©The Author(s) 2025.
World J Gastrointest Oncol. Oct 15, 2025; 17(10): 111339
Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.111339
Published online Oct 15, 2025. doi: 10.4251/wjgo.v17.i10.111339
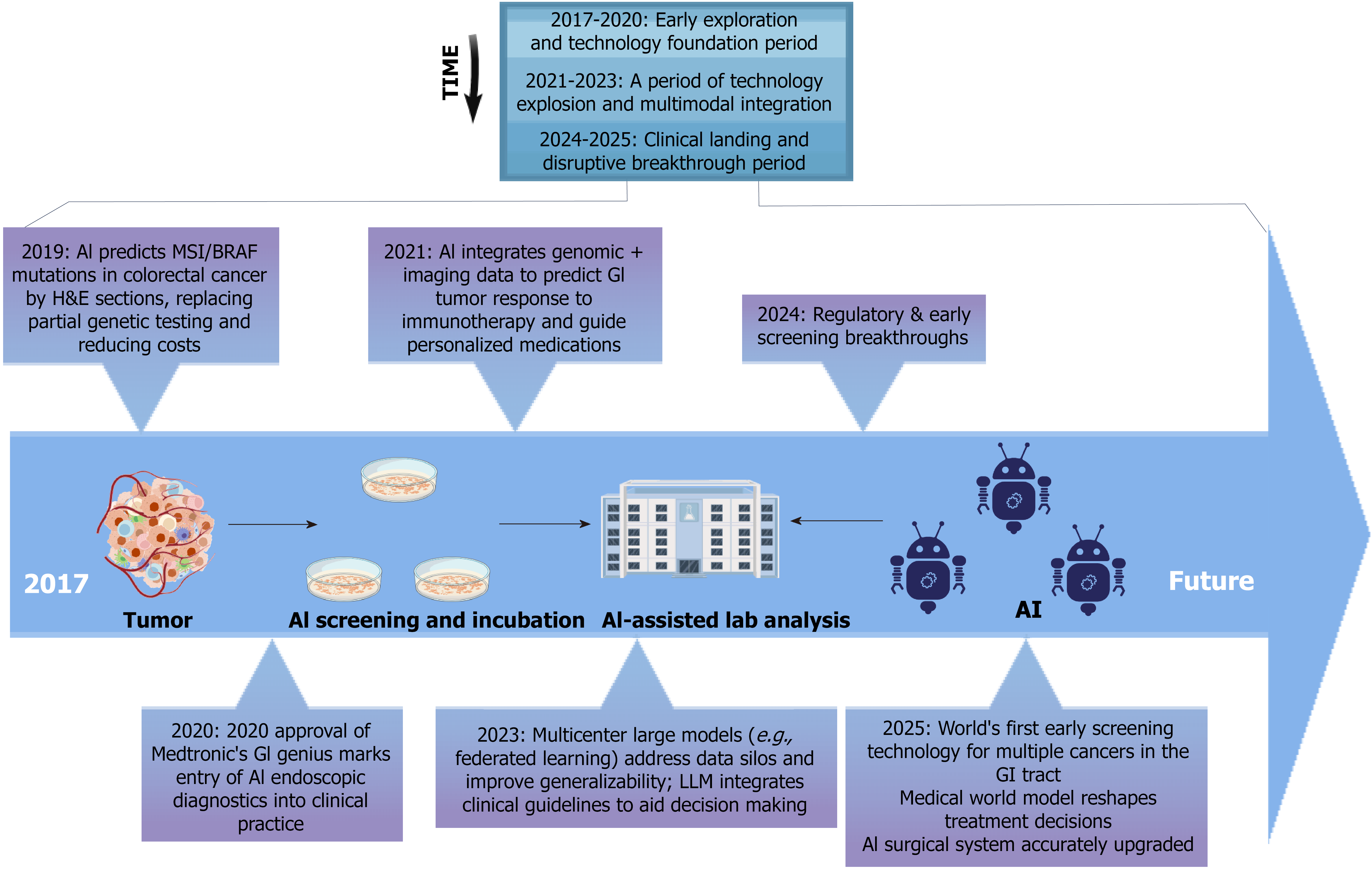
Figure 1 Milestones in artificial intelligence-driven clinical research for gastrointestinal cancers (2017-2025).
This timeline highlights major milestones in applying artificial intelligence (AI) to gastrointestinal cancer research and care from 2017 to 2025. Early efforts centered on predictive modeling using pathology and genomics. From 2020, AI tools entered clinical practice, including AI-assisted endoscopy and federated data integration. Recent advances (2023-2025) feature multimodal learning, guideline-informed language models, and early screening technologies, marking progress toward clinical adoption and regulatory readiness. AI: Artificial intelligence; GI: Gastrointestinal; LLM: Large language model.
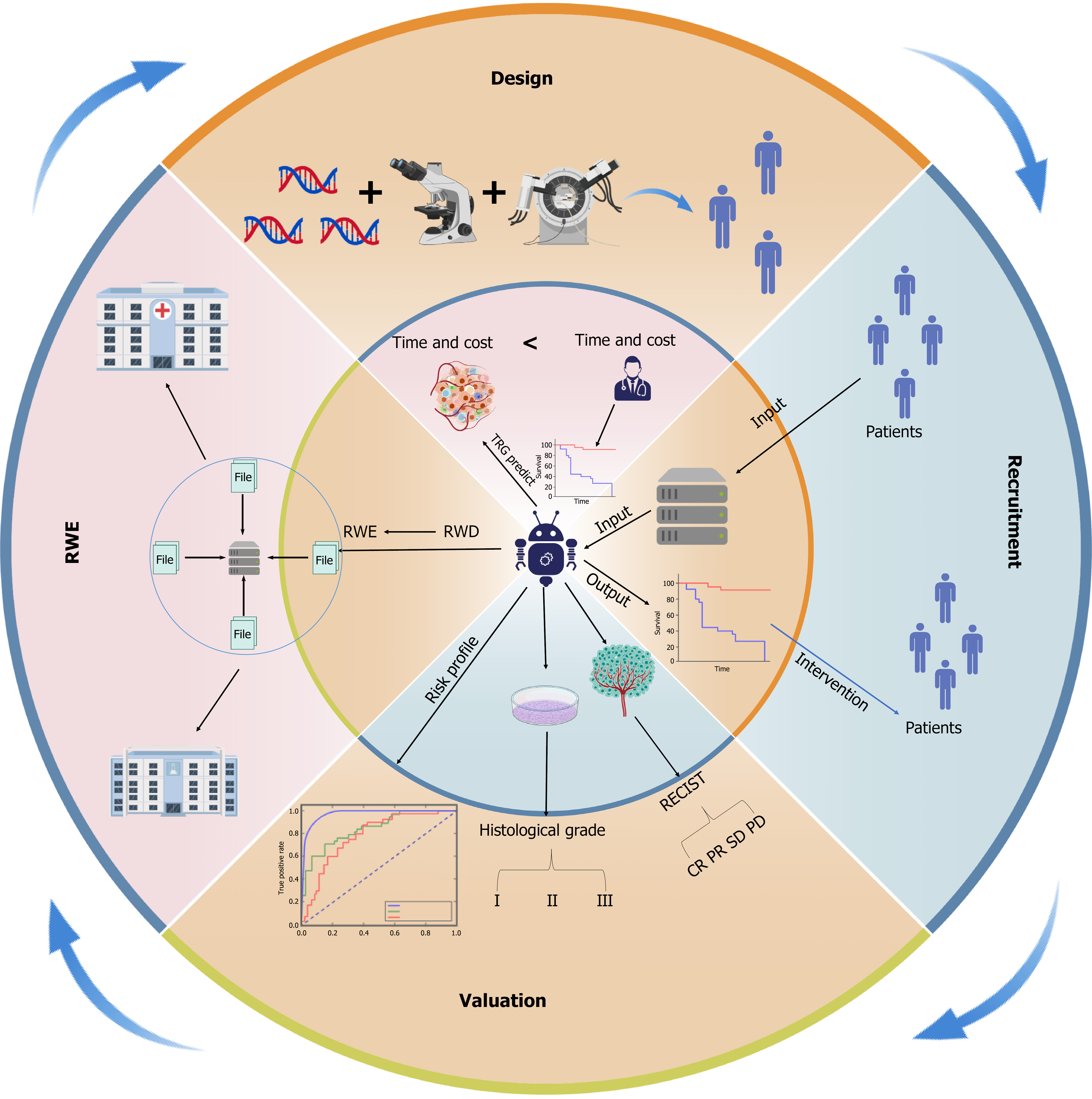
Figure 2 Artificial intelligence-powered transformation across the clinical trial continuum in gastrointestinal oncology.
Artificial intelligence (AI) is being integrated throughout the entire lifecycle of clinical trials in gastrointestinal cancer, from study design and patient recruitment to efficacy valuation and real-world evidence (RWE) generation. In the design phase, AI accelerates biomarker discovery and trial simulation using multimodal data, reducing both time and cost. During recruitment, AI-driven tools enhance eligibility matching, predict dropout risk, and optimize inclusion criteria. In the valuation phase, AI enables dynamic endpoint modeling, response assessment (e.g., Response Evaluation Criteria in Solid Tumours), and histopathological grading. Finally, real-world data can be converted into actionable RWE through AI-based natural language processing and federated learning, supporting post-trial validation and continuous learning cycles. This circular framework underscores the bidirectional flow between prospective trials and RWE in precision oncology. RWE: Real-world evidence; RWD: Real-world data; RECIST: Response Evaluation Criteria in Solid Tumours; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
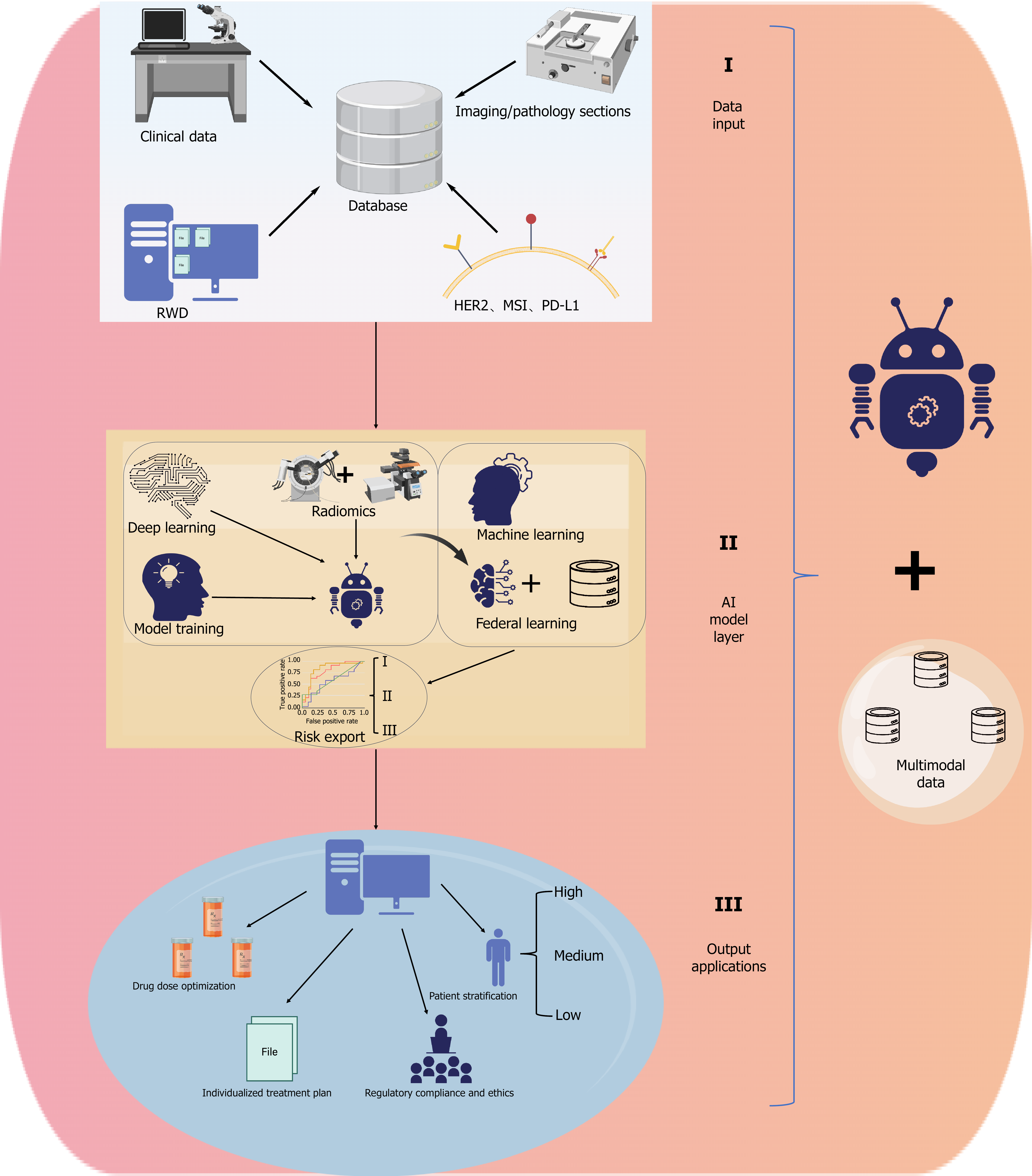
Figure 3 Multimodal data integration and artificial intelligence-powered clinical application framework in gastrointestinal oncology.
This diagram presents a structured framework for the integration of artificial intelligence (AI) in gastrointestinal cancer management. I: Multimodal data input includes clinical data, medical imaging, pathology slides, molecular biomarkers (e.g., HER2, MSI, PD-L1), and real-world data from electronic health records and wearable devices; II: The AI model layer incorporates deep learning, radiomics, machine learning, and federated learning to construct robust and privacy-preserving predictive models; III: Output applications span patient stratification, drug dose optimization, individualized treatment planning, and regulatory compliance—illustrating the translational path from data acquisition to clinical deployment.
- Citation: Wang Z, Zhang RY, Ji C, Zhang JY, Yue BT, Wang F. Revolutionizing gastrointestinal cancer research with artificial intelligence: From precision patient stratification to real-world evidence. World J Gastrointest Oncol 2025; 17(10): 111339
- URL: https://www.wjgnet.com/1948-5204/full/v17/i10/111339.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v17.i10.111339