©The Author(s) 2025.
World J Gastrointest Oncol. Nov 15, 2025; 17(11): 111814
Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.111814
Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.111814
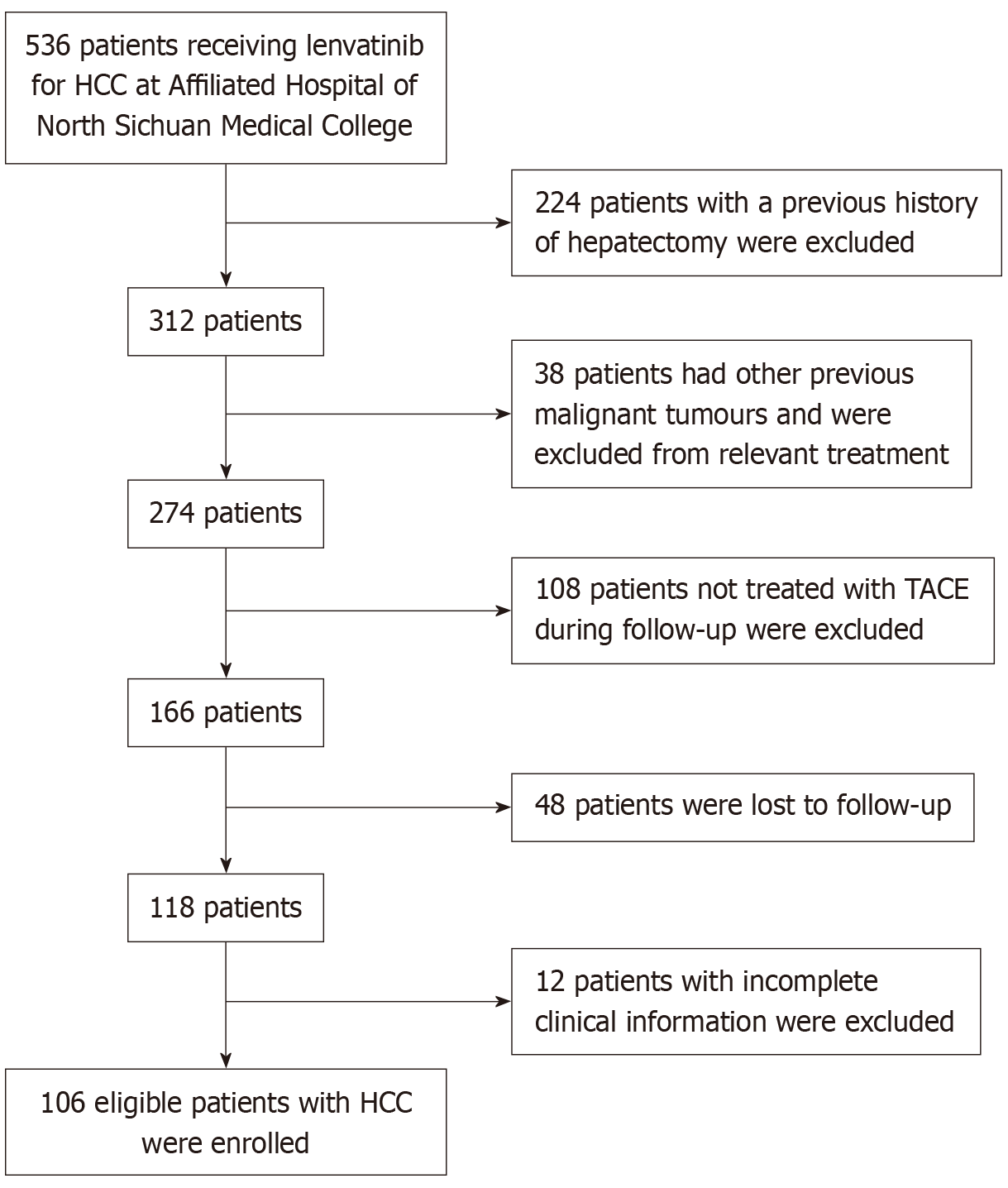
Figure 1 Validation cohort patient screening flowchart.
HCC: Hepatocellular carcinoma; TACE: Transcatheter arterial chemoembolization.
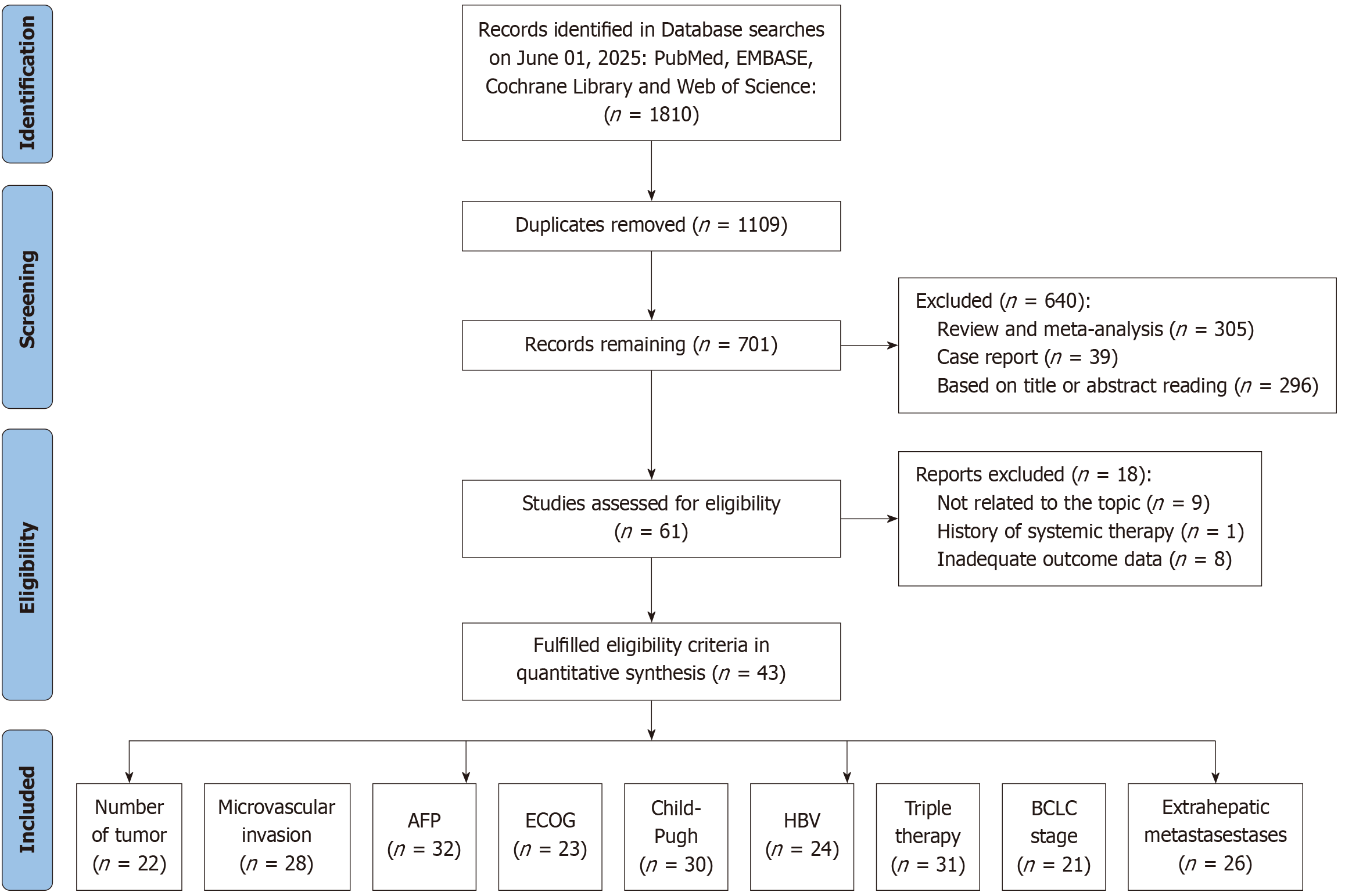
Figure 2 Literature search and screening process for prognostic risk factors associated with receiving transcatheter arterial chemoe
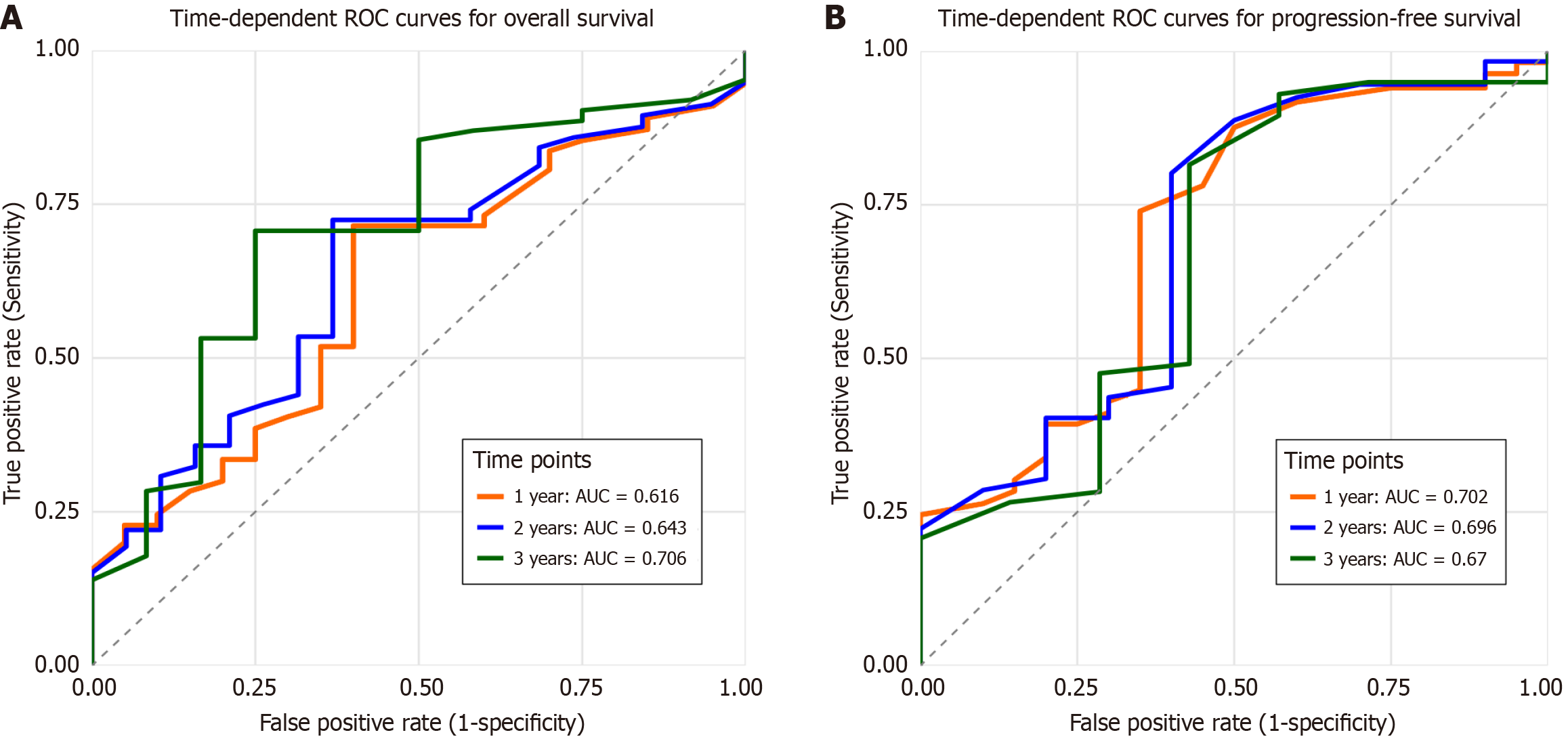
Figure 3 Receiver operating characteristic curves for prediction models in the validation cohort.
A: Overall survival; B: Progression-free survival. ROC: Receiver operating characteristic; AUC: Areas under the curve.
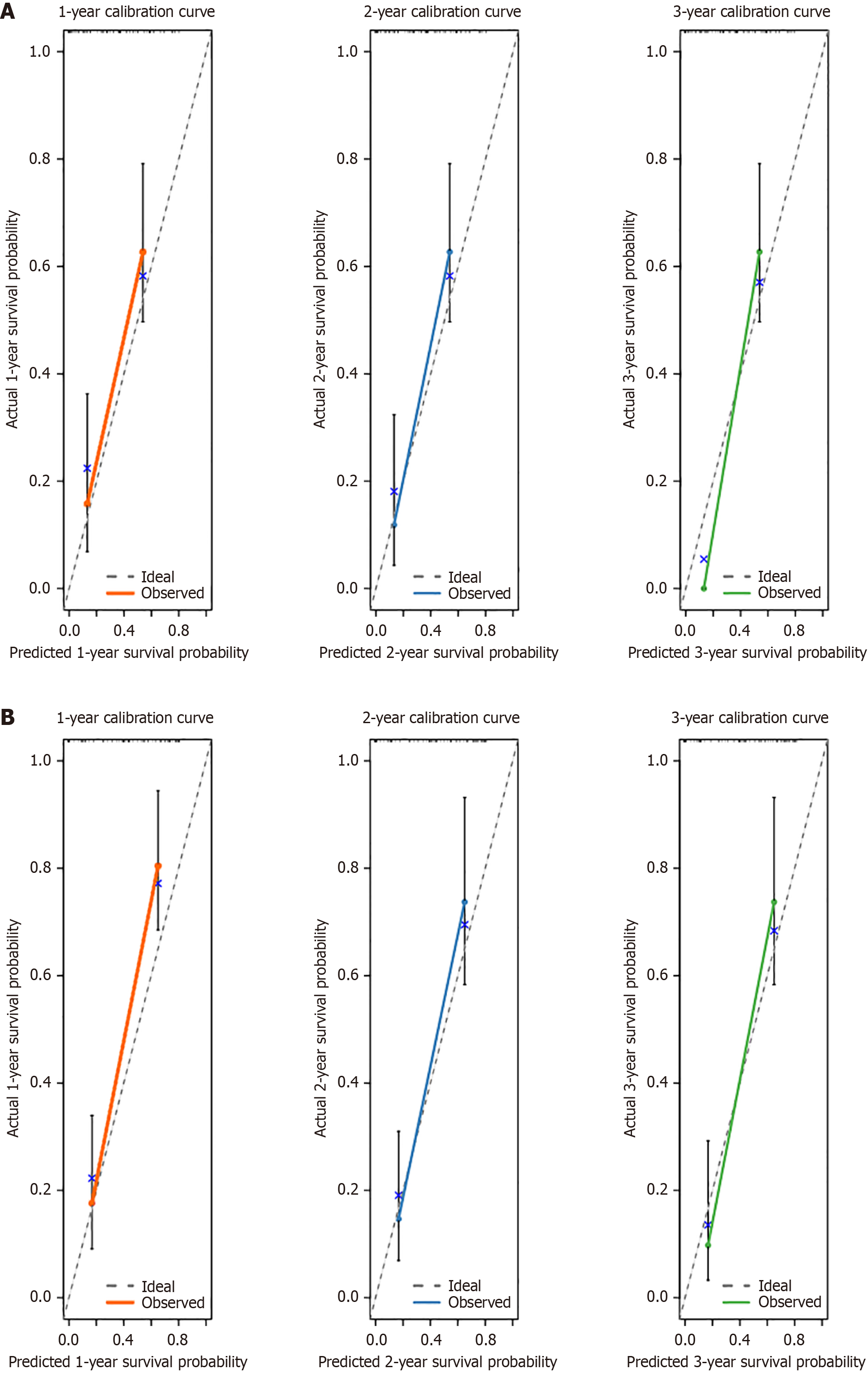
Figure 4 Prediction models for in the validation cohort, with 1-year, 2-year, and 3-year calibration curves.
A: Overall survival; B: Progression-free survival.
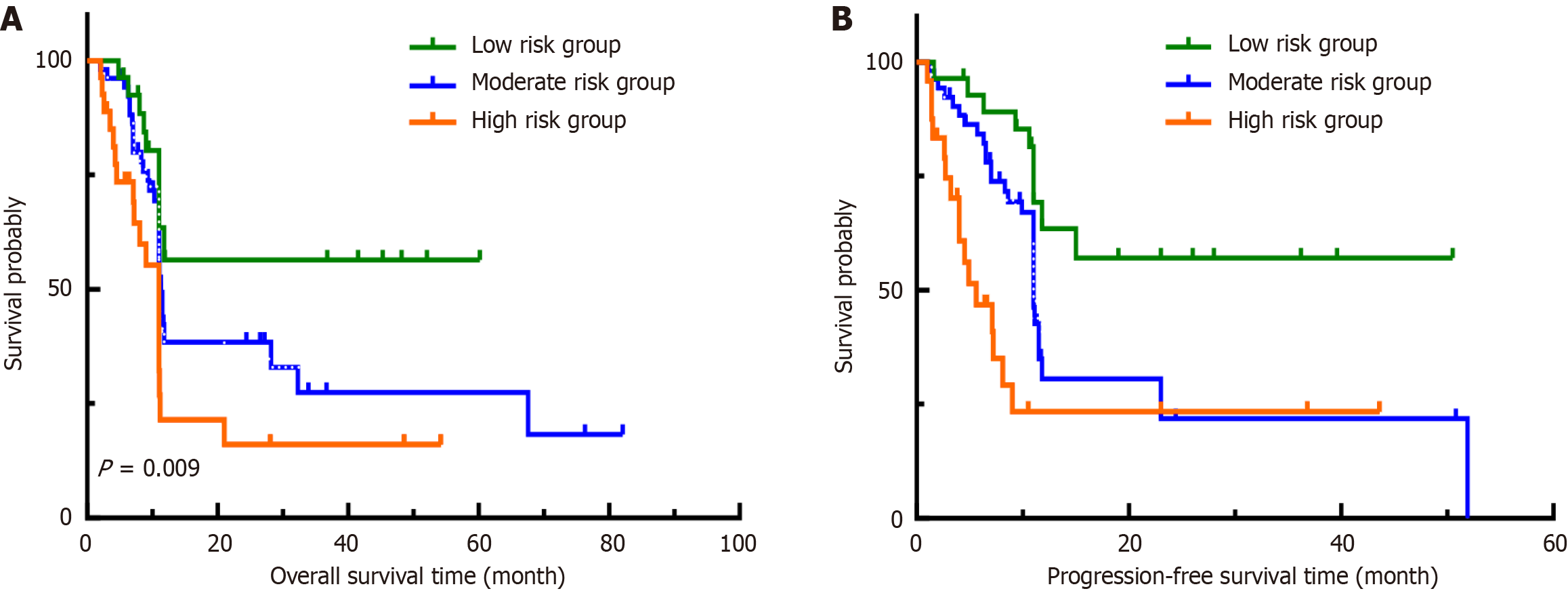
Figure 5 Kaplan-Meier survival curves for prediction models for the three risk groups in the validation group.
A: Overall survival; B: Pro
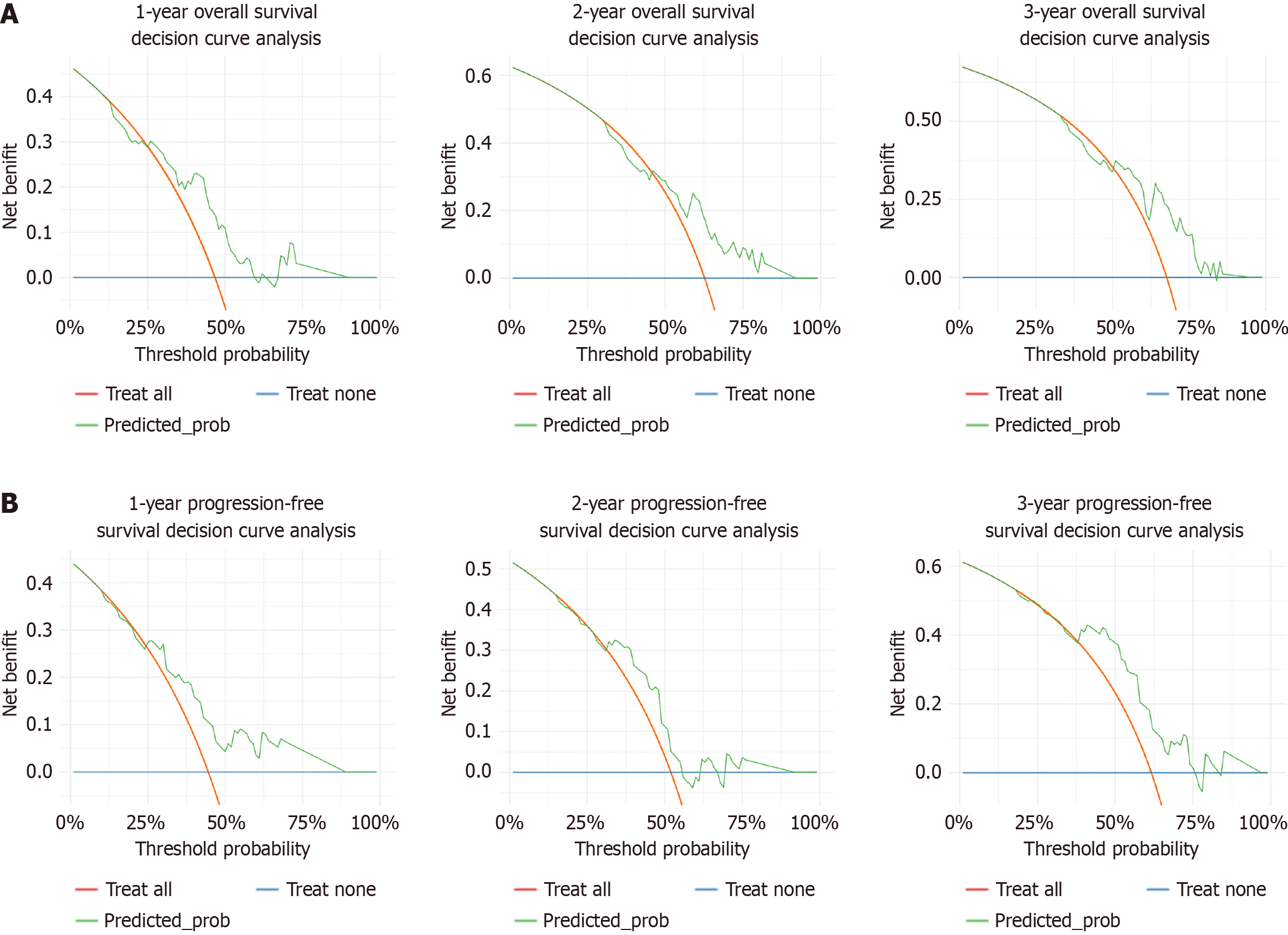
Figure 6 Prediction models for in the validation cohort, with 1-year, 2-year, and 3-year decision curves.
A: Overall survival; B: Progression-free survival.
- Citation: Yu JH, Yu J, Yu JX, Yang LF, Yan D, Liu Y, Xian JR, Yi PS. Personalized prognosis in unresectable hepatocellular carcinoma: Development and validation of a model for transcatheter arterial chemoembolization plus lenvatinib. World J Gastrointest Oncol 2025; 17(11): 111814
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/111814.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.111814