©The Author(s) 2026.
World J Gastrointest Oncol. Jan 15, 2026; 18(1): 113816
Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.113816
Published online Jan 15, 2026. doi: 10.4251/wjgo.v18.i1.113816
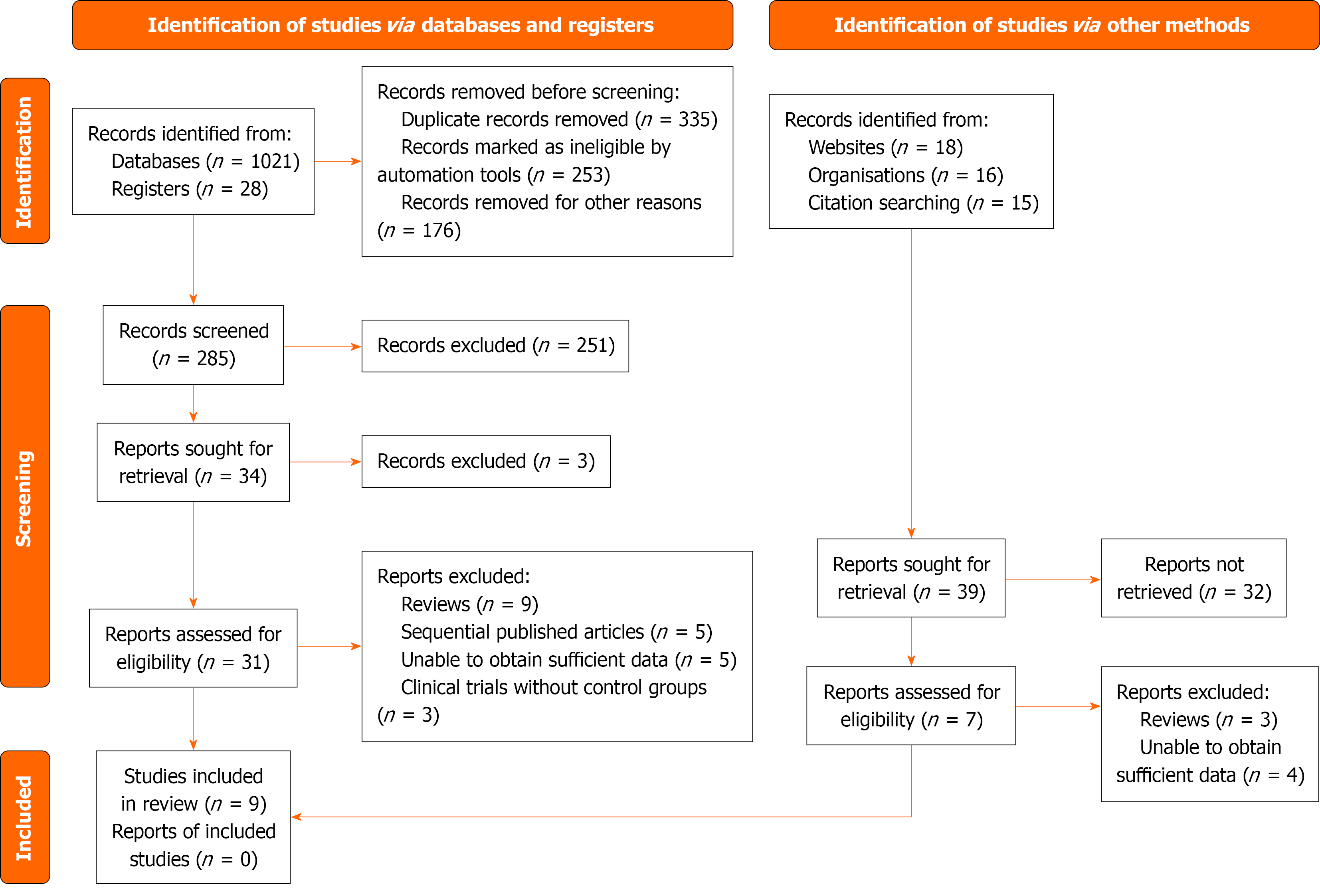
Figure 1 PRISMA flow diagram of study selection process.
This flowchart outlines the selection of studies included in the meta-analysis. A total of 1088 records were identified from databases and other sources. After removing duplicates and ineligible records, 9 studies met the inclusion criteria and were included in the final analysis.
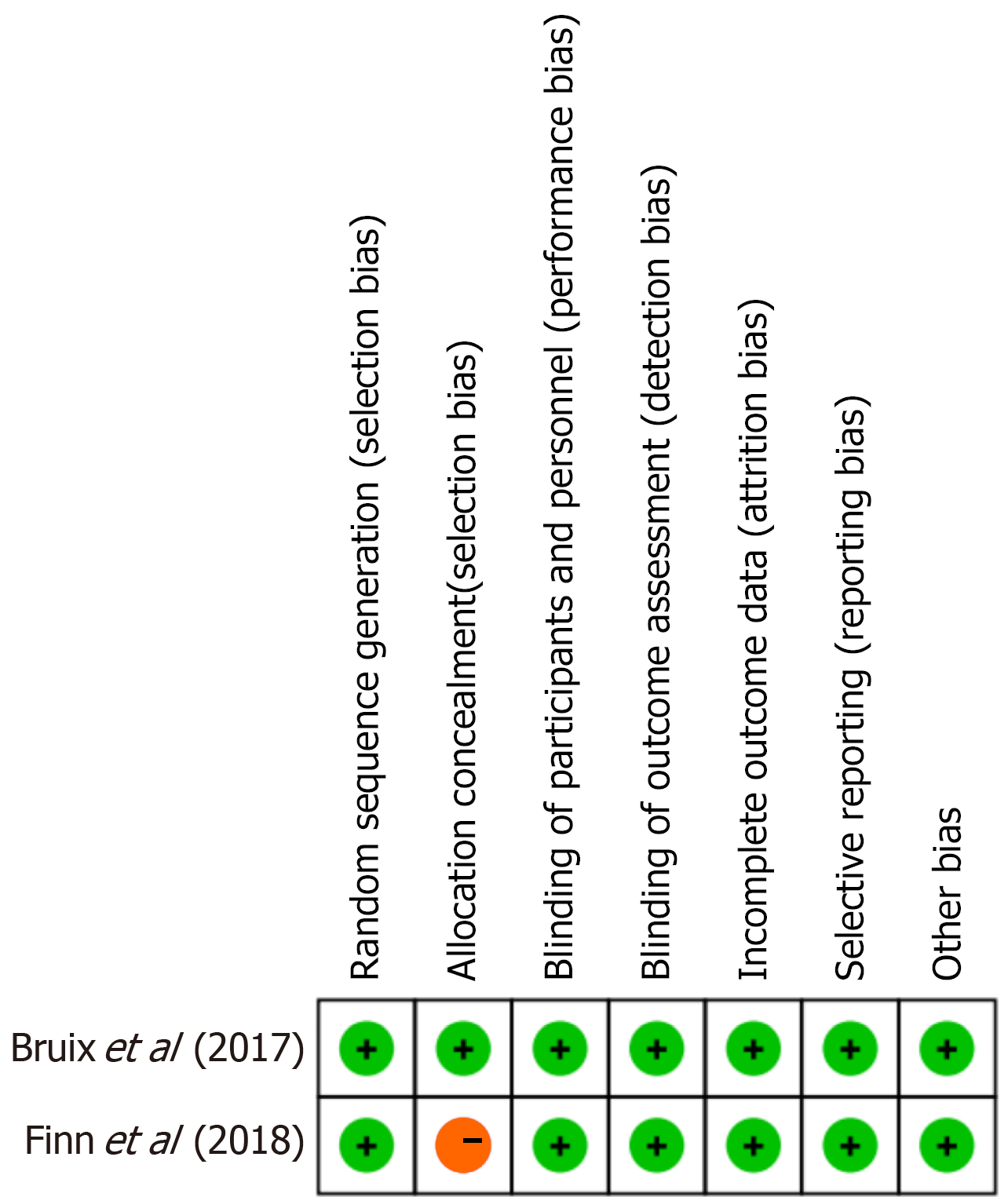
Figure 2 Risk of bias assessment for randomized controlled trials using the Cochrane RoB 2.
0 tool. The figure presents the domain-specific and overall risk of bias judgments for the two included randomized controlled trials. One trial exhibited high risk in allocation concealment, while both demonstrated low risk in other domains such as blinding, outcome measurement, and attrition.
Figure 3 Overall survival comparison between regorafenib and control treatments and sensitivity analysis of the pooled effect.
A: Forest plot of overall survival (OS) comparing regorafenib vs control therapies in patients with advanced hepatocellular carcinoma. A random-effects model was used due to moderate heterogeneity (I2 = 63.2%). Regorafenib was associated with a statistically significant improvement in OS (weighted mean difference = 2.54 months; 95% confidence interval: 0.26-4.81; P < 0.05); B: Sensitivity analysis of pooled OS results by sequential study exclusion. The analysis demonstrates the robustness of the OS effect estimate, showing consistent results with the omission of each individual study. CI: Confidence interval.
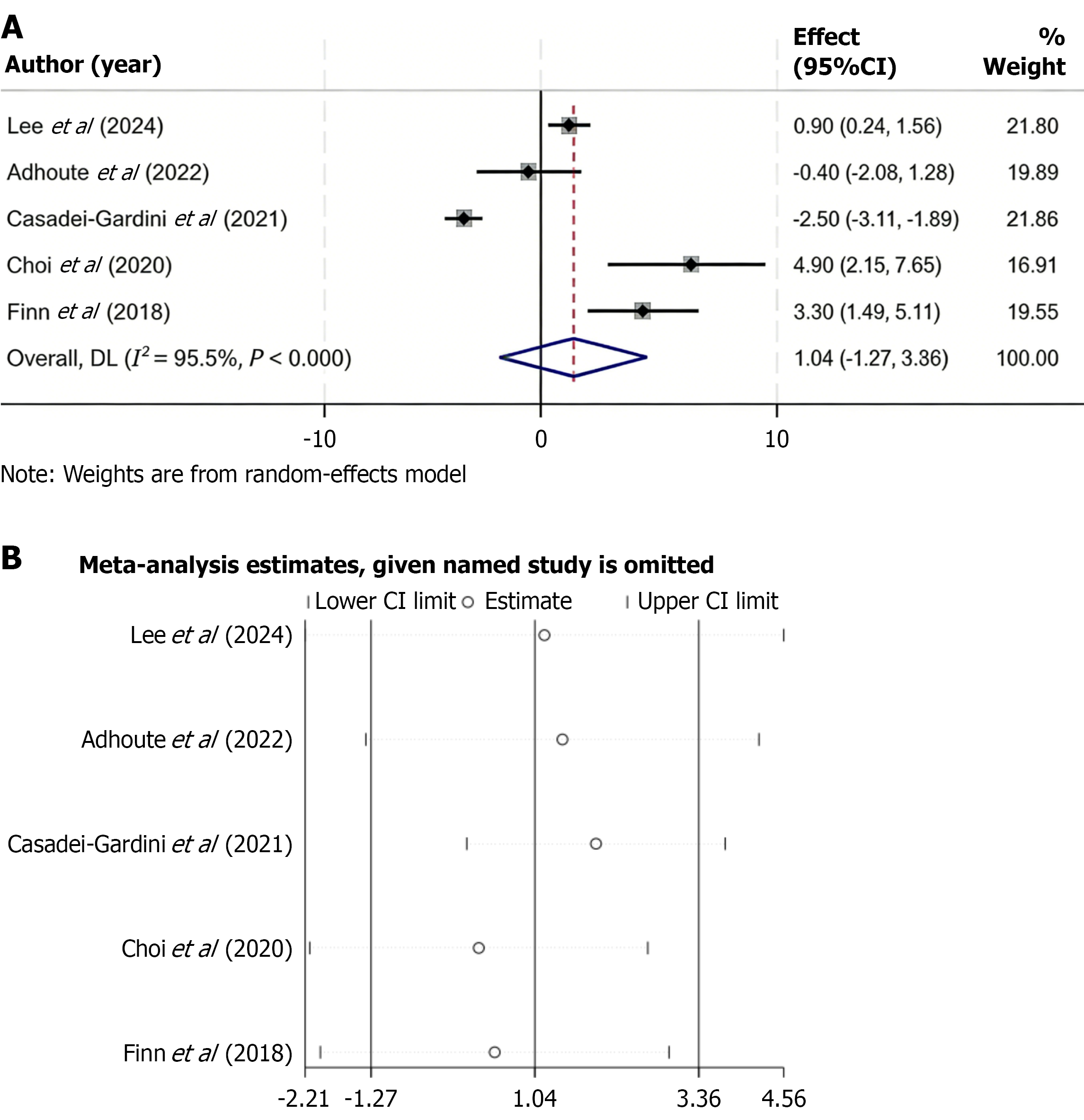
Figure 4 Progression-free survival comparison between regorafenib and control treatments and sensitivity analysis of the pooled estimate.
A: Forest plot of progression-free survival (PFS) comparing regorafenib vs control therapies. A random-effects model was applied due to substantial heterogeneity (I2 = 95.5%). No statistically significant difference in PFS was observed between groups (weighted mean difference = 1.04; 95% confidence interval: -1.27 to 3.36; P > 0.05); B: Sensitivity analysis of pooled PFS results by sequential study exclusion. The results remained stable across iterations, confirming that no individual study had a major impact on the overall pooled effect. CI: Confidence interval.
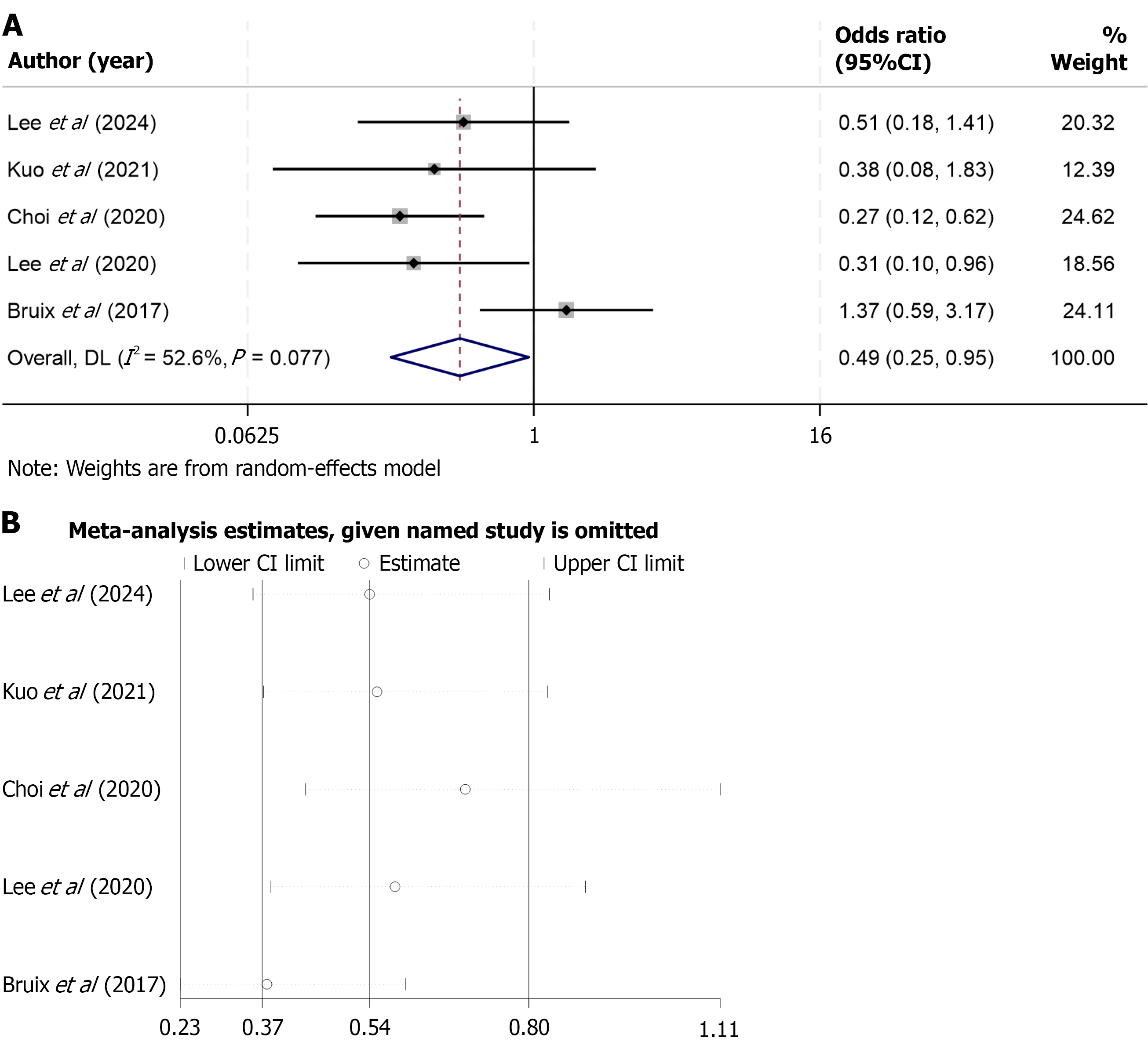
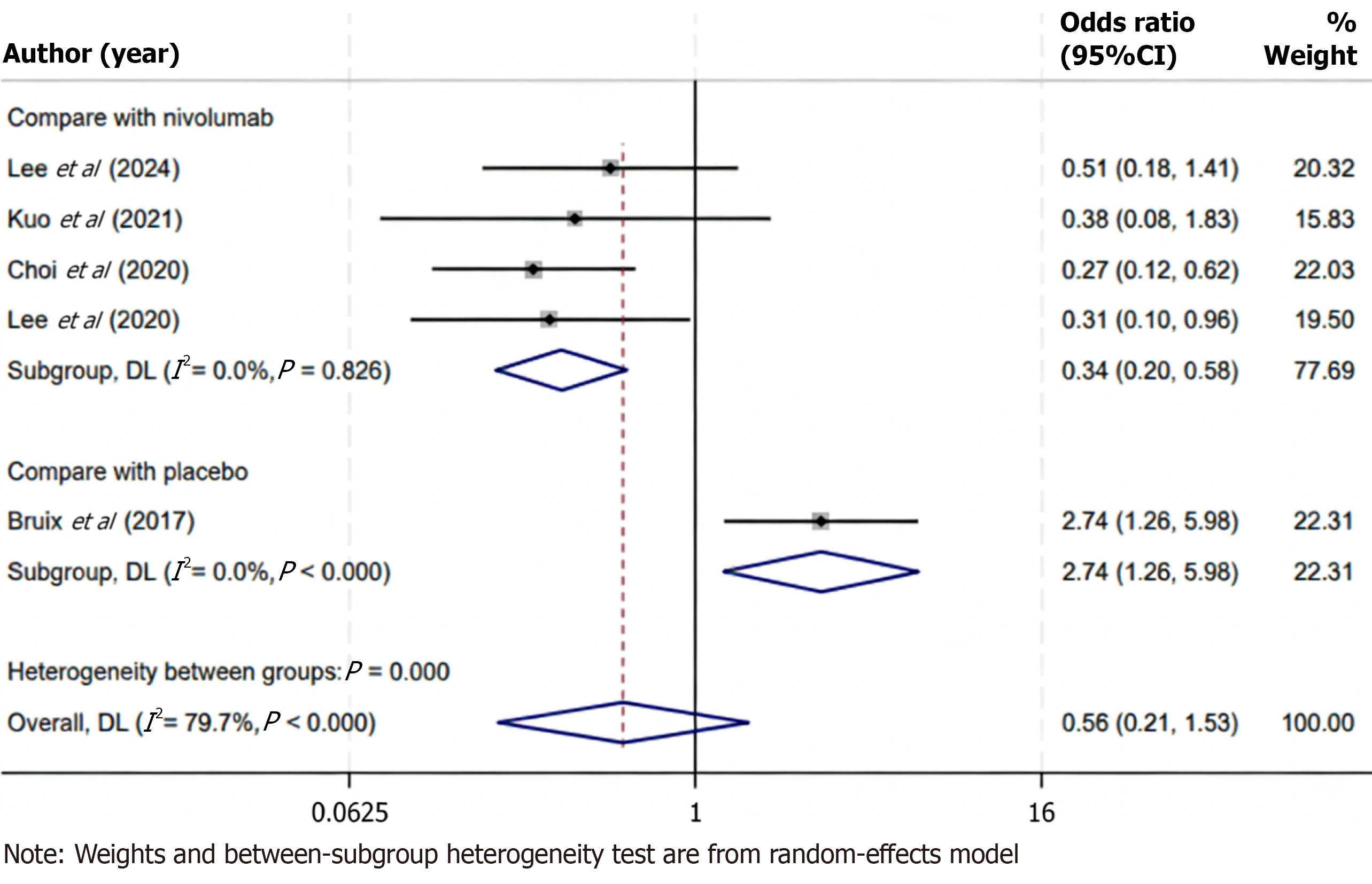
Figure 5 Forest plot of objective response rate for regorafenib vs control treatments, including subgroup analysis with nivolumab.
The overall analysis showed no significant difference in objective response rate (odds ratio = 0.56; 95% confidence interval: 0.21-1.53; P > 0.05; I2 = 79.7%). However, subgroup analysis demonstrated a significantly lower objective response rate with regorafenib compared to nivolumab (odds ratio = 0.34; 95% confidence interval: 0.20-0.58; P < 0.05; I2 = 0.0%). Subgroup analyses were conducted based on the type of comparator: Regorafenib vs nivolumab (n = 4 studies); regorafenib vs placebo (n = 1 study). The square markers in the forest plot have been scaled according to the inverse-variance weight of each study, where larger squares indicate studies with greater statistical weight. CI: Confidence interval.
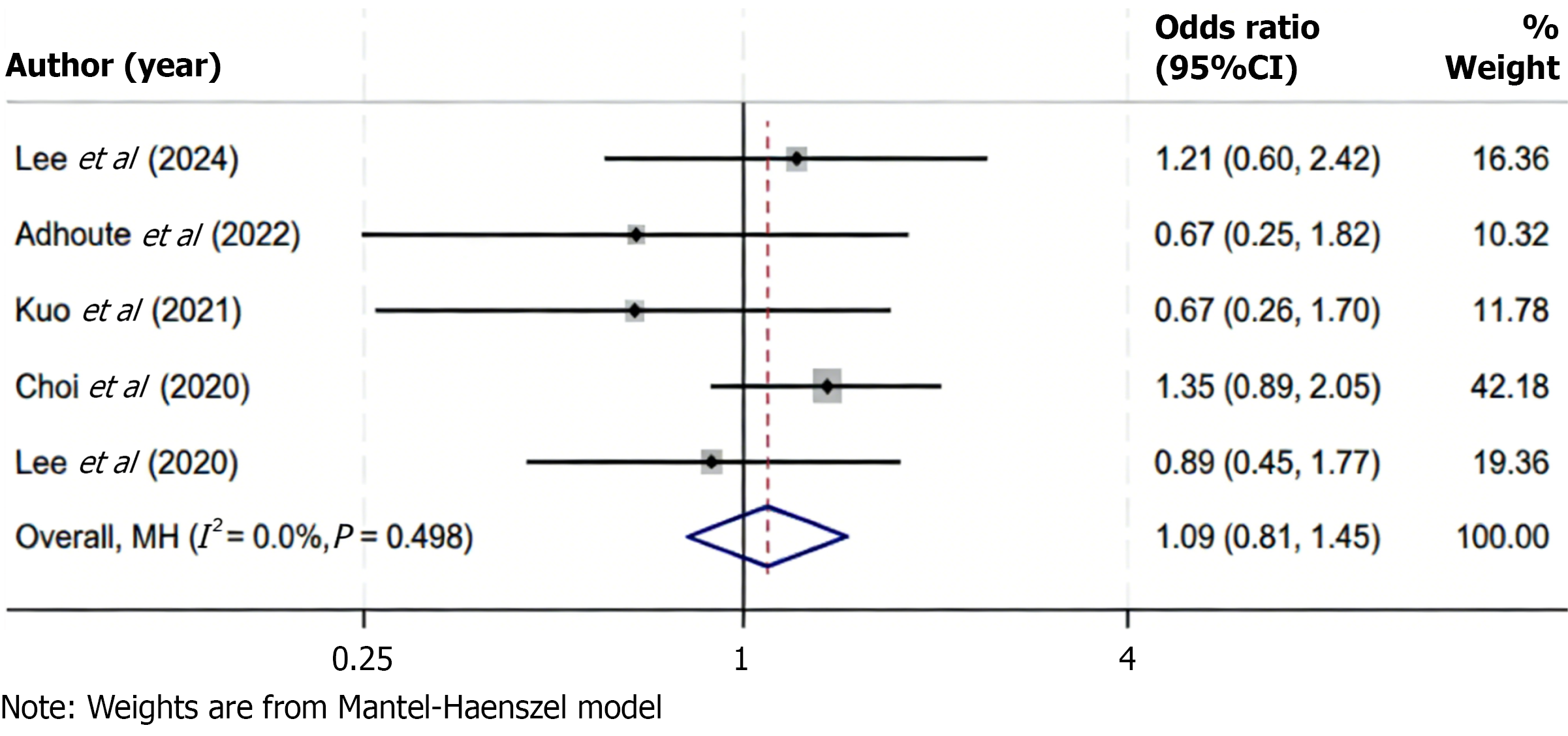
Figure 6 Forest plot of disease control rate comparing regorafenib and control therapies.
Using a fixed-effects model (I2 = 0.0%), the pooled analysis indicated no statistically significant difference in disease control rate between treatment groups (odds ratio = 1.09; 95% confidence interval: 0.81-1.45; P > 0.05), suggesting comparable disease stabilization efficacy. CI: Confidence interval.
- Citation: Cheng Z, Yue AM. Efficacy of regorafenib in the treatment of advanced hepatocellular carcinoma: A systematic review and meta-analysis. World J Gastrointest Oncol 2026; 18(1): 113816
- URL: https://www.wjgnet.com/1948-5204/full/v18/i1/113816.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v18.i1.113816