a small portion of pancreatic tissue is visible in the lower left corner. Foci of micropapillary carcinoma and undifferentiated carcinoma (UDC) demonstrate transitional areas between ductal adenocarcinoma and UDC, with metastatic deposits identified in a regional lymph node; B: A purplish-red appearance with a background of loose stroma, demonstrating a mixture of residual renal architecture and tumor infiltration. Preserved renal tubules and collecting ducts are visible on the right. At the same time, dense clusters of tumor cells with indistinct borders occupy the central and left regions, consistent with an invasive growth pattern. Focal necrosis is also observed; C: Section showing multiple rounds to oval glandular structures, representing moderately to well-differentiated ductal adenocarcinoma with micropapillary components. Tumor cells are unevenly distributed, and cellular density and heterogeneity vary across regions.
Figure 4 Immunohistochemical staining intensity of several molecular markers.
A: Immunohistochemical profiles of pancreatic ductal adenocarcinoma (PDAC) and pancreatic undifferentiated rhabdoid carcinoma (PURC). CK7 and SMARCB1 were positive in PDAC but negative in PURC, whereas CD10 and vimentin were positive in PURC but absent in PDAC. CK-pan and SMARCA4 were expressed in both PDAC and PURC. Ki-67 labeling was higher in PURC compared to PDAC; B: Fluorescence in situ hybridization analysis showing loss of SMARCB1 in a subset of tumor cells, consistent with rhabdoid carcinoma phenotype. Red probes denote SMARCB1, and green probes denote EWSR1. PDAC: Pancreatic ductal adenocarcinoma; PURC: Pancreatic undifferentiated rhabdoid carcinoma; H&E: Hematoxylin and eosin.
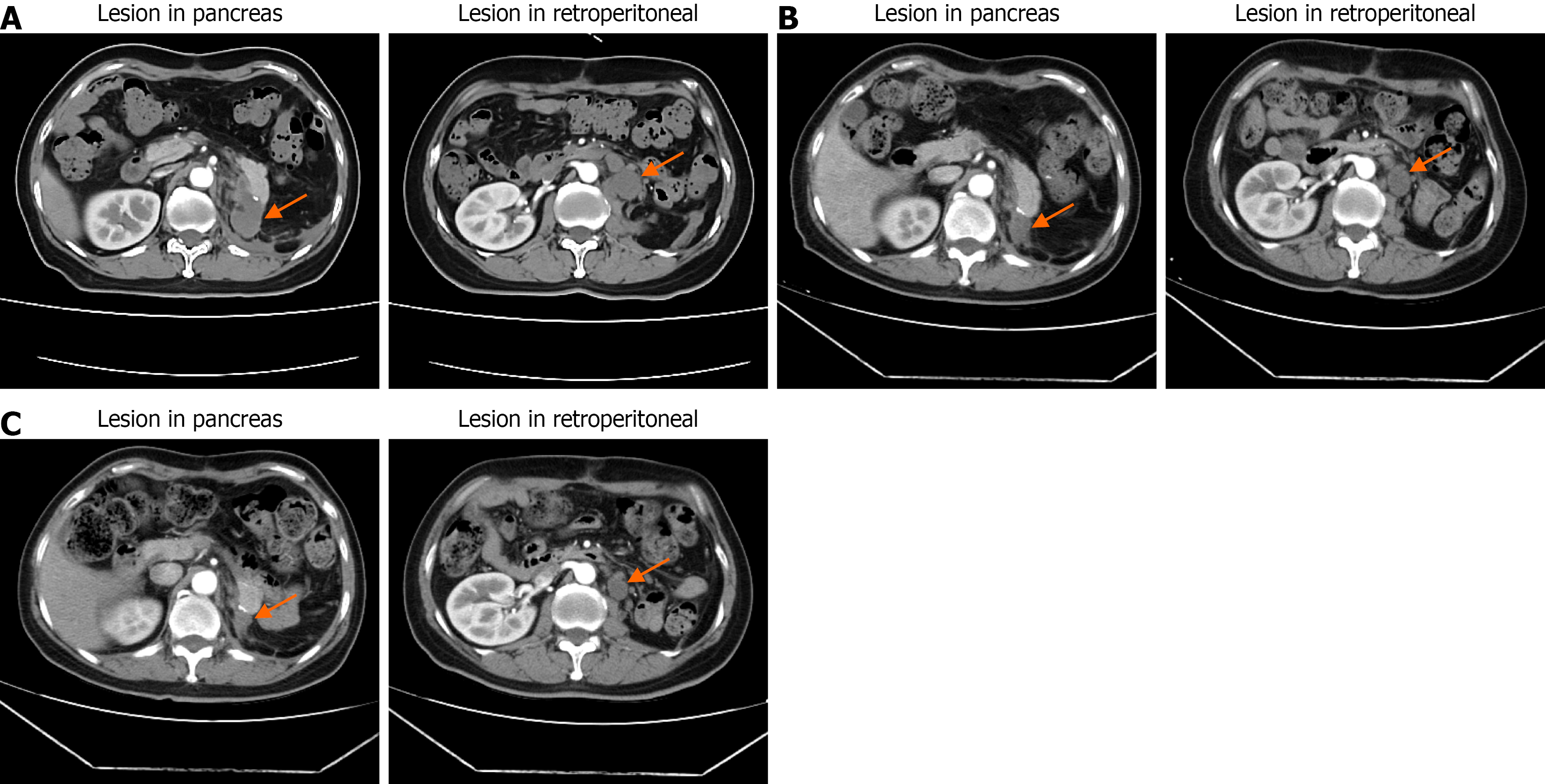
Figure 5 Representative computed tomography scans after surgery and during neoadjuvant therapy.
A: A recurrent mass at the pancreatic remnant and a retroperitoneal metastatic lesion after surgery; B and C: After neoadjuvant and targeted therapy, both the local recurrence and metastatic foci were reduced in size compared with pre-treatment imaging.
- Citation: Yao WQ, Ma XY, Wang GH. Clinicopathologic features of SMARCB1/INI1-deficient pancreatic undifferentiated rhabdoid carcinoma: A case report and review of literature. World J Gastrointest Oncol 2026; 18(1): 114021
- URL: https://www.wjgnet.com/1948-5204/full/v18/i1/114021.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i1.114021