©The Author(s) 2025.
World J Gastrointest Oncol. Nov 15, 2025; 17(11): 110468
Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.110468
Published online Nov 15, 2025. doi: 10.4251/wjgo.v17.i11.110468
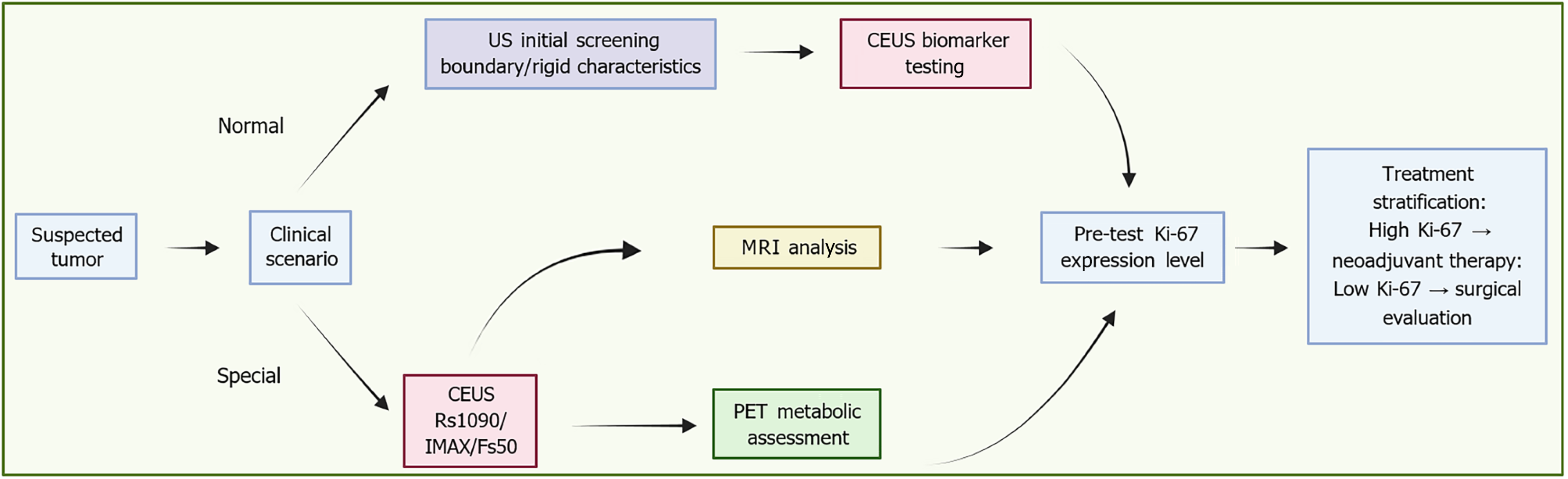
Figure 1 Imaging modalities and their contribution to Ki-67 assessment.
An integrated clinical pathway for tumor management involves initial screening via ultrasound to assess boundary and rigid features, followed by contrast-enhanced ultrasound biomarker testing. This testing includes quantitative parameters such as rise slope 10%-90%, maximum intensity, and falling slope 50%. Contrast-enhanced ultrasound testing serves as a trigger for multiparametric magnetic resonance imaging, which is used for apparent diffusion coefficient and Ki-67 assessment. Treatment is stratified by Ki-67 expression: High expression mandates neoadjuvant therapy, while low expression proceeds directly to surgical evaluation. The employment of advanced quantification techniques and positron emission tomography metabolic assessment further reinforces the precision oncology decision-making process throughout the pathway. US: Ultrasound; CEUS: Contrast-enhanced ultrasound; Rs1090: Rise slope 10%-90%; IMAX: Maximum intensity; Fs50: Falling slope 50%; MRI: Magnetic resonance imaging; PET: Positron emission tomography.
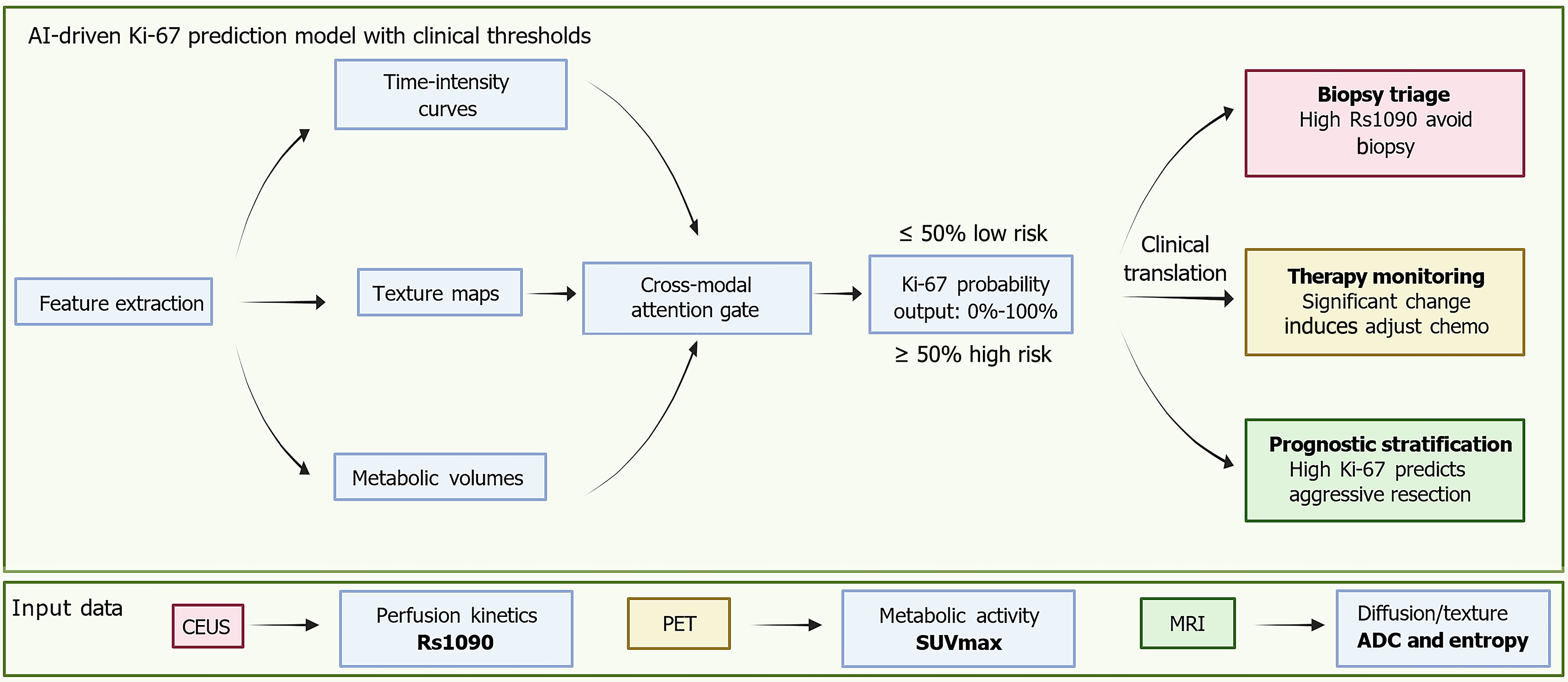
Figure 2 Unified predictive model for Ki-67 using multimodal imaging.
This schematic presents an artificial intelligence fusion model that integrates multimodal imaging data. These include contrast-enhanced ultrasound derived perfusion kinetics, specifically rise slope 10%-90%, positron emission tomography based metabolic activity represented by the maximum standardized uptake value, and magnetic resonance imaging derived diffusion and texture metrics such as apparent diffusion coefficient and entropy. The model extracts key imaging features including time-intensity curves, metabolic volumes, and texture maps, which are then synthesized using a cross-modal attention gate to generate a probabilistic Ki-67 output ranging from 0%-100%. Clinically, this artificial intelligence-derived output supports three major decisions. First, biopsy triage, where a high rise slope 10%-90% value may help avoid invasive procedures in patients at risk for biopsy-related complications. Second, therapy monitoring, where significant changes in perfusion parameters or rising maximum standardized uptake values during neoadjuvant treatment can prompt early adjustments to chemotherapy. Third, prognostic stratification, where high-probability Ki-67 spatial predictions assist in identifying aggressive tumor regions and support surgical margin planning. AI: Artificial intelligence; Rs1090: Rise slope 10%-90%; CEUS: Contrast-enhanced ultrasound; PET: Positron emission tomography; SUVmax: Maximum standardized uptake value; MRI: Magnetic resonance imaging; ADC: Apparent diffusion coefficient.
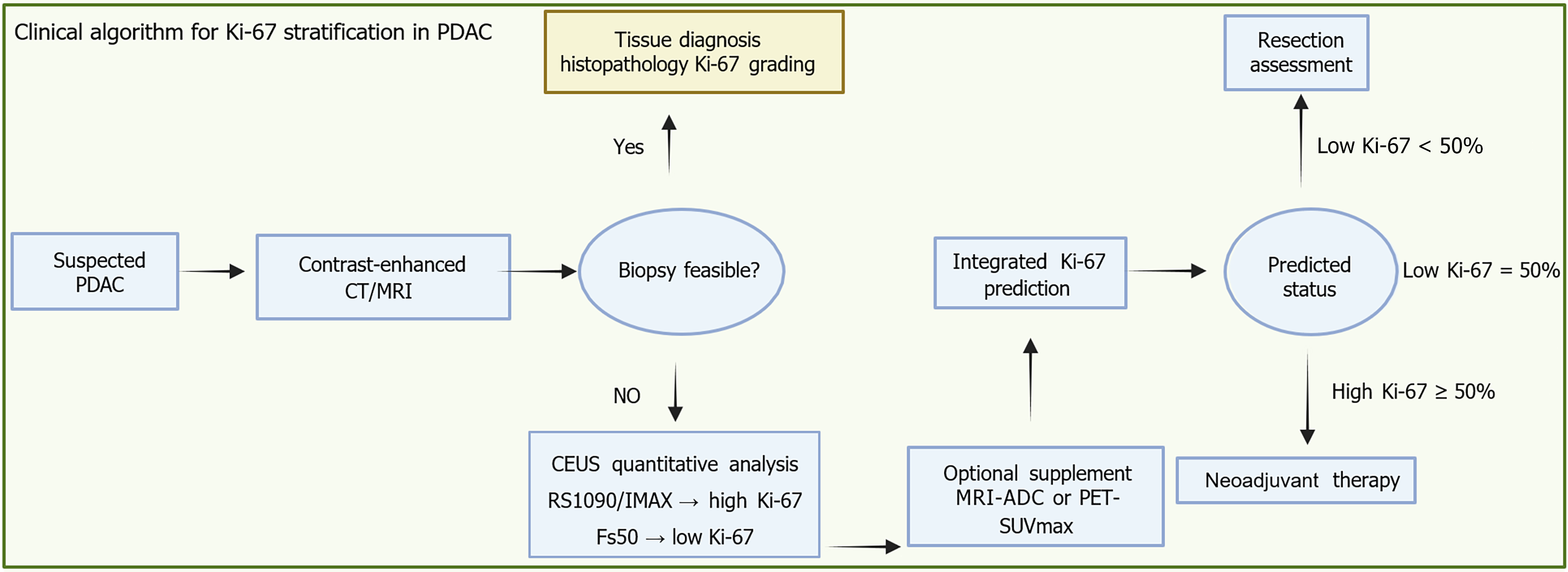
Figure 3 Integrated imaging biomarker pathway for Ki-67 prediction and treatment allocation in biopsy-contraindicated pancreatic ductal adenocarcinoma.
This clinical workflow outlines a decision-making pathway for suspected pancreatic ductal adenocarcinoma, integrating imaging biomarkers to support personalized treatment planning. Following initial contrast-enhanced computed tomography or magnetic resonance imaging for anatomical staging, the feasibility of tissue biopsy is assessed. If biopsy is possible, histopathological Ki-67 grading provides a direct evaluation of tumor proliferative activity. When biopsy is not feasible - such as in patients on anticoagulation therapy or with poor clinical condition - noninvasive contrast-enhanced ultrasound becomes essential. Quan
- Citation: Wu YX, Tian R, Li XW, Guo JY, Tang JF, Zhou CF. Emerging non-invasive imaging biomarkers of Ki-67 in pancreatic cancer: Toward predictive precision oncology. World J Gastrointest Oncol 2025; 17(11): 110468
- URL: https://www.wjgnet.com/1948-5204/full/v17/i11/110468.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i11.110468