Published online Jun 16, 2025. doi: 10.4253/wjge.v17.i6.104234
Revised: March 29, 2025
Accepted: May 13, 2025
Published online: June 16, 2025
Processing time: 179 Days and 16.8 Hours
The use of proton pump inhibitors (PPIs) with the intent of reducing gastric acidity to the desired therapeutic level for treating bleeding peptic ulcer still has several limitations.
To compare intravenous PPIs and oral potassium competitive acid blockers (PCABs) administered prior to endoscopic treatment of bleeding peptic ulcers.
This retrospective study involved 105 consecutive patients with non-variceal upper gastrointestinal bleeding (treated August 2023 to February 2024). Prior to emergency endoscopy, patients received either intravenous PPI (pantoprazole 80 mg bolus) or oral PCAB (tegoprazan 50 mg single-dose). Severity of bleeding was assessed using the Glasgow-Blatchford, Rockall, and AIMS65 scoring systems. Patients with severe comorbidities were excluded. Primary outcomes included need for therapeutic endoscopic intervention and occurrence of re-bleeding. Multivariate logistic regression was performed to adjust for potential confounding factors.
Total of the 105 patients, 61 received intravenous PPI injection and 44 received oral PCAB prior to emergency endoscopy. To minimize selection bias, bleeding severity was assessed using the Glasgow-Blatchford, Rockall and AIMS65 scores, with no statistically significant differences observed between the two groups. During emergency endoscopy performed within 48 hours, ulcer bed status was classified according to the Forrest classification. The proportion of lesions graded IIa or higher was significantly lower in the PCAB group (P < 0.001), as was the frequency of therapeutic endoscopy intervention (odds ratio = 0.272, 95% con
Pre-endoscopic PCAB administration is more effective than PPI injection for upper gastrointestinal bleeding and may reduce ulcer bleeding mortality.
Core Tip: This study compared the effectiveness of intravenous proton pump inhibitors (PPIs) and oral potassium competitive acid blockers (PCABs) in managing non-variceal upper gastrointestinal bleeding prior to emergency endoscopy. PPI injection and PCAB administration groups showed no significant differences in bleeding severity. However, the PCAB group demonstrated significantly fewer ulcer lesions with recent bleeding stigmata and required fewer therapeutic endoscopic interventions. Additionally, re-bleeding events were more frequent in the PPI group. These findings suggest that pre-endoscopic administration of PCABs may be more effective than PPIs and could potentially reduce mortality associated with ulcer bleeding.
- Citation: Lim NR, Chung WC. Intravenous proton pump inhibitors vs oral potassium competitive acid blockers before endoscopic treatment of bleeding peptic ulcers. World J Gastrointest Endosc 2025; 17(6): 104234
- URL: https://www.wjgnet.com/1948-5190/full/v17/i6/104234.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i6.104234
Effective management of bleeding peptic ulcers (BPUs) requires reducing stomach acidity and maintaining it at a hydrogen ion concentration index (pH) 6 of or higher. Gastric acid and pepsin inhibit platelet aggregation and promote clot dissolution, thereby contributing to both the onset and persistence of bleeding. Previous studies have shown that maintaining the gastric pH at or above 6 can significantly reduce the severity of the initial bleeding as well as the risk of re-bleeding in patients with BPUs[1,2].
Over the past two decades, pharmacological management of BPUs has focused on the potent suppression of gastric acid secretion using proton pump inhibitors (PPIs). Unlike histamine-2 receptor antagonists, PPIs are highly effective acid suppressants that do not lead to drug resistance and are widely used in the treatment of both non-bleeding and BPUs. A meta-analysis demonstrated that PPI therapy significantly reduced the risk of ulcer re-bleeding [-14.6%; 95% confidence interval (CI): -16.2 to -12.9] and the need for emergency surgery (-5.4%; 95%CI: -8.4 to -2.4)[3]. The same study also reported an association between PPI use and reduced mortality (-2.7%; 95%CI: -9.2 to 3.8)[3].
However, the clinical goal of maintaining gastric pH above 6 remains largely theoretical and has not been reliably validated as a marker of treatment effectiveness. What has been clearly demonstrated is the link between potent acid suppression therapy and reduced re-bleeding rates, rather than the maintenance of a specific gastric pH. Additionally, although continuous high-dose intravenous PPI therapy has been widely adopted for managing bleeding ulcers, several recent studies from North America have shown that even this regimen often fails to consistently maintain gastric pH above 6[4].
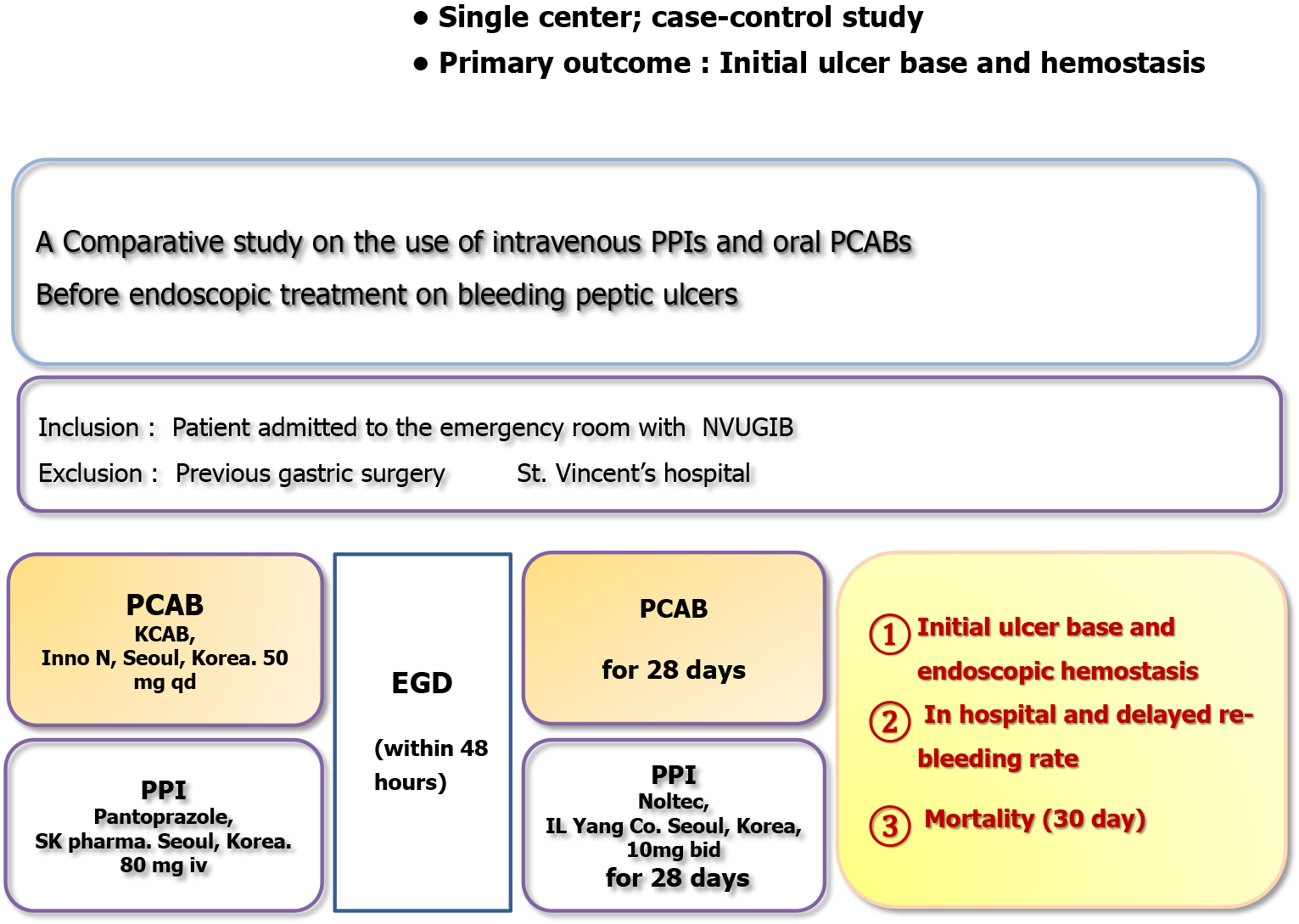
Potassium competitive acid blockers (PCABs) are a newer class of gastric acid secretion inhibitors that act by competitively binding to the K+ binding site of H+, K+- adenosine triphosphatase, thereby effectively suppressing gastric acid production[5]. Compared to traditional PPIs, PCABs offer several advantages: They are not affected by the existing level of acid secretion, achieve peak plasma concentration within approximately 1 hour, have a longer duration of action, and can be taken independently of meal timing[6]. These properties allow PCABs to maintain gastric acidity at a stable, elevated pH level. In clinical scenarios where PPIs may be insufficient to suppress acid secretion, due to various factors, PCABs provide a more reliable alternative. Given their ability to rapidly and effectively raise gastric pH to the therapeutic target of 6, it is important to further investigate the clinical efficacy of PCABs in the treatment of BPUs. The purpose of this study was to determine the effectiveness of oral PCAB (administered once daily) vs intravenous PPI therapy (administered as an initial bolus followed by continuous infusion for 48 hours) in reducing the recurrence of ulcer re-bleeding. Additionally, we aimed to evaluate the ulcer bed condition using Forrest classification and the frequency of endoscopic hemostasis during emergency endoscopy within 48 hours.
This study was conducted at St. Vincent’s Hospital, Catholic University School of Medicine (Suwon, Korea), between August 2023 and February 2024. A consecutive series of patients who presented to the emergency department with symptoms indicative of non-variceal upper gastrointestinal bleeding (NVUGIB), including hematemesis, melena, blood in nasogastric aspirates, or hypotension were enrolled. Inclusion was limited to patients with NVUGIB confirmed by clinical presentation and endoscopic findings. Exclusion criteria were as follows: Age under 18 years; pregnancy; variceal bleeding; post-endoscopic procedure bleeding; Mallory-Weiss tears; angiodysplasia; esophageal ulcers; gastric cancer; diverticular bleeding; suspected small bowel bleeding; history of gastric surgery; severe comorbidities, including advanced liver disease (Child-Pugh class B or C), chronic kidney disease requiring dialysis, or heart failure with ejection fraction < 20%; or refusal of nocturnal emergency endoscopy.
After appropriate resuscitation, all patients received either high-dose intravenous PPI therapy (pantoprazole 80 mg IV bolus followed by a continuous infusion of 8 mg/hour for 48 hours) or oral PCAB (tegoprazan 50 mg; HK Inno N, Seoul, Korea) prior to undergoing endoscopic treatment (Figure 1). Before initiating the study, the importance of early acid suppression in NVUGIB management was emphasized through continuous education of emergency room physicians. Patients presenting with NVUGIB were informed about both treatment options (IV PPI vs oral PCAB) though standardized education, and the choice of therapy was made at the discretion of the emergency physician. To minimize selection bias between the PPI and PCAB groups, the severity of bleeding was assessed using the Glasgow-Blatchford, Rockall and AIMS65 scores[7-9]. No statistically significant differences were found in these scores between the two groups.
Pantoprazole was chosen as the intravenous PPI for this study based on several key considerations. It is the most commonly used intravenous PPI for NVUGIB at our institution and in many clinical settings across Korea, enhancing the direct applicability of our findings to current practice. Pantoprazole also has a well-established pharmacokinetic profile and is associated with minimal cytochrome P450-mediated drug-drug interactions, making it a safe and practical option. Furthermore, a meta-analysis reported no consistent evidence that any PPI is superior to others in treating acute upper gastrointestinal bleeding[10], supporting the notion that the selection of pantoprazole would be unlikely to introduce bias or affect comparative outcomes.
Emergency esophagogastroduodenoscopy was performed within 48 hours of presentation. Ulcer beds were classified using the Forrest classification system, and endoscopic treatment was administered for lesions classified as Forrest IIa or higher. The endoscopic treatment methods included epinephrine injection, electrocoagulation, and hemostasis. For injection therapy, 5-15 mL of epinephrine diluted 1:10000 (0.5 to 2.0 mL/injection) was injected around the hemorrhagic scar. Electrocoagulation methods included argon plasma coagulation and bipolar electrocoagulation. Combination therapy was employed when monotherapy was ineffective or when multiple bleeding sites were observed. Initial hemostasis was defined as the absence of active bleeding following irrigation and a 3-minute observation period post-treatment. A second-look endoscopy was performed within 24 hours in all patients who received endoscopic therapy. To minimize bias, endoscopic images were reviewed by two independent authors to confirm Forrest classification.
In this study, re-bleeding was defined based on objective clinical and endoscopic criteria. Clinically, it included persistent melena, hematochezia, or the presence of fresh blood in vomit after the first emergency endoscopy during hospitalization. Re-bleeding was also defined endoscopically as the persistence of a high-risk (Forrest IIa or higher) during second-look endoscopy, necessitating additional endoscopic hemostasis. Additionally, signs of hemodynamic instability (systolic blood pressure < 90 mmHg, heart rate > 120 beats/minute), or a rapid decrease in hemoglobin of more than 2 g/dL, were also considered indicative of re-bleeding. All re-bleeding cases were confirmed via endoscopy. Helicobacter pylori (H.
All data were collected in standard forms and analyzed using computer-based statistical software (SPSS version 25.0; IBM, Armonk, NY, United States). Continuous variables were compared using a Student’s t-test, while differences between categorical (dichotomous) variables were evaluated using the χ2 test. To adjust for confounding factors in this retrospective study, differences in re-bleeding rates and therapeutic intervention rates between the two treatment groups were further analyzed using multivariate logistic regression. A P value < 0.05 was considered statistically significant.
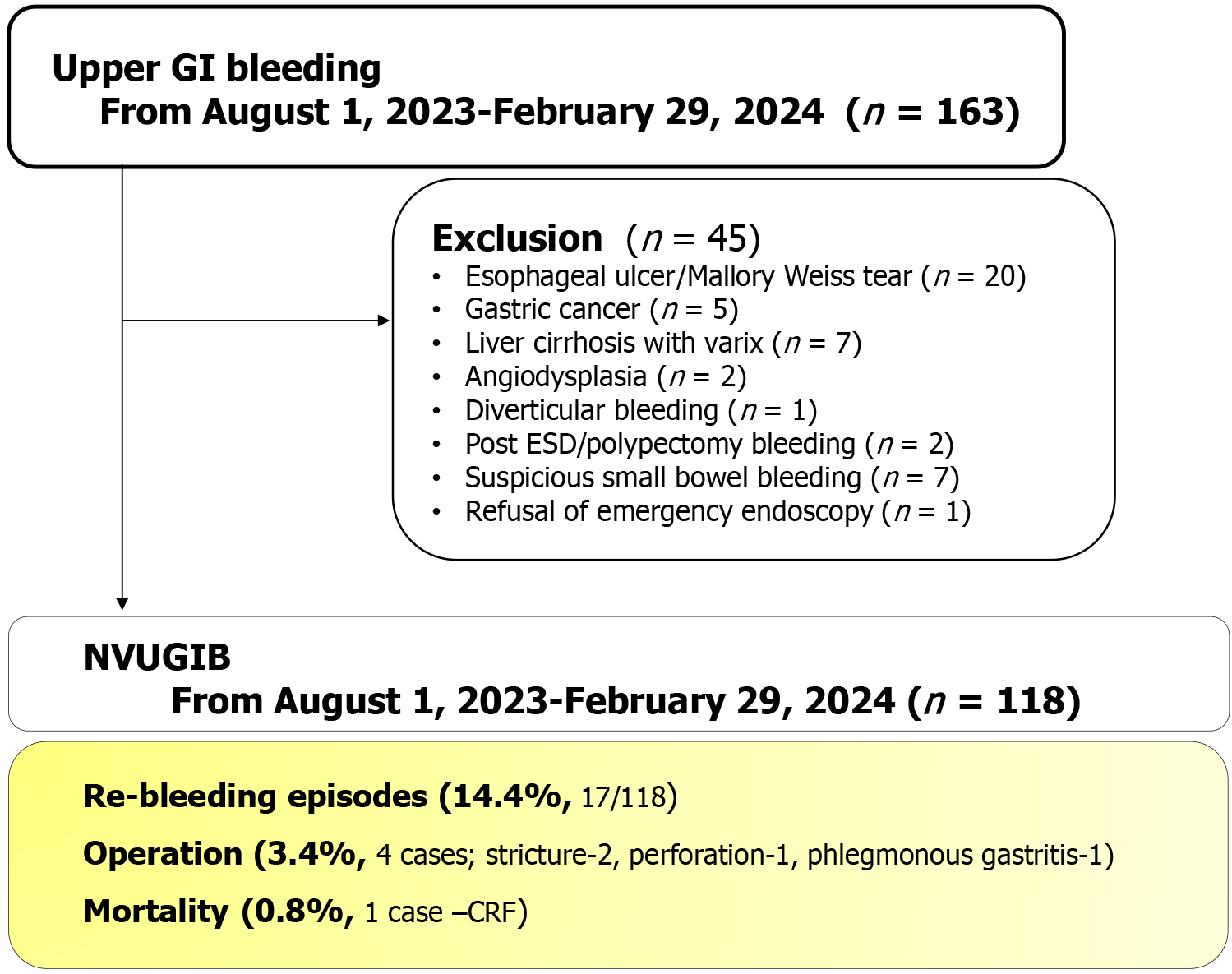
A total of 163 patients who presented to the emergency room with upper gastrointestinal bleeding were initially screened for inclusion in the study. After applying exclusion criteria, 118 patients were found to be eligible and included in the final analysis. Among them, 17 patients (14.4%) experienced re-bleeding. Four patients (3.4%) required surgical intervention due to complications, including 2 for stenosis, 1 for perforation, and 1 for phlegmonous gastritis. One patient with chronic renal failure died (0.8%) from massive re-bleeding (Figure 2).
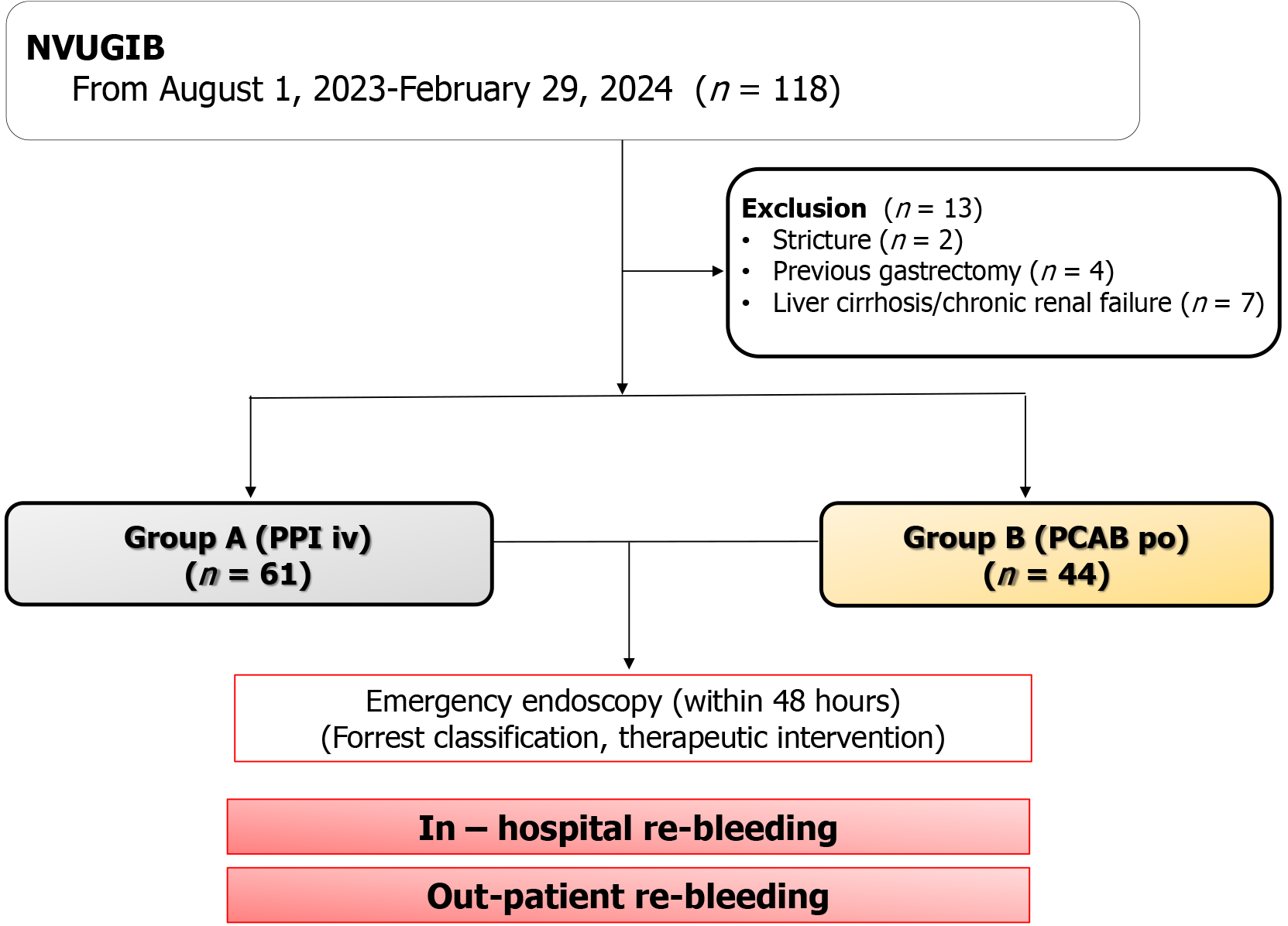
To assess the effectiveness of administering gastric acid suppressants prior to emergency endoscopy in patients with NVUGIB, patients with conditions that could significantly affect drug metabolism had been excluded. After applying these criteria, 105 patients remained for analysis. Of these, 61 patients received IV PPI therapy prior to endoscopy, while 44 patients received oral PCAB (Figure 3).
Baseline characteristics, including ASA physical status and the severity of bleeding (Glasgow-Blatchford score, Rockall score, and AIMS65 score) were comparable between the two groups, with no statistically significant differences (Table 1). Patients with severe bleeding tendencies were excluded to better isolate the efficacy of the medications. Only patients who were stable enough to undergo duodenoscopy were included; the only patients with ASA class 4 were those with a recent myocardial infarction. There were no significant differences between groups regarding NSAID or antithrombotic use, nor in the prevalence of H. pylori infection. Based on Forrest classification, the PPI group included 7 patients with stage Ia/Ib lesions and 20 with stage IIa lesions, while the PCAB group included 2 patients with stage Ib and 3 with stage IIa lesions.
| Before endoscopy (pre-treatment) | Intravenous injection of PPI (n = 61) | Per oral intake, PCAB (n = 44) | P value |
| Age (year), mean ± SD | 68.83 ± 15.96 | 71.21 ± 15.59 | 0.47 |
| Male sex, n (%) | 46 (75.4) | 26 (59.1) | 0.20 |
| Current smoker | 25 | 10 | 0.08 |
| Alcohol | 19 | 10 | 0.47 |
| Causes of bleeding | |||
| Gastric ulcer | 26 | 24 | 0.71 |
| Duodenal ulcer | 24 | 14 | - |
| Both | 11 | 6 | - |
| Helicobacter pylori | 32 | 15 | 0.12 |
| Anti-platelet agents | 16 | 12 | 0.25 |
| Anti-coagulant (NOAC) | 3 | 5 | 0.17 |
| NSAIDs/COX 2 inhibitors | 12 | 11 | 0.38 |
| ASA status | - | - | 0.12 |
| 1 | 6 | 5 | - |
| 2 | 44 | 25 | - |
| 3 | 9 | 14 | - |
| 4 | 2 | 0 | - |
| Glasgow-Blatchford score, mean ± SD | 10.27 ± 4.63 | 9.97 ± 4.15 | 0.75 |
| Rockall score, mean ± SD | 5.85 ± 2.18 | 5.30 ± 1.65 | 0.18 |
| AIMS65 score, mean ± SD | 1.38 ± 1.11 | 1.12 ± 0.57 | 0.17 |
| Therapeutic intervention | 33 | 10 | < 0.01 |
| Forrest classification | |||
| I and IIa | 27 | 5 | < 0.01 |
| Re-bleeding episode | 13 | 2 | 0.02 |
| In-hospital | 6 | 1 | - |
| Out-patient | 7 | 1 | - |
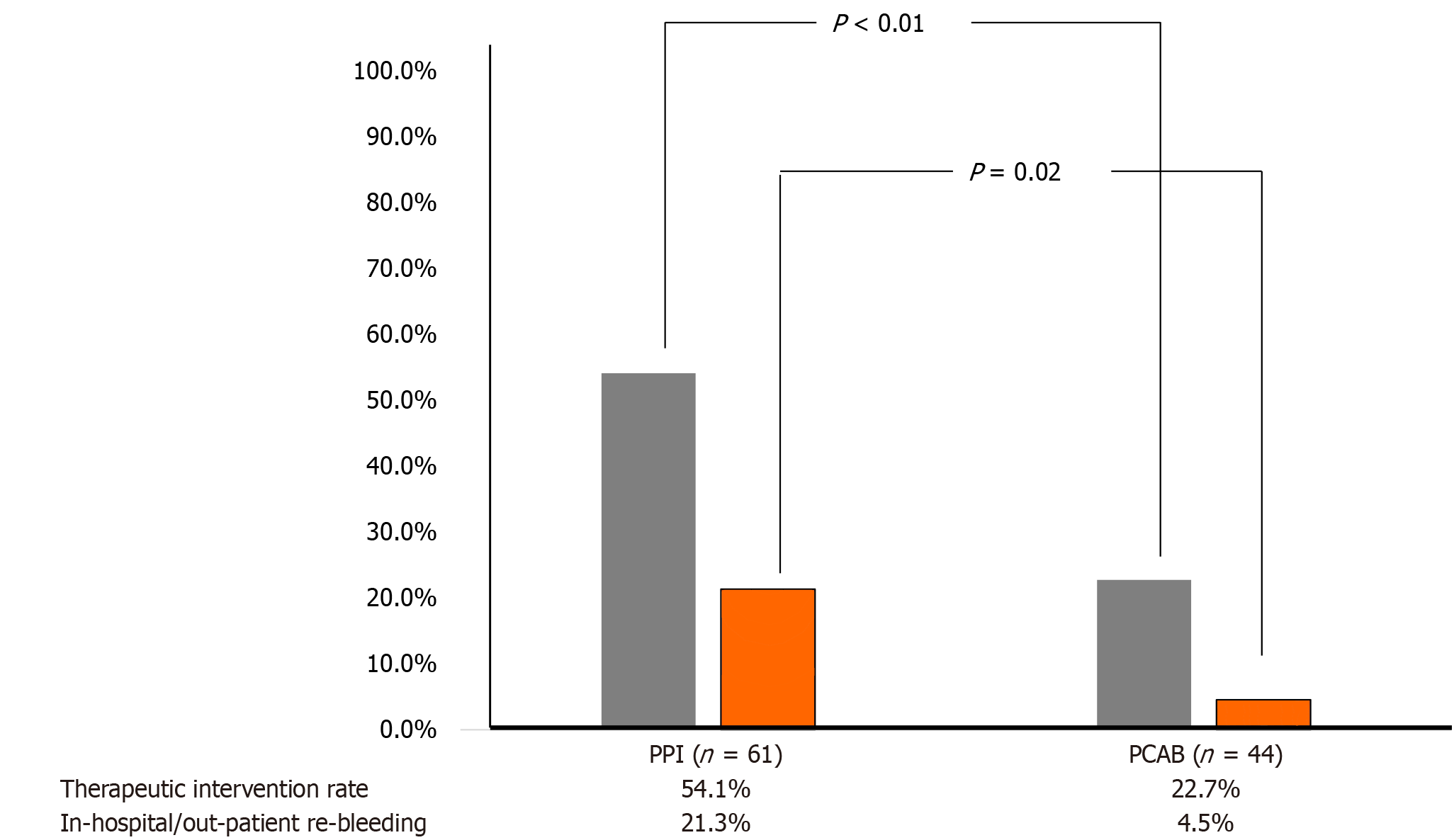
The frequency of therapeutic endoscopy, as determined by ulcer bed evaluation during emergency endoscopy, was significantly higher in the PPI group (54.1%, 33/61) compared to the PCAB group (22.7%, 10/44) (P < 0.01) (Figure 4). To assess the independent effect of PCAB relative to PPI therapy, we performed multivariate logistic regression analyses focusing on two primary outcomes: The need for therapeutic endoscopic intervention and re-bleeding (Tables 2 and 3). In the multivariate analysis related to therapeutic intervention (Table 2), after adjusting for potential confounders, including age, sex, H. pylori infection, and the use of anti-coagulants and anti-platelet agents, PCAB therapy was independently associated with significantly lower odds of requiring endoscopic therapy compared to PPI therapy [odds ratio (OR) = 0.272, 95%CI: 0.111-0.665, P = 0.004]. None of the other adjusted variables (age, sex, H. pylori infection status, anti-coagulant or anti-platelet use) were significantly associated with the need for therapeutic endoscopy.
| Variables | OR | 95%CI | P value |
| Age (year), mean ± SD | 1.005 | 0.972-1.039 | 0.777 |
| Male sex | 0.548 | 0.184-1.632 | 0.280 |
| Helicobacter pylori infection | 1.331 | 0.528-3.356 | 0.544 |
| Use of anti-coagulants | 0.994 | 0.193-5.120 | 0.994 |
| Use of anti-platelet | 1.299 | 0.493-3.423 | 0.596 |
| PCAB (vs PPI) | 0.272 | 0.111-0.665 | 0.004a |
| Variables | OR | 95%CI | P value |
| Age (year), mean ± SD | 1.001 | 0.950-1.054 | 0.977 |
| Male sex | 0.804 | 0.153-4.216 | 0.797 |
| Helicobacter pylori infection | 0.624 | 0.151-2.584 | 0.515 |
| Use of anti-coagulants | 9.206 | 1.144-74.111 | 0.037a |
| Use of anti-platelet | 2.243 | 0.618-8.131 | 0.219 |
| Forrest classification (≥ IIa) | 1.822 | 0.499-6.655 | 0.364 |
| PCAB (vs PPI) | 0.141 | 0.024-0.844 | 0.032a |
In-hospital re-bleeding occurred in 6 patients in the PPI group and 1 patient in the PCAB group. Outpatient re-bleeding requiring readmission was observed in 7 patients in the PPI group and 1 patient in the PCAB group. Overall, the re-bleeding rate was significantly higher in the PPI group (21.3%, 13/61) compared to the PCAB group (4.5%, 2/44), demonstrating a statistically significant difference (P = 0.02) (Figure 4).
In the multivariate analysis for re-bleeding (Table 3), PCAB treatment remained significantly protective against re-bleeding compared to PPI therapy (OR = 0.141, 95%CI: 0.024-0.844, P = 0.032), indicating an approximately 86% reduction in odds of re-bleeding with PCAB use. Additionally, use of anti-coagulants was identified as a significant independent risk factor for re-bleeding (OR = 9.206, 95%CI: 1.144-74.111, P = 0.037).
PPIs are currently regarded as the first-line pharmacological treatment for upper gastrointestinal bleeding. Numerous studies have demonstrated their ability to reduce both re-bleeding rates and the need for surgical intervention[3]. However, despite their widespread use and established benefits, serious complications such as mortality can still occur in patients with upper gastrointestinal bleeding even after PPI administration. While PPIs offer several advantages, they are also associated with notable limitations. These include a delayed onset of action, often requiring 3 days to 5 days to achieve maximum efficacy, and incomplete control of nocturnal acid secretion. Additionally, interindividual variability in drug efficacy, largely due to differences in cytochrome 2C19 genotype, and dependency on food timing for optimal administration further limit their effectiveness[11,12]. Mechanistically, PPIs are prodrugs that require protonation in acidic environments to become active and can only inhibit active proton pumps, leading to partial and sometimes inconsistent acid suppression. In contrast, PCABs have been developed to address these shortcomings. PCABs exhibit rapid onset of action, sustained acid suppression, and are less affected by cytochrome 2C19 polymorphisms or food intake. Given their pharmacodynamic advantages, PCABs are emerging as a promising alternative in the management of NVUGIB, potentially offering superior control of gastric acid secretion in the acute setting.
We acknowledge that comparing an intravenous PPI with an oral PCAB introduces methodological complexity, primarily due to differences in route of administration and drug pharmacokinetics. Intravenous delivery offers immediate bioavailability and a rapid onset of action, achieving target intragastric pH within 20 minutes, while oral administration depends on gastrointestinal absorption, which may delay onset and reduce bioavailability. This delay may be further exacerbated in patients with severe hypovolemia, as reduced blood flow to the gastrointestinal tract can lead to gastrointestinal mucosal hypoxia, cellular damage, and delayed gastric emptying. Despite these theoretical disad
A previous large-scale study, which included 13498 patients treated for NVUGIB between 2004 and 2011, reported a re-bleeding rate of 13%-18% and a 30-day mortality of 11.0%[15]. Similarly, a Danish nationwide cohort study conducted between 2006 and 2014 and involving 19258 patients with BPUs found a re-bleeding rate of 10.8% and a 30-day mortality rate of 10.2%, consistent with earlier studies[16]. In contrast, data from Korean clinical settings have shown more favorable outcomes. A large-scale prospective registry, the Korean Peptic Ulcer Bleeding Registry, collected data from 904 patients across multiple centers between 2014 and 2015, with 897 patients included in the final analysis[17]. That study reported a re-bleeding rate of 7.1% and a notably lower 30-day mortality rate of 1%. The improved outcomes in the Korean cohort may be attributed to advances in endoscopic techniques, early intervention, and the routine use of pre-endoscopic intravenous PPI therapy. In comparison, our study showed a higher overall re-bleeding rate, which may partially reflect a broader definition of re-bleeding. Specifically, our criteria included not only clinical signs of recurrent bleeding but also endoscopic findings during second-look procedures within 48 hours, where additional hemostasis was performed.
To date, no studies have directly compared PCAB and PPI therapies in patients with acute upper gastrointestinal bleeding. However, a recent study investigating ulcers that developed after endoscopic submucosal resection, rather than peptic ulcers, reported a re-bleeding rate of 1.3% in the PCAB group compared to 10.0% in the PPI group[18]. Our findings are consistent with those results, and are especially noteworthy given that they pertain specifically to patients with BPUs. This study also highlighted practical challenges in shifting clinical paradigms. In real-world settings, emergency physicians and internists have traditionally favored fasting and intravenous medication in upper gast
In recent years, interest in “green endoscopy” has been growing, as the climate crisis becomes an increasingly urgent global concern. Governments, corporations, and individuals are now actively seeking strategies to reduce their environmental impact, and the medical field is no exception. Endoscopy, as a resource-intensive procedure, contributes significantly to the carbon footprint of healthcare delivery. A notable audit by Namburar et al[20] evaluated all endoscopic procedures performed over a 5-day period, categorizing institutions as either low-volume (≤ 2000 proce
This study has several important limitations. First, its non-randomized, retrospective design, conducted in a single-center setting with a relatively small sample size, limits the generalizability of our findings. To establish broader clinical relevance and inform guideline development for the management of upper gastrointestinal bleeding, well-designed prospective randomized controlled trials are warranted. To mitigate the risk of selection bias, we performed comprehensive baseline comparisons between the treatment groups using multiple validated bleeding severity scores (Glasgow-Blatchford, Rockall, and AIMS65). Herein, we also expanded Table 1 to provide detailed patient characteristics. These comparisons revealed no statistically significant differences between groups, suggesting a reasonably balanced baseline despite the lack of randomization. Due to the differing routes of drug administration (intravenous for PPI vs oral for PCAB), blinding of patients and treating physicians was not feasible. To address this limitation, we adopted standardized assessment protocols for endoscopic findings, employing the Forrest classification, and used objective criteria to define re-bleeding, including hemodynamic instability and hemoglobin level changes. Additionally, endoscopists were not involved in initial treatment decisions, reducing the risk of treatment-related bias during endoscopic evaluation. Another limitation is that our study population consisted solely of Korean patients, which limits ethnic and racial diversity. Genetic polymorphisms, particularly involving CYP2C19, are more prevalent in Asian populations, where 15%-20% are rapid metabolizers, potentially diminishing the efficacy of PPIs. In contrast, PCABs are less affected by CYP2C19 metabolism, offering a potential pharmacogenetic advantage in this population. Nevertheless, validation in more ethnically diverse cohorts is essential to ensure generalizability across different populations.
In conclusion, the method of performing PCAB before emergency endoscopy is simple and effective, and it is expected to contribute to green endoscopy, which has been receiving attention recently.
| 1. | Green FW Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74:38-43. [PubMed] |
| 2. | Brunner G, Luna P, Hartmann M, Wurst W. Optimizing the intragastric pH as a supportive therapy in upper GI bleeding. Yale J Biol Med. 1996;69:225-231. [PubMed] |
| 3. | Bardou M, Toubouti Y, Benhaberou-Brun D, Rahme E, Barkun AN. Meta-analysis: proton-pump inhibition in high-risk patients with acute peptic ulcer bleeding. Aliment Pharmacol Ther. 2005;21:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 4. | Metz DC, Amer F, Hunt B, Vakily M, Kukulka MJ, Samra N. Lansoprazole regimens that sustain intragastric pH > 6.0: an evaluation of intermittent oral and continuous intravenous infusion dosages. Aliment Pharmacol Ther. 2006;23:985-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Shin JM, Inatomi N, Munson K, Strugatsky D, Tokhtaeva E, Vagin O, Sachs G. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438). J Pharmacol Exp Ther. 2011;339:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 126] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 6. | Hori Y, Matsukawa J, Takeuchi T, Nishida H, Kajino M, Inatomi N. A study comparing the antisecretory effect of TAK-438, a novel potassium-competitive acid blocker, with lansoprazole in animals. J Pharmacol Exp Ther. 2011;337:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 131] [Article Influence: 8.7] [Reference Citation Analysis (1)] |
| 7. | Blatchford O, Murray WR, Blatchford M. A risk score to predict need for treatment for upper-gastrointestinal haemorrhage. Lancet. 2000;356:1318-1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 666] [Cited by in RCA: 709] [Article Influence: 27.3] [Reference Citation Analysis (2)] |
| 8. | Rockall TA, Logan RF, Devlin HB, Northfield TC. Risk assessment after acute upper gastrointestinal haemorrhage. Gut. 1996;38:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 888] [Cited by in RCA: 919] [Article Influence: 30.6] [Reference Citation Analysis (1)] |
| 9. | Saltzman JR, Tabak YP, Hyett BH, Sun X, Travis AC, Johannes RS. A simple risk score accurately predicts in-hospital mortality, length of stay, and cost in acute upper GI bleeding. Gastrointest Endosc. 2011;74:1215-1224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 351] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 10. | Scally B, Emberson JR, Spata E, Reith C, Davies K, Halls H, Holland L, Wilson K, Bhala N, Hawkey C, Hochberg M, Hunt R, Laine L, Lanas A, Patrono C, Baigent C. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol. 2018;3:231-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (17)] |
| 11. | Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther. 2006;23 Suppl 2:2-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 260] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 12. | Strand DS, Kim D, Peura DA. 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver. 2017;11:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 219] [Cited by in RCA: 435] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 13. | Laine L, Shah A, Bemanian S. Intragastric pH with oral vs intravenous bolus plus infusion proton-pump inhibitor therapy in patients with bleeding ulcers. Gastroenterology. 2008;134:1836-1841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 79] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Yang E, Kim S, Kim B, Kim B, Kim Y, Park SS, Song GS, Yu KS, Jang IJ, Lee S. Night-time gastric acid suppression by tegoprazan compared to vonoprazan or esomeprazole. Br J Clin Pharmacol. 2022;88:3288-3296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 15. | Rosenstock SJ, Møller MH, Larsson H, Johnsen SP, Madsen AH, Bendix J, Adamsen S, Jensen AG, Zimmermann-Nielsen E, Nielsen AS, Kallehave F, Oxholm D, Skarbye M, Jølving LR, Jørgensen HS, Schaffalitzky de Muckadell OB, Thomsen RW. Improving quality of care in peptic ulcer bleeding: nationwide cohort study of 13,498 consecutive patients in the Danish Clinical Register of Emergency Surgery. Am J Gastroenterol. 2013;108:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Laursen SB, Stanley AJ, Laine L, Schaffalitzky de Muckadell OB. Rebleeding in peptic ulcer bleeding - a nationwide cohort study of 19,537 patients. Scand J Gastroenterol. 2022;57:1423-1429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Kim JS, Kim BW, Park SM, Shim KN, Jeon SW, Kim SW, Lee YC, Moon HS, Lee SH, Jung WT, Kim JI, Kim KO, Park JJ, Chung WC, Kim JH, Baik GH, Oh JH, Kim SM, Kim HS, Yang CH, Jung JT, Lim CH, Song HJ, Kim YS, Kim GH, Kim JH, Chung JI, Lee JH, Choi MH, Choi JK. Factors Associated with Rebleeding in Patients with Peptic Ulcer Bleeding: Analysis of the Korean Peptic Ulcer Bleeding (K-PUB) Study. Gut Liver. 2018;12:271-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Kagawa T, Iwamuro M, Ishikawa S, Ishida M, Kuraoka S, Sasaki K, Sakakihara I, Izumikawa K, Yamamoto K, Takahashi S, Tanaka S, Matsuura M, Hasui T, Wato M, Inaba T. Vonoprazan prevents bleeding from endoscopic submucosal dissection-induced gastric ulcers. Aliment Pharmacol Ther. 2016;44:583-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Chiu PW, Ng EK, Cheung FK, Chan FK, Leung WK, Wu JC, Wong VW, Yung MY, Tsoi K, Lau JY, Sung JJ, Chung SS. Predicting mortality in patients with bleeding peptic ulcers after therapeutic endoscopy. Clin Gastroenterol Hepatol. 2009;7:311-6; quiz 253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 20. | Namburar S, von Renteln D, Damianos J, Bradish L, Barrett J, Aguilera-Fish A, Cushman-Roisin B, Pohl H. Estimating the environmental impact of disposable endoscopic equipment and endoscopes. Gut. 2022;71:1326-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/