©The Author(s) 2024.
World J Gastrointest Oncol. Dec 15, 2024; 16(12): 4685-4699
Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4685
Published online Dec 15, 2024. doi: 10.4251/wjgo.v16.i12.4685
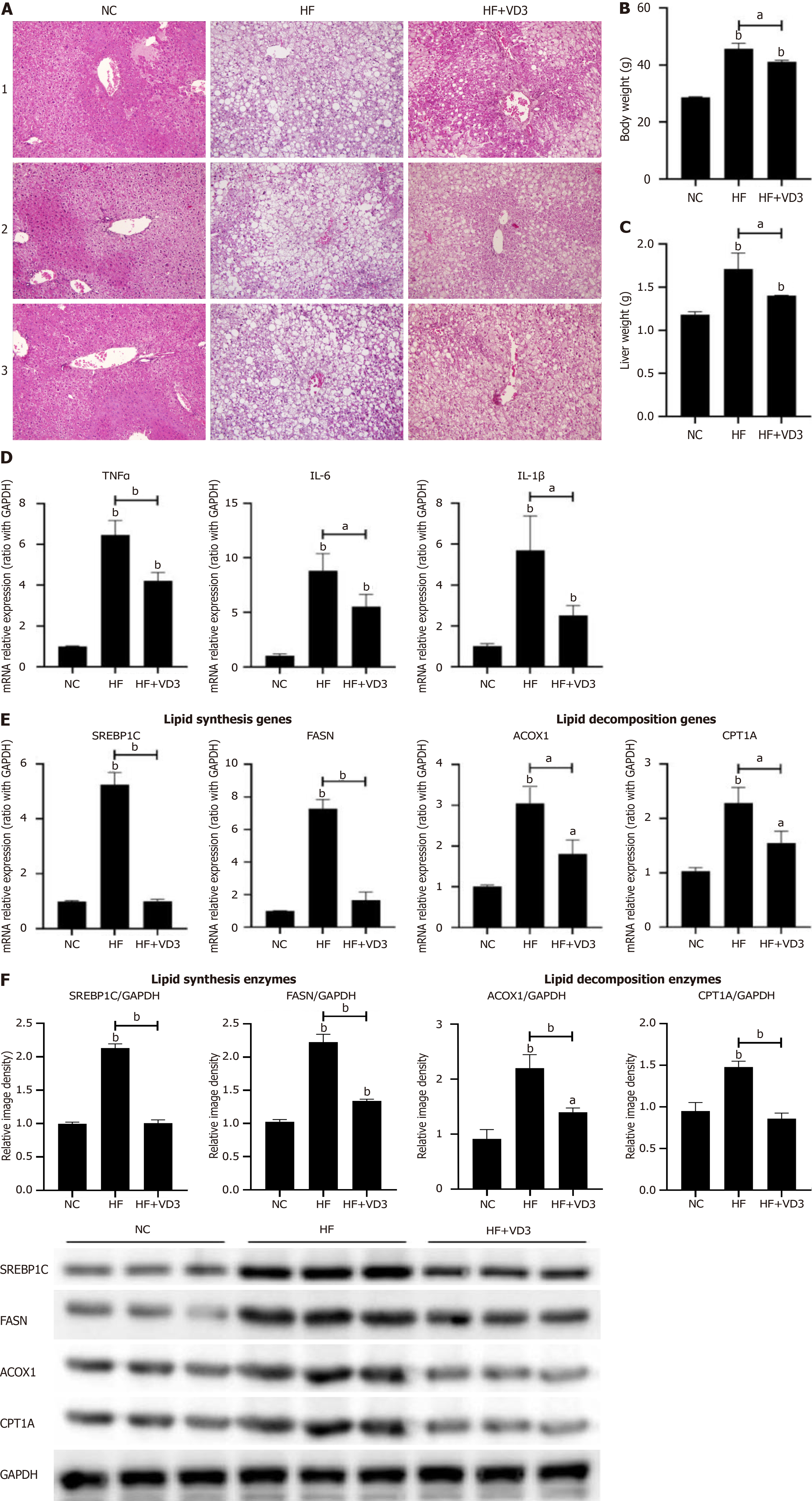
Figure 1 Supplementation with 1,25-dihydroxy-vitamin D improves hepatic steatosis and lipid metabolism in non-alcoholic fatty liver disease.
Wild-type C57BL/6 mice were fed normal control diet or high-fat (HF) diet for 16 weeks, or HF diet plus 1,25-dihydroxy-vitamin D (20 µg/kg) by oral gavage every alternate day for 16 weeks. Phosphate-buffered saline by oral gavage served as a control (n = 10/group). A: Hepatic steatosis determined by hematoxylin & eosin staining (200 ×); B: Body weight of mice; C: Liver weight of mice; D: Hepatic proinflammatory cytokines expression; E: Hepatic lipid metabolism genes mRNA expression; F: Hepatic lipid metabolism enzymes protein expression. Values are mean ± SEM, aP < 0.05, bP < 0.01, n = 10 animals per group; NC: Normal control; HF: High-fat; VD3: 1,25-dihydroxy-vitamin D; TNFα: Tumor necrosis factor α; IL: Interleukin; SREBP1C: Sterol-regulatory element binding protein 1C; FASN: Fatty acid synthetase; ACOX1: Acyl-CoA oxidase 1; CPT1A: Carnitine palmitoyltransferase 1A.
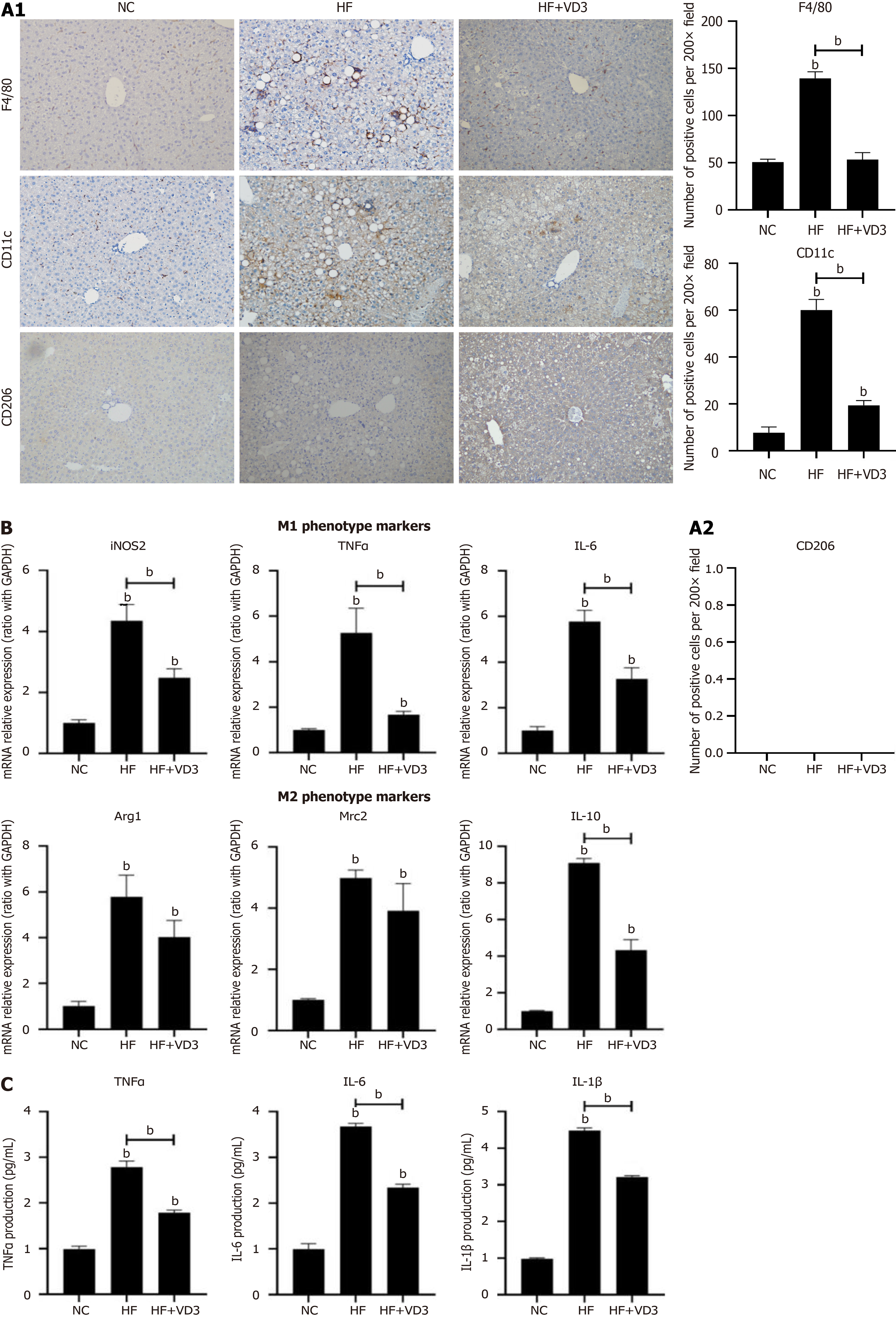
Figure 2 Supplementation with 1,25-dihydroxy-vitamin D decreases the proinflammatory M1 polarization of hepatic macrophages in non-alcoholic fatty liver disease.
Wild-type C57BL/6 mice were fed either normal control (NC) diet or high-fat (HF) diet for 16 weeks, or HF diet plus 1,25-dihydroxy-vitamin D [1,25(OH)2D3] (20 µg/kg) by oral gavage every alternate day for 16 weeks. Phosphate-buffered saline by oral gavage served as a control (n = 10/group). Hepatic macrophages were isolated from mice administered NC diet, HF diet alone or plus 1,25(OH)2D3. A: M1/M2 phenotype of hepatic macrophages determined by immunohistochemical staining (200 ×); B: M1/M2 gene marker expression on hepatic macrophages; C: Proinflammatory cytokine secretion from hepatic macrophages. Values are mean ± SEM, aP < 0.05, bP < 0.01, n = 10 animals per group; NC: Normal control; HF: High-fat; VD3: 1,25-dihydroxy-vitamin D; iNOS: Inducible NO synthase; TNFα: Tumor necrosis factor α; IL: Interleukin; Arg1 Arginine 1; Mrc2: Macrophage mannose receptor 2.
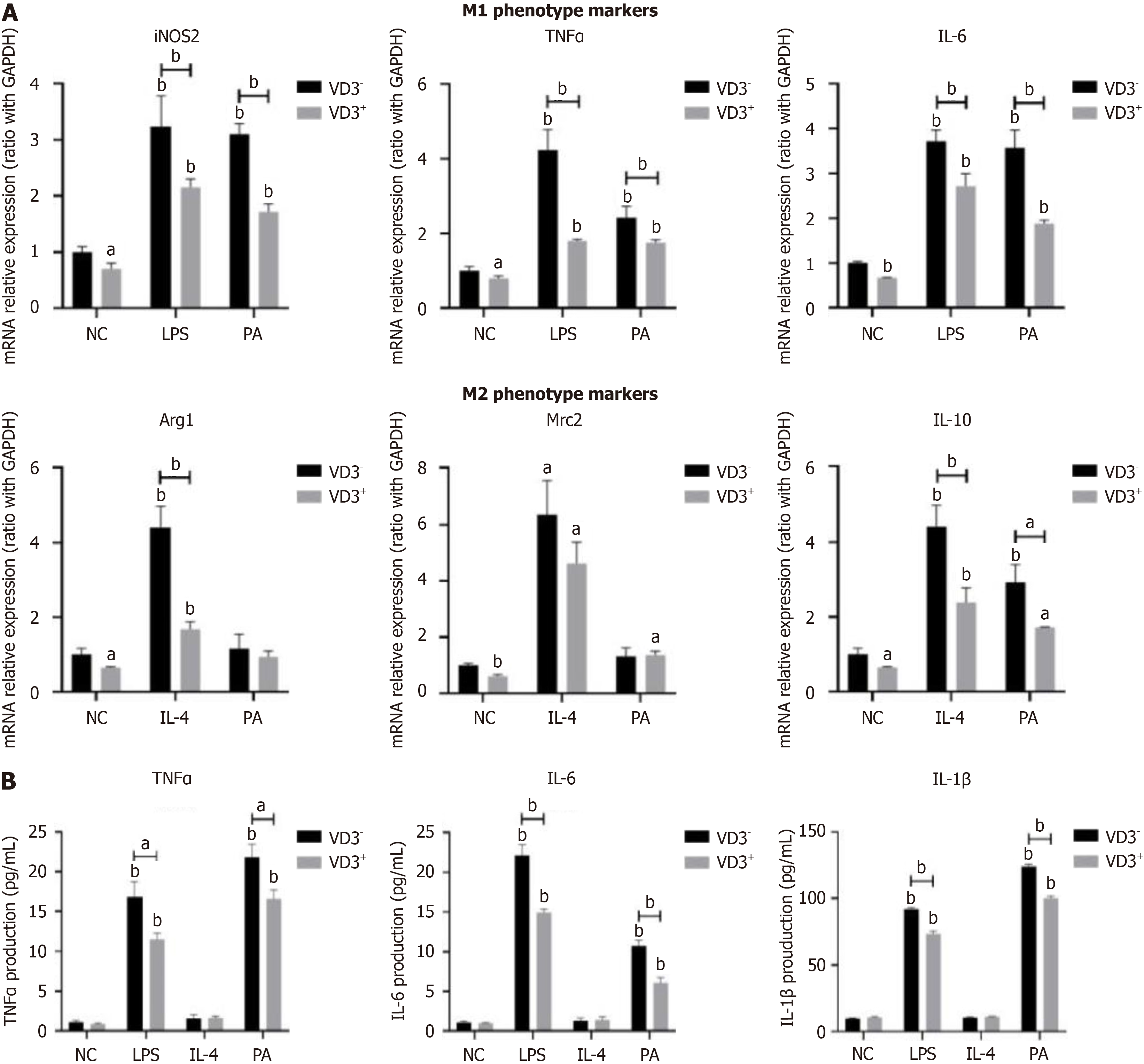
Figure 3 Administration of 1,25-dihydroxy-vitamin D inhibits fatty-acid-induced proinflammatory M1 polarization of macrophages.
RAW264.7 macrophages were incubated with palmitic acid (0.5 mmol/L) for 24 hours or Dulbecco's modified Eagle’s medium as a normal control. Lipopolysaccharide (100 ng/mL) or interleukin-4 (5 ng/mL) treatment served as positive controls for macrophage M1 or M2 polarization. For administration of 1,25-dihydroxy-vitamin D (1,25(OH)2D3), RAW264.7 macrophages were incubated with 1,25(OH)2D3 (VD3, 20 ng/mL) for a further 24 hours. A: M1/M2 gene marker expression on RAW264.7 macrophages; B: Proinflammatory cytokine secretion from RAW264.7 macrophages. Values are mean ± SEM, aP < 0.05, bP < 0.01, n = 3 experiments; NC: Normal control; PA; Palmitic acid; VD3: 1,25-dihydroxy-vitamin D; LPS: Lipopolysaccharide; IL: Interleukin; iNOS: Inducible NO synthase; TNFα: Tumor necrosis factor α; Arg1 Arginine 1; Mrc2: Macrophage mannose receptor 2.
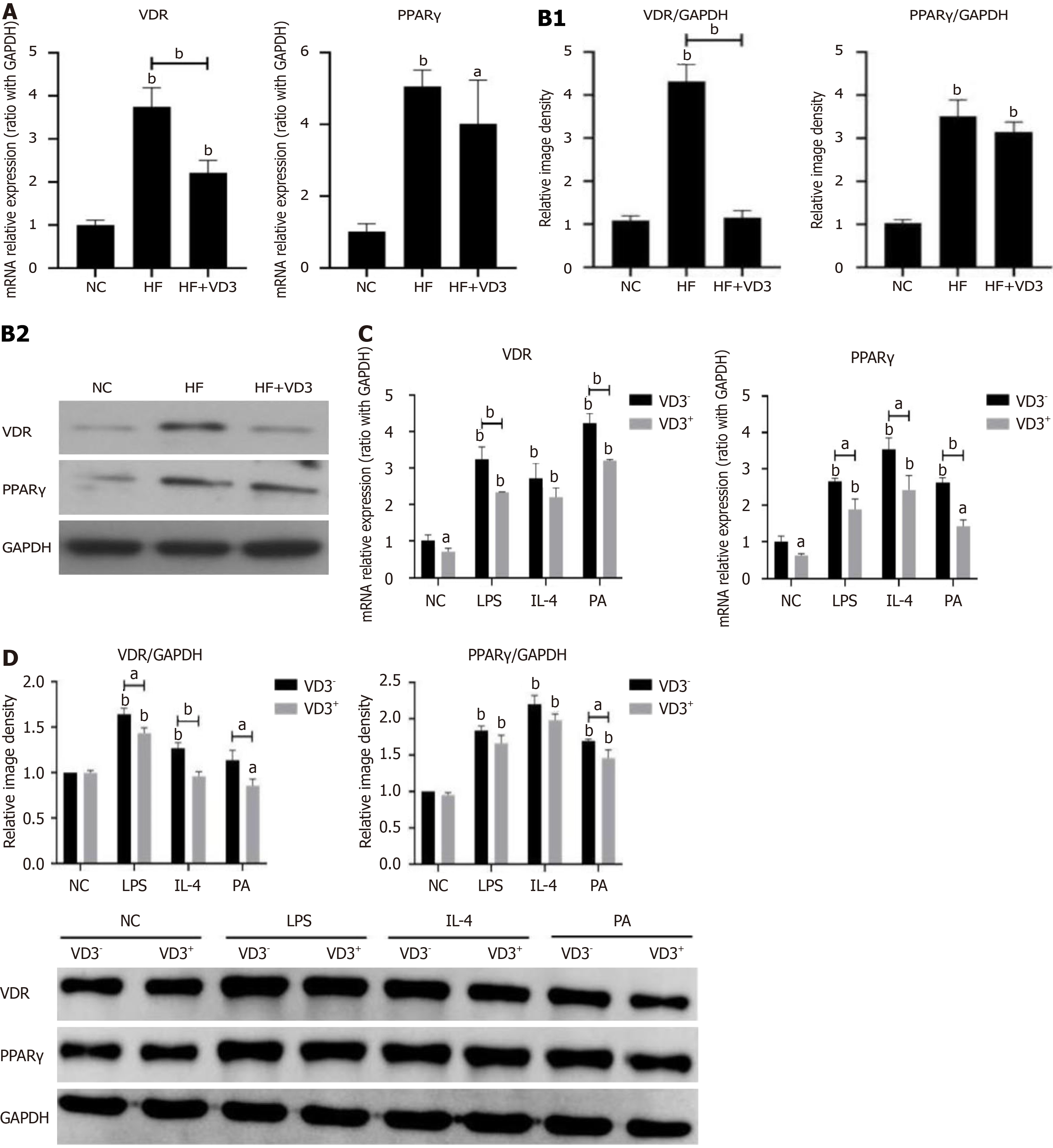
Figure 4 Effect of vitamin D receptor-peroxisome proliferator activated receptor γ pathway modulating 1,25-dihydroxy-vitamin D administration on high-fat diet/fatty acid-induced hepatic macrophage polarization.
Wild-type C57BL/6 mice were fed either normal control (NC) diet or high-fat (HF) diet for 16 week, or HF diet plus 1,25-dihydroxy-vitamin D [1,25(OH)2D3] (20 µg/kg) by oral gavage every alternate day for 16 weeks. Phosphate-buffered saline by oral gavage served as a control (n = 10/group). Hepatic macrophages were isolated from mice administered NC diet, HF diet alone or plus 1,25(OH)2D3. RAW264.7 macrophages were incubated with palmitic acid (0.5 mmol/L) for 24 hours or Dulbecco's modified Eagle’s medium as a normal control. Lipopolysaccharide (100 ng/mL) or interleukin-4 (5 ng/mL) treatment served as positive controls for macrophage M1 or M2 polarization. For administration of 1,25(OH)2D3, RAW264.7 macrophages were incubated with 1,25(OH)2D3 (20 ng/mL) for a further 24 hours. A: MRNA expression of vitamin D receptor (VDR)-peroxisome proliferator activated receptor γ on hepatic macrophages; B: Protein expression of VDR and PPARγ on hepatic macrophages; C: MRNA expression of VDR and PPARγ on RAW264.7 macrophages; D: Protein expression of VDR and PPARγ on RAW264.7 macrophages. Values are mean ± SEM, aP < 0.05, bP < 0.01, n = 10 animals per group, n = 3 experiments; VDR: Vitamin D receptor; PPARγ: Peroxisome proliferator activated receptor γ; NC: Normal control; HF: High-fat; VD3: 1,25-dihydroxy-vitamin D; PA: Palmitic acid; LPS: Lipopolysaccharide; IL: Interleukin.
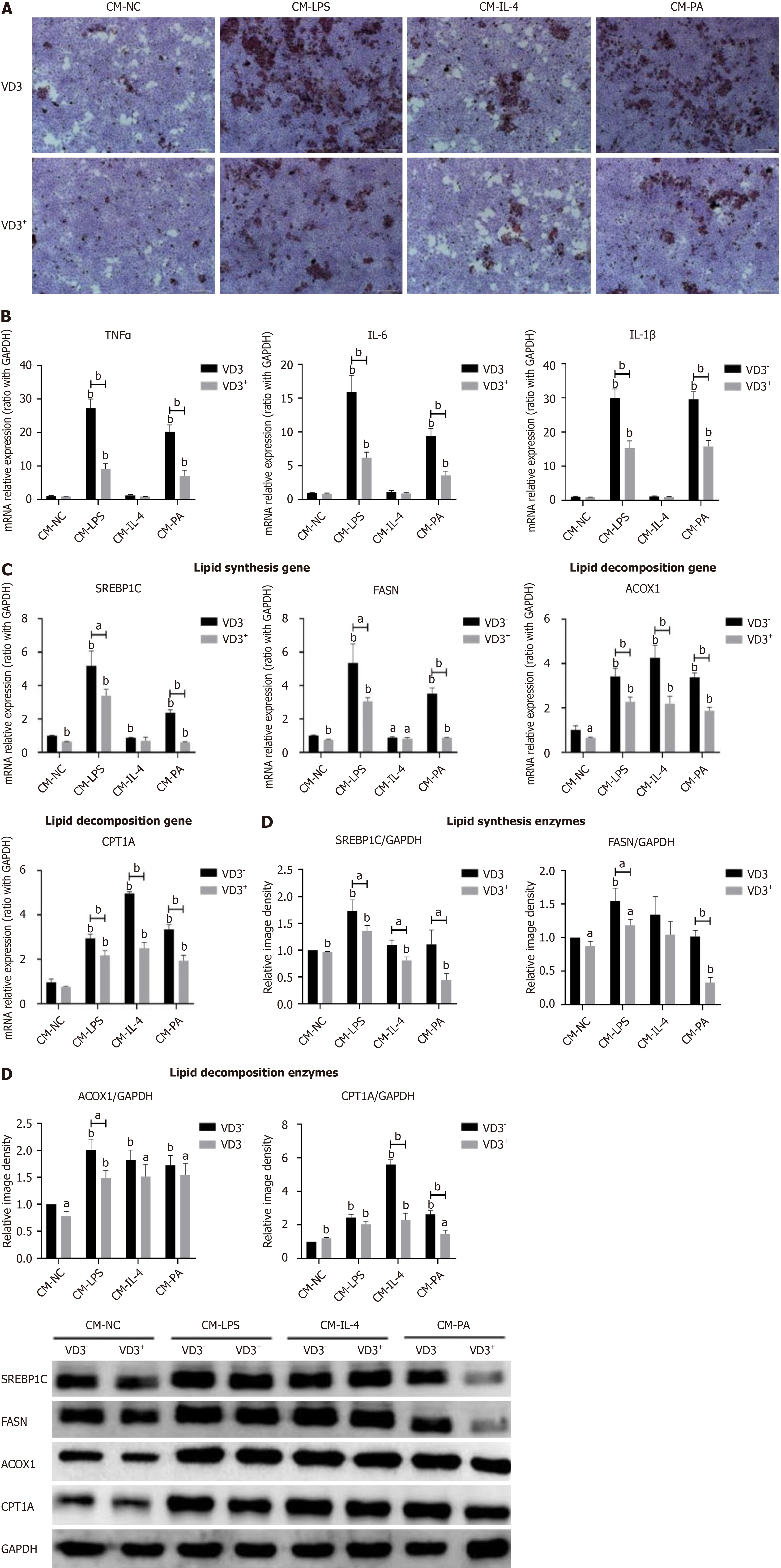
Figure 5 Administration of 1,25-dihydroxy-vitamin D inhibiting fatty-acid-induced proinflammatory M1 polarization of macrophages directly relieves lipid accumulation and metabolism in hepatocytes.
RAW264.7 macrophages were incubated with palmitic acid (0.5 mmol/L) for 24 hours or Dulbecco's modified Eagle’s medium as a normal control. Lipopolysaccharide (100 ng/mL) or interleukin-4 (5 ng/mL) treatment served as positive controls for macrophage M1 or M2 polarization. For administration of 1,25-dihydroxy-vitamin D [1,25(OH)2D3], RAW264.7 macrophages were incubated with 1,25(OH)2D3 (20 ng/mL) for a further 24 hours. The cell culture supernatants were collected and prepared for conditioned media (CMs). AML12 hepatocytes were treated with different CMs from RAW264.7 macrophages for 24 hours. A: Lipid accumulation in AML12 hepatocytes determined by Oil Red O staining (200 ×); B: Proinflammatory cytokines mRNA expression on AML12 hepatocytes; C: Lipid metabolism genes mRNA expression on AML12 hepatocytes; D: Lipid metabolism enzymes protein expression on AML12 hepatocytes. Values are mean ± SEM, aP < 0.05, bP < 0.01, n = 3 experiments; CM: Conditioned media; NC: Normal control; LPS: Lipopolysaccharide; IL: Interleukin; PA: Palmitic acid; VD3: 1,25-dihydroxy-vitamin D; TNFα: Tumor necrosis factor α; SREBP1C: Sterol-regulatory element binding protein 1C; FASN: Fatty acid synthetase; ACOX1: Acyl-CoA oxidase 1; CPT1A: Carnitine palmitoyltransferase 1A.
- Citation: Luo WJ, Dong XW, Ye H, Zhao QS, Zhang QB, Guo WY, Liu HW, Xu F. Vitamin D 1,25-Dihydroxyvitamin D3 reduces lipid accumulation in hepatocytes by inhibiting M1 macrophage polarization. World J Gastrointest Oncol 2024; 16(12): 4685-4699
- URL: https://www.wjgnet.com/1948-5204/full/v16/i12/4685.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i12.4685