Published online Jun 16, 2025. doi: 10.4253/wjge.v17.i6.104539
Revised: March 18, 2025
Accepted: May 7, 2025
Published online: June 16, 2025
Processing time: 167 Days and 21 Hours
Neuroendocrine tumors (NETs) are rare malignancies arising from the diffuse neuroendocrine system, often in the gastroenteropancreatic (GEP) tract. GEP-NETs, primarily involving the intestines (50%) and pancreas (30%), may occa
To evaluate the diagnostic role of EUS in characterizing GEP-NETs based on clinical, histopathological, tumor grading, and site-specific differences.
This single-center retrospective descriptive study was conducted at Aga Khan University Hospital, Karachi, a tertiary care hospital, from January 2021 to December 2023. Fourteen adult patients (≥ 18 years) with suspected NETs who underwent EUS and were diagnosed via histopathology were included. Data on demographics, clinical features, radiological findings, and histopathological characteristics were collected. Descriptive analysis was performed using SPSS version 23, with descriptive statistics expressed as means ± SD for continuous variables and frequencies/percentages for categorical data.
A total of 14 adult GEP-NETs patients who underwent EUS were included, with a mean age of 52 ± 14 years and the majority being male (71.4%). Common clinical presentations included weight loss (85.7%) and abdominal pain (78.6%). Computed tomography scans were performed in 92.9% of cases, with pancreatic masses detected in 42.9% of patients. EUS-guided fine needle biopsy (FNB) had a 100% diagnostic yield. The pancreas was the most common tumor site (57.1%). Histopathology revealed 78.6% of cases as well-differentiated NETs with 42.9% being grade II. Metastases were seen in 57.1% of patients, with the liver being the most common site. Surgical interventions were performed in 28.6% of patients, and all patients were alive at the time of study analysis.
EUS, with accurate imaging and effective EUS-FNB, is the gold standard for GEP-NET diagnosis, aiding tumor assessment and prognosis. Larger studies are needed to validate its impact on management outcomes.
Core Tip: Despite its proven diagnostic utility, data on endoscopic ultrasonography (EUS) in resource-constrained settings remains limited. This study evaluates the role of EUS in diagnosing gastroenteropancreatic (GEP) neuroendocrine tumors (NETs) at a tertiary hospital in Pakistan. Among 14 patients, EUS-guided fine-needle biopsy achieved a 100% diagnostic yield, with the pancreas as the most common tumor site (57.1%). Histopathology revealed 78.6% as well-differentiated NETs, and metastases were observed in 57.1% of cases, primarily to the liver. EUS proved invaluable in detecting small lesions, assessing tumor depth, and guiding management, reinforcing its role as the gold standard for GEP-NET diagnosis in low-resource settings.
- Citation: Ayesha S, Karim MM, Shahid AH, Rehman AU, Uddin Z, Abid S. Diagnostic role of endoscopic ultrasonography in defining the clinical features and histopathological spectrum of gastroenteropancreatic neuroendocrine tumors. World J Gastrointest Endosc 2025; 17(6): 104539
- URL: https://www.wjgnet.com/1948-5190/full/v17/i6/104539.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i6.104539
Neuroendocrine tumors (NETs) are a diverse group of malignancies that originate from the secretory cells of the diffuse neuroendocrine system[1]. Generally, NETs are rare, slow-growing tumors constituting 0.5% of all malignancies and 2% of total malignancies related to the gastrointestinal tract (GI). However, depending on the tumor’s location, extent, and classification, their growth rate can sometimes be rapid[2].
NETs usually arise from the gastroenteropancreatic (GEP) tract and the bronchopulmonary tree. Based on their site of origin, NETs may exhibit distinct clinical behaviors. One classification, based on embryonic derivation, distinguishes between foregut (gastroduodenal), midgut (jejunal, ileal, and cecal), and hindgut (distal colonic and rectal) tumors[1].
Mostly, GEP-NETs involve the intestines and pancreas, comprising around 50% and 30% of cases, respectively. GEP-NETs constitute approximately 66.7% of all neuroendocrine neoplasm cases[3,4]. Most GEP-NETs, around 95%, occur sporadically, while 5% of cases are part of syndromes such as multiple endocrine neoplasia type 1, neurofibromatosis type 1, and von Hippel–Lindau syndrome[4]. Majority of the GEP-NETs cases may present as non-functioning, while a few exist as hormonally functioning tumors with symptoms related to the hypersecretion of peptides and biogenic amines, causing characteristic hormonal syndromes[2,5]. Intestinal and pancreatic NETs are functional, secreting hormones in only 20% and 10%-30% of cases, respectively[3].
Neuroendocrine cells have chromogranin A (CGA), synaptophysin and neuron-specific enolase in their cytoplasmic dense core granules, which are useful for diagnosing these tumors. Ki-67 index and mitotic index correlate with cellular proliferation and are essential for disease prognosis[2].
A significant number of GEP-NETs are now diagnosed at early and asymptomatic stages. It has been reported that the incidence and prevalence of NETs have been increasing[2]. The considerable rise in the incidence of GEP-NETs over the last few decades is most likely due to the evolving utilization of endoscopy and other advanced modalities[6].
Computed tomography (CT) scans are crucial in detecting pancreatic NETs with a sensitivity ranging from 64% to 82%, however it struggles to detect lesions smaller than 1 cm and poses a risk of potential complications[7,8]. Magnetic resonance imaging offers superior imaging quality with a sensitivity of 90%, yet its availability and interpretation rely heavily on the operator[9]. Conventionally, such lesions are biopsied using transabdominal ultrasound, which limits detection and precise staging, especially for pancreatic and small intestine NETs[8]. Whereas endoscopic ultrasonography (EUS) is shown to be highly accurate in visualizing these small active NETs that may not be visible on CT scans or transabdominal ultrasound. It also effectively identifies the presence of multiple lesions in individuals diagnosed with MEN-1 syndrome[10]. The availability of EUS plays a major role in the evaluation, diagnosis and management of these tumors. EUS allows for the assessment of the depth of GI-NETs, presence of locoregional lymph nodes and improved detection of pancreatic NETs[6].
This study evaluates the role of EUS in diagnosing GEP-NETs, exploring distinct clinical presentation, histopathological features, tumor grading and site-specific differences within the GI, in patients presenting to our tertiary care hospital in a low- and middle-income country, Pakistan.
A single-center retrospective observational study was conducted in the Gastroenterology section of the Department of Medicine at Aga Khan University Hospital (AKUH) in Karachi, Pakistan. The study included all inpatient and outpatient individuals with suspected NETs who underwent EUS for the diagnosis of GEP-NETs from January 2021 to December 2023.
After obtaining approval from the institutional ethical review committee (ERC), patient data were retrieved from medical records of all adult patients of either gender who were clinically and radiologically indicated for EUS, underwent endoscopy, and were diagnosed with NETs through histopathological analysis, irrespective of outcomes. Data for patients under 18 years of age were excluded. The reviewed data included patient demographics, clinical characteristics, findings on radiological imaging and histopathological features. The collected demographic and clinical data encompassed age, gender, signs and symptoms, clinical features and indications for EUS, duration from symptom onset to diagnosis, and any complications associated with the diagnostic procedure.
The diagnostic evaluation comprised endoscopic findings, with histopathological features of the tumors also being included into the data collection procedure. The histopathological features encompassed information regarding the lesion site, histological characteristics, and grade differentiation. Histopathological classification of these tumors was done according to the World Health Organization (WHO) classification. According to the WHO, NETs are categorized into well-differentiated NETs (WD-NETs), poorly differentiated neuroendocrine carcinomas, and mixed neuroendocrine-non-neuroendocrine neoplasms. WD-NETs are further classified as low (G1), intermediate (G2), and high (G3) grade based on the mitotic count per 2 mm² and/or the proliferative index Ki-67, and/or the presence of necrosis[11].
All descriptive analyses were performed using the SPSS version 23. Descriptive data were obtained and expressed as means with standard deviations for continuous variables, provided the data met normality assumptions, as tested by histograms and the Kolmogorov-Smirnov test. The distribution of categorical variables was computed as frequencies and percentages.
This study was approved by the AKUH-ERC, with ethical code number 6874-25427. All patient identifiers were eliminated during data entry, and patient confidentiality was fully and completely respected.
At our center, 437 EUS procedures were performed between January 2021 and December 2023, based on clinical and radiological indications. Of these, 14 patients who underwent EUS and were diagnosed with NETs through histopathological analysis were included in the study. Majority of patient were males 10 (71.4%) while 4 patients (28.6%) were females. All patients were adult above 33 years ranging from 34 to 74 years. The mean age of the patients was 52 ± 14 years. Weight loss was the most common clinical presentation, observed in 12 out of 14 patients (85.7%), followed by abdominal pain, which was reported in 11 patients (78.6%). The mean duration from symptom onset to disease diagnosis was 4 weeks (2 to 8 weeks). The mean size of the lesion was reported as 4.5 ± 2.4 cm, with a range from 0.4 cm to 9.8 cm (Table 1).
| Variable | Frequency (No.) | Percentage (%) |
| Total | 14 | 100.00 |
| Age, mean ± SD | 52.0 ± 14.0 (range 34-74) | |
| Gender | ||
| Male | 10 | 71.40 |
| Female | 4 | 28.60 |
| Clinical presentations | ||
| Abdominal pain | 11 | 78.60 |
| Weight loss | 12 | 85.70 |
| Jaundice | 2 | 14.30 |
| Generalized weakness | 1 | 7.10 |
| Dyspepsia | 1 | 7.10 |
| Size of lesion, mean ± SD | 4.5 ± 2.4 (range 0.4-9.8) | |
| Duration from symptom onset to disease diagnosis, weeks | 4 (range 2-8) | |
| Metastatic type of tumor | ||
| Yes | 8 | 57.10 |
| No | 6 | 42.90 |
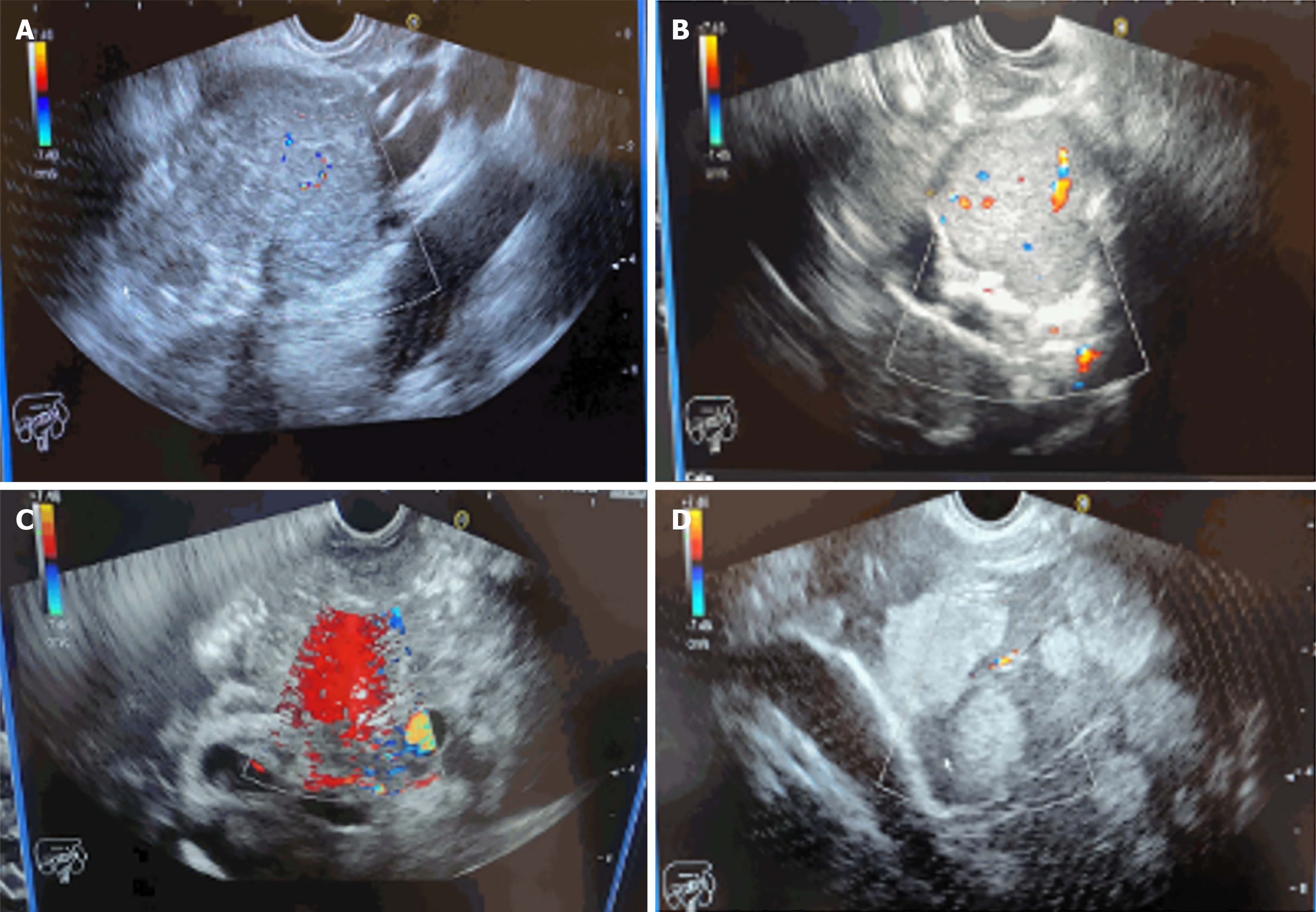
Initially, 13 out of 14 patients (92.9%) underwent CT scans, while the remaining 1 patient underwent esophagogastroduodenoscopy (EGD). Lesions were successfully detected in all patients, indicating the need for EUS to be performed for a definitive diagnosis. CT scan findings showed a pancreatic mass in 6 out of 14 patients (42.9%). Additionally, pancreatic cyst was detected in 1 patient (7.1%). Among the 6 patients with detected pancreatic mass, liver metastatic lesions and lymphadenopathy were each observed in 1 patient (7.1%). In one patient (7.1%) who underwent EGD, an extrinsic mass compressing the pyloric area was detected. Subsequently, all patients underwent EUS guided fine needle biopsy (FNB) procedure in conscious state without any complications and achieved a 100% diagnostic yield (Table 2, Figure 1).
| Variable | Frequency (No.) | Percentage (%) |
| Total | 14 | 100.0 |
| CT scan status | ||
| Yes | 13 | 92.9 |
| No | 1 | 7.1 |
| CT scan successfully identifies lesion | ||
| Yes | 13 | 100.0 |
| EGD | ||
| Yes | 1 | 7.1 |
| No | 13 | 92.9 |
| Indication for EUS based on CT and EGD findings | ||
| Pancreatic mass | 6 | 42.9 |
| Pancreatic cyst | 1 | 7.1 |
| Pancreatic tail lesion and ductal dilation | 1 | 7.1 |
| Porta hepatis mass | 2 | 14.3 |
| Paraduodenal lesion | 1 | 7.1 |
| Liver lesion | 1 | 7.1 |
| Mediastinal lymphadenopathy | 1 | 7.1 |
| Extrinsic mass compressing pyloric area | 1 | 7.1 |
| EUS diagnosis | ||
| EUS guided FNB | 14 | 100.0 |
| Complications | 0 | 0.0 |
| EUS procedure’s setting | ||
| Endoscopy suite | 13 | 92.9 |
| In-patient | 1 | 7.1 |
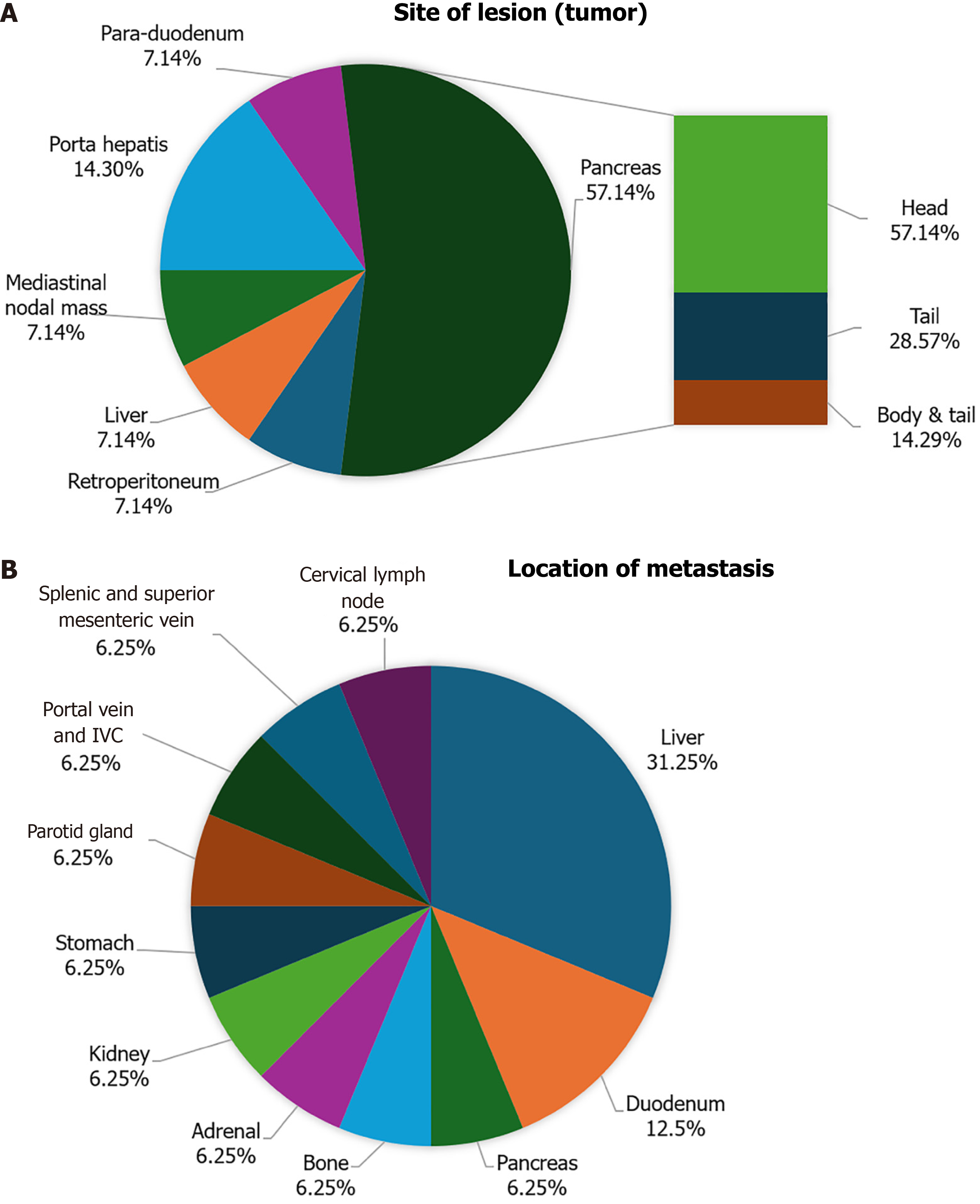
In our study, the pancreas was identified as the most common tumor site, with 8 patients (57.1%) affected. This was followed by porta hepatis, where 2 patients (14.3%) had tumors (Figure 2A). Among the patients diagnosed with pancreatic tumors, 5 out of 7 patients (57.1%) had lesions in the head of the pancreas, whereas 2 patients (28.6%) had lesions in the pancreatic tail, and 1 patient (14.3%) had lesions in both the body and tail of the pancreas. Out of 14 patients, 8 patients (57.1%) had GEP-NETs that metastasized to other sites. Among these tumors, the most common site of metastasis was the liver, with 5 patients (35.7%) affected, followed by the duodenum, which was affected in 2 patients (14.3%; Figure 2B).
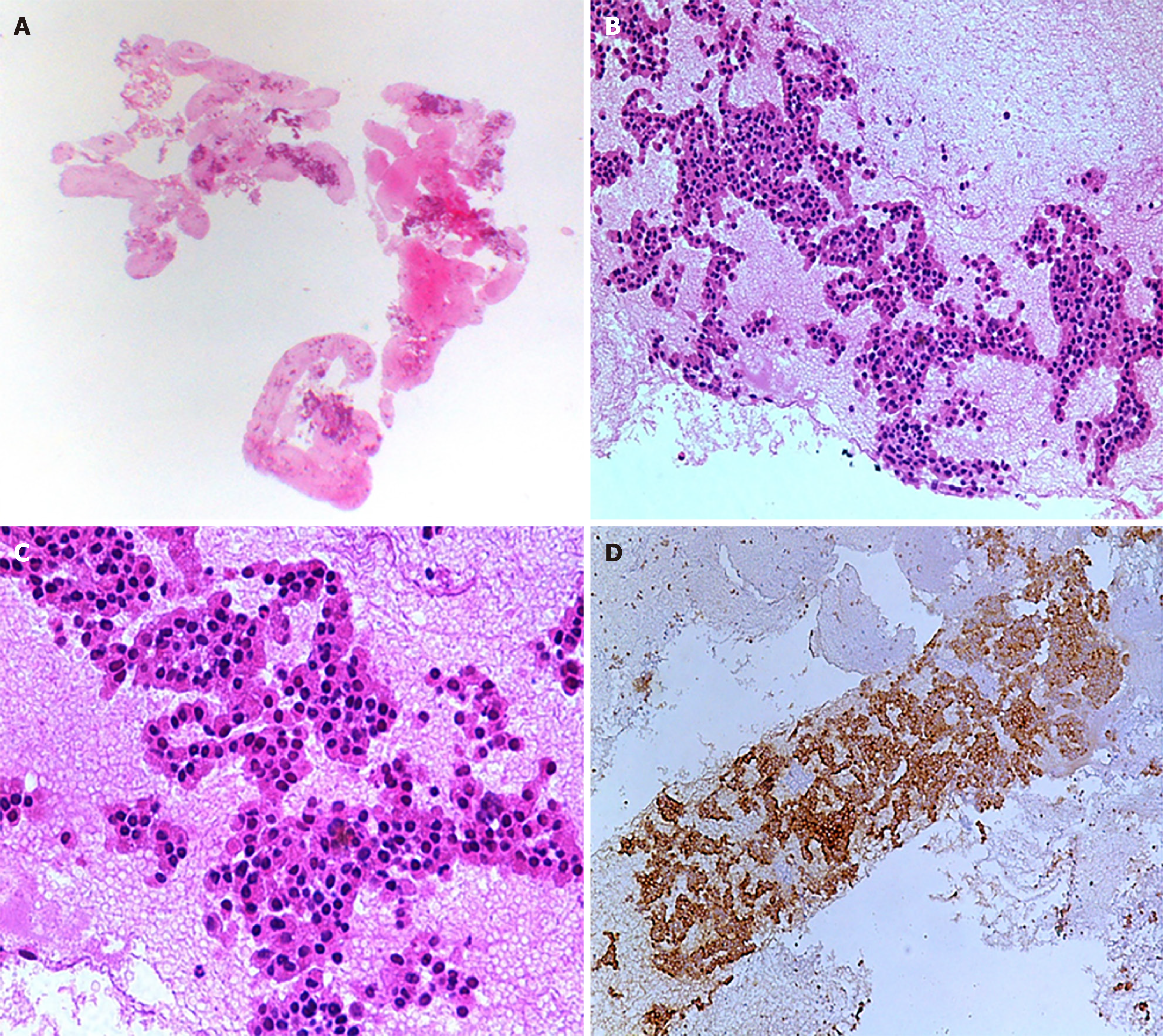
Histopathological diagnosis was established utilizing immunohistochemistry (IHC) and morphological evaluation (Figure 3). Out of 14 patients, tumor cells from 13 patients (92.9%) were found to be positive for cytokeratin AE1/AE3 staining, while synaptophysin staining was positive in all 14 patients. Among 6 patients (42.9%), CGA staining was performed, and 5 out of these 6 patients (35.7%) showed positive results. The Ki-67 index was assessed in all 14 patients, with 8 graded as G2 (57.2%), 3 as G3 (21.4%), and 3 as G1 (21.4%). Final histopathology analysis showed a higher prevalence of WD-NETs, found in 11 out of 14 patients (78.6%). In contrast, poorly differentiated tumors were seen in 3 patients (21.4%). Among the WD-NETs, further subclassification into grades showed that the majority were grade II, observed in 6 out of 14 patients (42.9%), followed by grade I in 4 patients (28.6%) and grade III in 1 patient (7.1%). For the poorly differentiated tumors, the majority were of the small cell type, found in 2 patients (14.3%), with one case (7.1%) being of the large cell type (Table 3).
| Variable | Frequency (No.) | Percentage (%) |
| Total | 14 | 100.0 |
| Histopathology types | ||
| Well differentiated tumor | 11 | 78.6 |
| Grade 1 | 4 | 28.6 |
| Grade 2 | 6 | 42.9 |
| Grade 3 | 1 | 7.1 |
| Poorly differentiated tumor | 3 | 21.4 |
| Small cell | 2 | 14.3 |
| Large cell | 1 | 7.1 |
| Cytokeratin AE1/AE3 | ||
| Positive | 13 | 92.9 |
| Ki-67 | ||
| Positive | 14 | 100.0 |
| G1 (≤ 2%) | 3 | 21.4 |
| G2 (3%-20%) | 8 | 57.2 |
| G3 (> 20%) | 3 | 21.4 |
| Synaptophysin | ||
| Positive | 14 | 100.0 |
| Chromogranin A | ||
| Positive | 5 | 35.7 |
| Negative | 1 | 7.1 |
| Not done | 8 | 57.2 |
Out of 15 patients, 4 (28.6%) underwent distinct surgical interventions. Specifically, 1 patient (7.1%) underwent a Whipple procedure, another underwent paraganglioma excision with right adrenalectomy, a third patient had an excision of a deep cervical lymph node, and the fourth patient underwent a laparotomy with radical excision of a paraduodenal NET. Out of the 14 patients, 2 (14.3%) underwent chemotherapy. All patients were alive at the time of study analysis (Table 4).
| Variable | Frequency (No.) | Percentage (%) |
| Total | 14 | 100.0 |
| Surgical procedure | ||
| Yes | 4 | 28.6 |
| No | 10 | 71.4 |
| Surgical procedure type | ||
| Whipple procedure | 1 | 7.1 |
| Paraganglioma excision with right adrenalectomy | 1 | 7.1 |
| Excision of deep cervical lymph node | 1 | 7.1 |
| Laparotomy and radical excision of paraduodenal neuroendocrine tumor | 1 | 7.1 |
| Additional management type | ||
| Chemotherapy | 2 | 14.3 |
| Palliative care | 1 | 7.1 |
| Vital status | ||
| Alive | 14 | 100.0 |
NETs are slow-growing neoplasms that are uncommon and are distinct in their histological, biological, and clinical characteristics. The incidence of NETs has been increasing over the last few decades[2]. Patients with NETs may present with symptoms due to primary tumor invasion, metastasis, or secretion of hormonally active substances by the tumor[5]. These tumors are most often diagnosed by endoscopists and are frequently recognized as incidental findings. The primary sites for GEP-NETs include the pancreas, intestine, and stomach[12]. Our study reports on 14 patients diagnosed with GEP-NETs who presented to the AKUH, a tertiary care center in Pakistan, over a three-year period. In a descriptive study conducted at two tertiary care hospitals in Brazil, 27 patients with suspected NETs were reported[13]. In another study, only 18 patients with GI-NETs were reported between 1993 and 2011[14]. Our center has reported a significant number of NET cases compared to other institutions, likely due to our role as a tertiary referral center for NETs.
GEP-NETs are common among adults in their 50s and 60s[15]. Similarly, the average age of our patients in this study was 52 years. A significantly increased incidence of GEP-NETs is observed in males, accounting for 71.4% of cases, which aligns with a previous study where 56.3% of the patients diagnosed with GEP-NETs were male[16]. In our study, the male-to-female ratio was 2.5 (10:4), which is significantly higher compared to the 1.1 ratio reported in the previous study[17]. Additionally, 64.3% of our GEP-NET patients initially presented with complaints of abdominal pain accompanied by weight loss, which were also common presenting complaints reported in previous studies[15]. In our study, all patients were symptomatic, leading to a significantly shorter average time from the onset of these specific symptoms to diagnosis, which was around 28 days, compared to 388 days in the previous study[18]. However, this timeframe is significantly comparable to a study conducted at a larger German tertiary care hospital, where the median time from symptoms to diagnosis was reported as 19.5 days[19].
GEP-NETs are among the most prevalent tumors related to the GI tract, primarily including those of the stomach and pancreas cases[20]. In our study, the most common site of the tumor was the pancreas, reported in 57.1% of patients, which is consistent with a previous study that found the pancreas to be the most frequent tumor site, constituting 37% of all cases[21]. Additionally, 57.1% of our patients had distant metastatic lesions at the time of diagnosis. This finding aligns with the literature, which indicates that nearly 50% of GEP-NET cases present with distant metastatic lesions[3]. Among the metastatic sites, the liver was the most common, with metastases occurring in around 35.7% of cases in our study. A previous study reported liver metastases rates ranging between 25% and 39%[22]. In our study, the average size of the lesions was found to be 4.5 ± 2.4 cm. It has been previously established that distant metastasis is associated with lesions greater than 3 cm[23]. Similarly, in our study, all patients with lesions larger than 3 cm were diagnosed with metastatic tumors. Interestingly, some patients with non-metastatic tumors also had lesions greater than 3 cm.
The primary diagnostic modalities relied mainly on CT, with PET scan performed in only one patient as the combined imaging modality. However, an upper GI endoscopy was initially performed on a patient who presented with chronic dyspepsia and weight loss, revealing an extrinsic mass compressing pyloric area, for which EUS was subsequently performed. PET scanning was found to be an accurate imaging modality for detecting distant metastases, with an accuracy rate of 90.4%[24]. In one patient who underwent a PET scan, a distant metastatic lesion was not found. The reported sensitivity and specificity of CT scanning range from 61%-93% and 71%-100%, respectively[25]. In our study, all patients underwent a CT scan except for one. A previous study reported that detecting tumor lesions smaller than 2 cm via CT scanning was challenging. However, in our study, CT scans successfully detected lesions smaller than 2 cm in two patients. Despite this, the exact depth, nature and extent of the tumors, along with regional lymph node involvement, were not identified by CT scanning, which is essential for tumor staging. Previous studies have also reported that CT scan sensitivity is relatively poor for detecting bone and hepatic metastases, at around 61% and 79%, respectively[25].
EUS, a combined technique of endoscopy and ultrasound, is utilized to address limitations such as tumor localization, invasion, and staging[26]. In a previous study, the diagnostic sensitivity for EUS fine-needle aspiration, EUS-FNB, and the combination of both methods was reported as 88.4%, 94.3%, and 100%, respectively[23]. In our study, all patients who underwent EUS guided FNB procedures had tumor lesions successfully detected, achieving a 100% diagnostic yield. This is consistent with previous studies reporting 90% sensitivity for EUS and showing that all cases of NETs were detected by EUS[23,27]. Previous studies have found that EUS is suitable for detecting lesions smaller than 2 cm, which is supported by our study's findings[28]. Tumor lesions of 1.2 cm, and as small as 4 mm in two patients were successfully detected by EUS. In one study, the sensitivity of EUS for detecting pancreatic NETs in the head, body, and tail was reported as 92.6%, 78.9%, and 40%, respectively, making it the most sensitive modality for detecting lesions in the pancreatic head[9]. Our study supports these findings, as the most common lesion site in pancreatic NETs was the head of the pancreas, reported in 57.1% of all pancreatic NET cases.
In addition to imaging modalities, EUS-guided FNB was found to be essential for histopathological and IHC analysis by providing sufficient sampling tissue with preserved architecture, thereby enhancing diagnostic yield[29]. In IHC analysis, all our patients tested positive for synaptophysin staining, while cytokeratin AE1/AE3 staining was positive in 92.9% of cases. This finding also supported by the previous studies reporting positivity for synaptophysin and other IHC markers, which are crucial for a definitive diagnosis. In our study, the Ki-67 index was assessed in all 14 patients, with most having G2 tumors in 57.2% of the cases, whereas a previous study found most tumors to be G1/G2. CGA is considered the most specific IHC marker, providing insight into tumor prognosis[9]. High levels of CGA are associated with poor tumor progression, including increased tumor burden and shorter survival among these patients[30]. CGA staining was performed in 42.9% of cases, showing a positive result in only 35.7% of total cases. 21.4% of patients were diagnosed with poorly differentiated tumors, which were associated with a Ki-67 index greater than 10. According to the literature, the Ki-67 index was found to be greater than 20% in patients with poorly differentiated tumors[31]. In one study, 91.6% of patients were found to be alive and healthy during the follow-up period[23]. Similarly, our study showed an overall favorable prognosis, with 100% of patients alive and healthy during the follow-up period.
In our study, four patients underwent distinct surgical resection procedures. Of these, only two were previously diagnosed with metastatic lesions. In a previous study, the five-year overall survival (OS) rates for complete, incomplete, and unresectable disease were found to be 94%, 79%, and 43%, respectively[32]. In a study related to pancreatic NETs, the prognosis was found to be good after resection, with a recurrence rate of 21%-42%[23]. Larger tumor size, high Ki-67 index, and tumor grading were associated with shorter OS, progression-free survival (PFS), and recurrence-free survival (RFS)[21]. In our study, all four patients who underwent surgical procedures had lesions larger than 3 cm but not exceeding 8 cm. Among these patients, the Ki-67 index was not reported to be more than 5%, except in one case where it was found to be 90%, categorizing the tumor as poorly differentiated NETs. All patients who underwent surgical resection procedures during our study period experienced RFS. However, longitudinal follow-up is required in the future to make a definitive conclusion regarding long-term OS, PFS, and RFS among these patients.
It was reported that adjuvant therapy was not recommended for patients with completely resected tumors in studies conducted earlier. However, chemotherapy was found to be suitable for poorly differentiated NETs[20]. Therefore, two out of the three patients with poorly differentiated NETs underwent chemotherapy. Of these two patients, one had also previously undergone excision of a deep cervical lymph node. The remaining one patient with poorly differentiated NETs received palliative therapy.
In our study, EUS emerged as the gold standard for diagnosing GEP-NETs, providing precise imaging and effective EUS-FNB for histopathological and IHC analysis. We highlighted its utility and safety in resource-limited settings, advocating a shift from conventional transabdominal or CT guided biopsy methods to this minimally invasive, guideline-recommended EUS approach for improved tumor localization, staging, and prognosis prediction. However, a larger comparative prospective study with longitudinal follow-up and appropriate statistical analysis is needed in the future. Such a study would help determine prognostic factors for GEP-NET progression and explore the association between EUS-FNB findings and their direct impact on management plan.
| 1. | Cives M, Strosberg JR. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J Clin. 2018;68:471-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 424] [Article Influence: 53.0] [Reference Citation Analysis (1)] |
| 2. | Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020;12:791-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 153] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (20)] |
| 3. | Fernandez CJ, Agarwal M, Pottakkat B, Haroon NN, George AS, Pappachan JM. Gastroenteropancreatic neuroendocrine neoplasms: A clinical snapshot. World J Gastrointest Surg. 2021;13:231-255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (3)] |
| 4. | De Muzio F, Pellegrino F, Fusco R, Tafuto S, Scaglione M, Ottaiano A, Petrillo A, Izzo F, Granata V. Prognostic Assessment of Gastropancreatic Neuroendocrine Neoplasm: Prospects and Limits of Radiomics. Diagnostics (Basel). 2023;13:2877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 5. | Yazdanpanah O, Surapaneni S, Shanah L, Kabashneh S. Diagnosis and Management of Gastrointestinal Neuroendocrine Tumors: A Comprehensive Literature Review. Cureus. 2021;13:e14006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 6. | Nabi Z, Lakhtakia S, Reddy DN. Current status of the role of endoscopy in evaluation and management of gastrointestinal and pancreatic neuroendocrine tumors. Indian J Gastroenterol. 2023;42:158-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, Scheiman JM. Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol. 2000;95:2271-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 235] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Walczyk J, Sowa-Staszczak A. Diagnostic imaging of gastrointestinal neuroendocrine neoplasms with a focus on ultrasound. J Ultrason. 2019;19:228-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 9. | Haghighi S, Molaei M, Foroughi F, Foroutan M, Dabiri R, Habibi E, Mohammad Alizadeh AH. Role of endoscopic ultrasound in evaluation of pancreatic neuroendocrine tumors--report of 22 cases from a tertiary center in Iran. Asian Pac J Cancer Prev. 2012;13:4537-4540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Wang J, Benhammou JN, Ghassemi K, Kim S, Sedarat A, Farrell J, Pisegna JR. Endoscopic Ultrasound-Guided Fine Needle Aspiration Accurately Diagnoses Smaller Pancreatic Neuroendocrine Tumors Compared To Computer Tomography-Guided Fine Needle Aspiration. J Gastroenterol Pancreatol Liver Disord. 2017;4:1-7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Borga C, Businello G, Murgioni S, Bergamo F, Martini C, De Carlo E, Trevellin E, Vettor R, Fassan M. Treatment personalization in gastrointestinal neuroendocrine tumors. Curr Treat Options Oncol. 2021;22:29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Chin JL, O'Toole D. Diagnosis and Management of Upper Gastrointestinal Neuroendocrine Tumors. Clin Endosc. 2017;50:520-529. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 13. | Costa RDD, Kemp R, Santos JSD, Costa DAPD, Ardengh JC, Ribas-Filho JM, Ribas CAPM. The Role of Conventional Echoendoscopy (EUS) in Therapeutic Decisions in Patients with Neuroendocrine Gastrointestinal Tumors. Arq Bras Cir Dig. 2020;33:e1512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | León de Zayas B, del Olmo García, Ramos Prol A, Argente Pla M, Muñoz Vicente M, Gilsanz Peral A, Merino Torres J. Neuroendocrine tumors of the gastrointestinal tract: a descriptive study. Endocr Abstr. 2012;29:880. |
| 15. | Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991-1002. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 405] [Cited by in RCA: 529] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 16. | Rafique Z, Qasim A, Zafar A, Ali S, Chughtai AS, Atiq A. Clinicopathological Features of Neuroendocrine Tumors in Gastroenteropancreatic Tract: A Single Center Study. Cureus. 2022;14:e27384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 17. | Bazarbashi S, Aseafan M, Elgazzar T, Alkhayat M, Alghabban A, Abdelgawad MI, Alshamsan B, Alshibany A, Elhassan T, Aljubran A, Alzahrani A, Alhindi H, Raef H. Characteristics and treatment results of patients with gastroenteropancreatic neuroendocrine tumors in a tertiary care centre. BMC Endocr Disord. 2023;23:74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Koca E, Koch C, Husmann G, Bojunga J. Time from first symptoms to diagnosis in GEP-NET patients: Results from a large German tertiary referral center. J Clin Oncol. 2020;38:610. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Koch C, Koca E, Filmann N, Husmann G, Bojunga J. Time from first tumor manifestation to diagnosis in patients with GEP-NET: Results from a large German tertiary referral center. Medicine (Baltimore). 2021;100:e27276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Díez M, Teulé A, Salazar R. Gastroenteropancreatic neuroendocrine tumors: diagnosis and treatment. Ann Gastroenterol. 2013;26:29-36. [PubMed] |
| 21. | Aliyev A, Ibrahimli A, Mammadov E, Azizova N, Huseynli T, Babazada I, Hajiyev A, Samadov E, Jafarova A. Gastroenteropancreatic Neuroendocrine Tumors: Demographical, Clinicopathological, and Survival Data From Azerbaijan. Eurasian J Med Oncol. 2024;8:130-134. [DOI] [Full Text] |
| 22. | Hermans BCM, de Vos-Geelen J, Derks JL, Latten L, Liem IH, van der Zwan JM, Speel EM, Dercksen MW, Dingemans AC. Unique Metastatic Patterns in Neuroendocrine Neoplasms of Different Primary Origin. Neuroendocrinology. 2021;111:1111-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Akhlaq K, Khan H, Ali Z, Atiq M, Riyaz S, Raja U, Kiani A. Pancreatic Neuroendocrine Tumors: Spectrum of Clinical Presentation from a Tertiary Referral Center in Pakistan. Middle East J Cancer. 2024;15:145-152. [DOI] [Full Text] |
| 24. | Sharma P, Singh H, Bal C, Kumar R. PET/CT imaging of neuroendocrine tumors with (68)Gallium-labeled somatostatin analogues: An overview and single institutional experience from India. Indian J Nucl Med. 2014;29:2-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Pavel M, Öberg K, Falconi M, Krenning EP, Sundin A, Perren A, Berruti A; ESMO Guidelines Committee. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:844-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 465] [Cited by in RCA: 793] [Article Influence: 132.2] [Reference Citation Analysis (0)] |
| 26. | Kim MK. Endoscopic ultrasound in gastroenteropancreatic neuroendocrine tumors. Gut Liver. 2012;6:405-410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Reddy Y, Willert RP. Endoscopic ultrasound: what is it and when should it be used? Clin Med (Lond). 2009;9:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Rogers HK, Shah SL. Role of Endoscopic Ultrasound in Pancreatic Cancer Diagnosis and Management. Diagnostics (Basel). 2024;14:1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 29. | Constantinescu A, Ilie-Stan CM, Şandru V, Ungureanu BS, Gheonea DI, Ciurea T, Plotogea OM, Pavel C, Enache V, Munteanu MA, Constantinescu G. A morphological and immunohistochemical study of the endoscopic ultrasound-fine-needle biopsy samples from solid pancreatic masses: a single center study. Rom J Morphol Embryol. 2021;62:723-731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Gut P, Czarnywojtek A, Fischbach J, Bączyk M, Ziemnicka K, Wrotkowska E, Gryczyńska M, Ruchała M. Chromogranin A - unspecific neuroendocrine marker. Clinical utility and potential diagnostic pitfalls. Arch Med Sci. 2016;12:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Vyas M, Tang LH, Rekhtman N, Klimstra DS. Alterations in Ki67 Labeling Following Treatment of Poorly Differentiated Neuroendocrine Carcinomas: A Potential Diagnostic Pitfall. Am J Surg Pathol. 2021;45:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Ali J, Rahat A, Shah MH, Sajjad M, Malik I, Ikram SI, Ul Qamar MF. The Clinicopathologic Characteristics and Outcomes of Gastroentero-pancreatic Neuroendocrine Tumors - Experience from A Tertiary Cancer Center. Gulf J Oncolog. 2022;1:7-14. [PubMed] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/