Published online Dec 16, 2025. doi: 10.4253/wjge.v17.i12.110432
Revised: July 12, 2025
Accepted: October 17, 2025
Published online: December 16, 2025
Processing time: 193 Days and 9.3 Hours
Endoscopic biliary drainage for malignant hilar biliary obstruction (MHBO) re
To compare different stent types and drainage strategies, including the use of adjunctive therapies, in patients with MHBO treated endoscopically.
We retrospectively analyzed 164 patients with MHBO (Bismuth types 3–4) who underwent exclusive endoscopic drainage. Patients were grouped by stent type—uncovered self-expandable metal stents (UCSEMS), bilateral plastic stents, or a mixed approach (fully covered self-expandable metal stents + plastic)—as well as by drainage strategy (unilateral/bilateral) and use of radiofrequency ablation (RFA) or chemotherapy.
Patients receiving UCSEMS had significantly longer overall survival compared to those with plastic stents or the mixed approach (P < 0.0001). Mean stent occlusion times were 80 days (bilateral plastic), 84.4 days (mixed approach), and 122.5 days (UCSEMS; P < 0.0001). The mean number of ERCP reinterventions was highest in the UCSEMS group (5.4) compared to bilateral plastic (2.5) and mixed approach group (4.5; P < 0.0001). Patients who received RFA or chemotherapy had sign
Bilateral UCSEMS stenting appears most effective for palliative treatment of MHBO. Adjunctive use of RFA and chemotherapy may further enhance survival, supporting a personalized, multidisciplinary approach.
Core Tip: This retrospective cohort study analyzed 164 patients with malignant hilar biliary obstruction (MHBO) treated through endoscopic intervention using different stent types and drainage strategies. Uncovered self-expandable metal stents were associated with the longest survival and stent patency. Adjunctive therapies such as radiofrequency ablation and chem
- Citation: Pietrzak J, Pertkiewicz J, Kozieł S, Babski P, Ligocka J, Przybyłkowski A. Endoscopic treatment of malignant hilar biliary obstruction: A retrospective cohort study. World J Gastrointest Endosc 2025; 17(12): 110432
- URL: https://www.wjgnet.com/1948-5190/full/v17/i12/110432.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i12.110432
Malignant hilar biliary obstructions (MHBOs) may result from cholangiocarcinoma, gallbladder carcinoma, hepatocellular carcinoma, pancreatic cancer, or metastatic lymph nodes at the liver hilum. Due to the challenging location, the variability of biliary anatomy, and the hilum’s role as the confluence of the bile ducts, achieving effective drainage requires careful planning and specific considerations. For biliary drainage to be successful, more than 50% of the liver volume must be drained, which often necessitates bilateral drainage[1,2]. A comparison of technical success rates between MHBO (80%-90%)[3] and distal biliary obstruction (95%-98%)[4] highlights the complexity of stenting in MHBO. However, technical difficulty does not always correlate with clinical outcomes in practice. The reported technical success rate of up to 90% in MHBO appears to be overestimated due to the broad definitions used in many studies, which consider any biliary duct cannulation as a success, without specifying critical details. This may overlook essential aspects of effective stenting in MHBO.
Despite guidelines clearly indicating the superiority of bilateral drainage using uncovered self-expandable metal stents (UCSEMS), their placement remains technically demanding for endoscopists[5,6]. Reinterventions may be more difficult compared to the scheduled, regular replacement of plastic stents or fully covered self-expandable metal stents (FCSEMS). Furthermore, with advances in chemotherapy[7] and the development of adjunctive therapies such as radiofrequency ablation (RFA), patients treated with UCSEMS often live beyond the typical occlusion period of these stents.
Currently, two classical approaches to MHBO stenting are used, depending on technical feasibility: Unilateral drainage with plastic stents or UCSEMS, and bilateral drainage using the same stent types[8]. A third approach—bilateral drainage with double FCSEMS—has shown promising results in a few studies but has not yet been widely adopted by specialists[9-13]. To date, no single cohort study has directly compared plastic stents, FCSEMS, and UCSEMS in MHBO patients. This study aimed to evaluate different stent types and adjunctive therapies, focusing on their impact on overall survival (OS), stent patency, frequency of reinterventions, and incidence of complications.
This retrospective analysis covers 164 consecutive patients who were treated between 2016 and 2024 at the Department of Gastroenterology and Internal Medicine, Medical University of Warsaw. The inclusion criteria for the study were a diagnosis of MHBO Bismuth 3 to 4 in a cholangiogram confirmed by two endoscopists, a diagnosis of malignancy confirmed by histopathological examination, or, in the case of hepatocellular carcinoma, typical radiological criteria as per European Association for the Study of the Liver Barcelona 2001 guidelines, endoscopic treatment performed exclusively in our endoscopy unit, and the availability of complete medical records and patient follow-up. Baseline characteristics of the study population, including demographics, cancer etiology, diagnostic approach, number of endoscopic retrograde cholangiopancreatography (ERCP) procedures, and adverse events, are summarized in Table 1.
| Characteristics | Mean ± SD or n (%) | |
| Sex (women/men) | 86 (52.4)/78 (47.6) | |
| Age (year) | 67 ± 65.6 | |
| ≤ 60 | 45 (27.4) | |
| > 60 | 119 (72.6) | |
| Etiology | Cholangiocarcinoma | 102 (62.2) |
| Gallbladder carcinoma | 36 (21.9) | |
| Colorectal cancer - metastases | 19 (11.6) | |
| Breast cancer - metastases | 2 (1.2) | |
| HCC | 2 (1.2) | |
| Non-small-cell lung cancer | 1 (0.6) | |
| Neuroendocrine carcinoma | 1 (0.6) | |
| Pancreatic cancer | 1 (0.6) | |
| Method of obtaining a histopathological result | ERCP | 90 (54.9) |
| Surgery | 50 (30.5) | |
| CT-guided biopsy | 17 (10.4) | |
| USG-guided biopsy | 2 (1.2) | |
| HCC criteria | 2 (1.2) | |
| Percutaneous transhepatic biliary drainage | 1 (0.6) | |
| Paracentesis | 1 (0.6) | |
| Endoscopic ultrasound | 1 (0.6) | |
| Time of progression to the next Bismuth degree (day) | 117.3 ± 83 | |
| Number of ERCP procedures before diagnosis | 1.2 ± 1.0 | |
| Total number of ERCP procedures | 4.0 ± 3.0 | |
| Chemotherapy | 86 (52.4) | |
| Radiofrequency ablation | 26 (15.9) | |
| Patient presenting adverse events other then recurrent biliary obstruction | Pancreatitis | 40 (24.4) |
| Bleeding | 15 (9.1) | |
| Perforation | 2 (1.2) | |
| Liver abscesses | 20 (12.2) | |
| Cholecystitis | 2 (1.2) | |
Patients were excluded from the study if they had incomplete data or were lost to follow-up after endoscopic treatment, lacked histopathological confirmation of malignancy, were treated with surgical resection, or died within one month following stent placement.
The primary endpoint of the study was OS, measured from the date of the first ERCP procedure to the date of death. Secondary endpoints included the incidence of cholangitis post-ERCP, failure rates, other post-procedural complications, total number of procedures, and mean stent patency duration. Cholangitis was defined as an increase in temperature
All ERCP procedures were performed under full anesthesia with endotracheal intubation. The endoscopists performing the procedures were highly experienced, having conducted more than 3000 ERCPs and performing over 300 ERCPs annually. No formal protocol dictated the drainage approach, but bilateral stenting was consistently preferred and performed whenever technically feasible. Unilateral drainage was used only when placement of two stents was not possible due to anatomical or technical constraints. The preferred stenting strategy was bilateral UCSEMS placement whenever technically feasible, followed by FCSEMS and plastic stents. Bilateral plastic stenting was reserved for cases where anatomical constraints or limited ductal space prevented the safe placement of metal stents. Additionally, a temporal trend was observed in our center, with an increasing preference for UCSEMS-based bilateral drainage strategies after 2018, reflecting evolving procedural expertise and availability of equipment. To prevent PEP, 100 mg of rectal diclofenac was administered prior to the procedure. For planned procedures without cholangitis, patients received perioperative antibiotic prophylaxis with ampicillin-sulbactam. In cases of cholangitis, patients received antibiotics based on bile or blood culture results or broad-spectrum empirical therapy initiated earlier. Duodenoscopes used for ERCP were from Olympus Medical Systems Corp., Tokyo, Japan. The stents used included plastic stents (7-10 Fr in diameter and 10-15 cm in length, primarily straight Boston Scientific), uncovered SEMS (6-10 mm in diameter and 8-12 cm in length; Boston Scientific, Cook Medical, MicroTech), and fully covered SEMS (6-10 mm in diameter and 8-12 cm in length; Boston Scientific). All stents were inserted using a transpapillary approach. RFA was performed using the HabibTM EndoHPB catheter (EMcision Ltd, London, United Kingdom) with standard ERCP duodenoscopes, delivering 10 Watts for 90 seconds per cycle.
The study was conducted in accordance with institutional and national ethical standards and with the 1964 Helsinki Declaration and its later amendments.
All statistical analyses were performed using STATISTICA (version 12.0, StatSoft Inc.) and Microsoft Excel. Quantitative variables were described using the mean, standard deviation, median, range, and 95% confidence intervals. Categorical variables were presented as counts and percentages. Normality was assessed with the Shapiro-Wilk test, and variance homogeneity with Levene’s or Brown-Forsythe’s test. Group comparisons used the t-test or Mann-Whitney U test (for two groups), and ANOVA or Kruskal-Wallis test (for multiple groups), with Tukey’s or Dunn’s post hoc tests, re
Patients were categorized into three groups based on the stent implantation strategy: UCSEMS (n = 47), bilateral plastic (n = 61), and mixed approach (n = 55). In the UCSEMS group, two UCSEMS were placed—one into each hepatic duct. In the bilateral plastic group, two plastic stents were placed in the same manner. The mixed group included patients in whom a combination of a FCSEMS and a plastic stent was used. In this approach, the FCSEMS was typically placed in the left hepatic duct, due to its fewer side branches, and the plastic stent in the right duct. Patients were assigned to this group when a mixed stenting strategy was technically feasible—that is, when there was sufficient space for safe and effective FCSEMS placement, and no sectoral ducts were at risk of being obstructed by the stent.
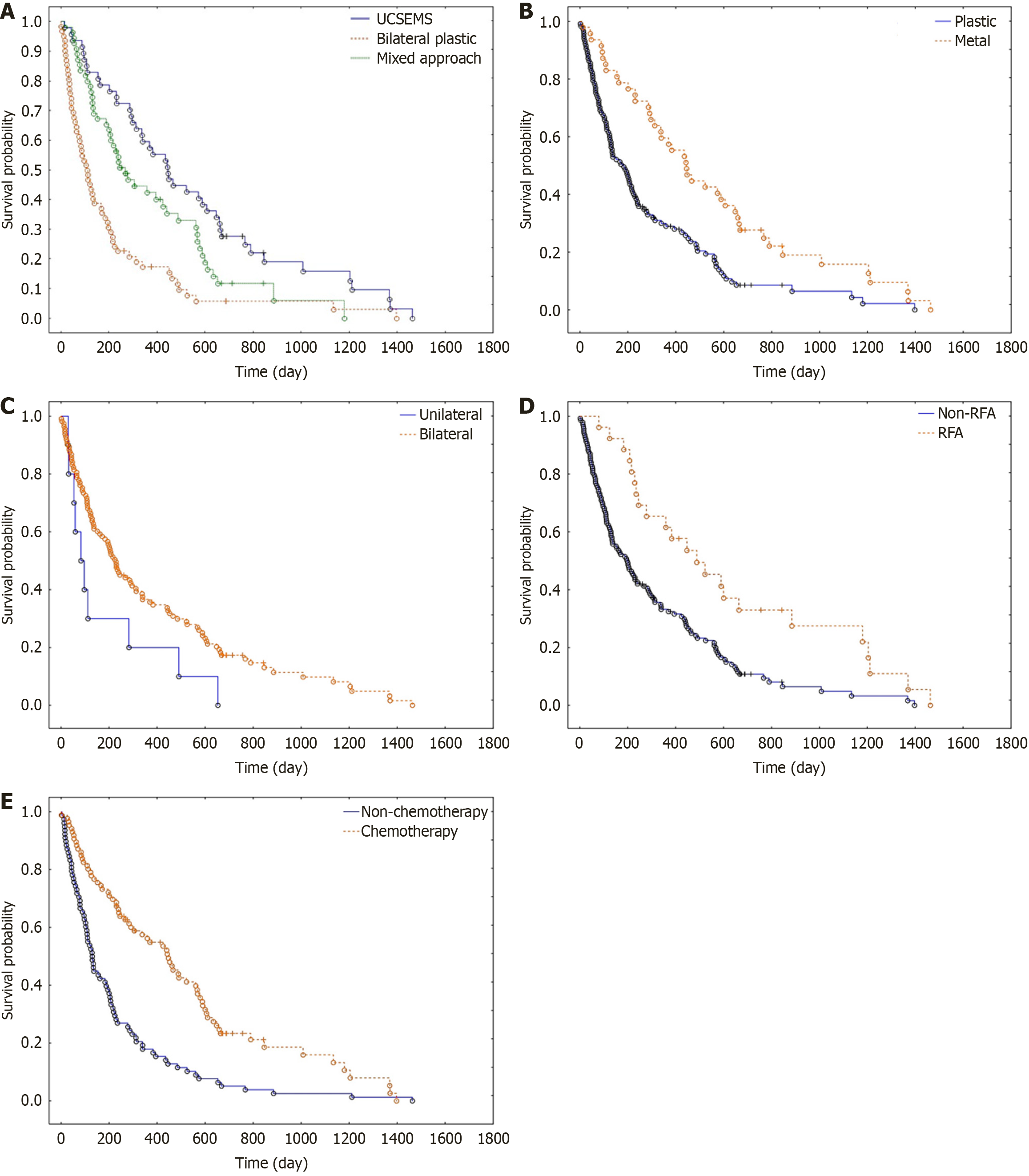
OS: The log-rank test demonstrated significantly higher survival in the UCSEMS group compared to plastic stents (P < 0.0001). The log-rank test also showed significantly higher survival in the UCSEMS group compared to the mixed approach group (P = 0.0242). Additionally, the log-rank test indicated significantly higher survival in the mixed approach group compared to the plastic group (P = 0.0008). The median survival time was 445 days, 110.5 days and 245 days for the UCSEMS, plastic and mixed approach groups, respectively (P < 0.0001; Figure 1A).
Total number of interventions: The mean number of interventions was 5.4 ± 3.7, 2.5 ± 2.1 and 4.5 ± 2.9 in the UCSEMS, bilateral plastic and mixed approach group respectively (P < 0,0001; Table 2).
| Number of ERCP | Number of failures | Episodes of cholangitis | Occlusion time in days | |
| Stent implantation strategy | ||||
| UCSEMS | 5.4 ± 3.7 | 0.7 ± 1.0 | 2.1 ± 2.0 | 122.5 ± 124.8 |
| Plastic | 2.5 ± 2.1 | 0.6 ± 1.1 | 0.8 ± 0.9 | 80.0 ± 99.9 |
| Mixed approach | 4.5 ± 2.9 | 0.5 ± 0.8 | 1.7 ± 1.5 | 84.4 ± 91.3 |
| P value | < 0.0001 | 0.7354 | 0.0001 | 0.0066 |
| Drainage approach | ||||
| Unilateral | 1.9 ± 0.9 | 1.5 ± 1.0 | 0.7 ± 0.8 | 115.6 ± 195.1 |
| Bilateral | 4.0 ± 3.4 | 0.4 ± 0.9 | 1.5 ± 1.7 | 99.4 ± 111.4 |
| P value | 0.0276 | 0.0001 | 0.2412 | 0.2748 |
| Stent type | ||||
| Plastic | 3.4 ± 2.7 | 0.5 ± 1.0 | 1.2 ± 1.3 | 82.1 ± 95.5 |
| Metal | 5.4 ± 3.7 | 0.7 ± 1.0 | 2.1 ± 2.0 | 122.5 ± 124.8 |
| P value | 0.0001 | 0.5364 | 0.0076 | 0.0032 |
| Adjunctive therapy | ||||
| RFA | 7.6 ± 4.2 | 0.5 ± 1.0 | 3.0 ± 2.4 | 79.6 ± 43.7 |
| Non-RFA | 3.3 ± 2.4 | 0.6 ± 1.0 | 1.2 ± 1.2 | 96.3 ± 113.9 |
| P value | < 0.0001 | 0.4589 | 0.0002 | 0.5418 |
| Chemotherapy | 3.1 ± 2.5 | 0.4 ± 0.6 | 1.7 ± 1.7 | 103.6 ± 101.9 |
| Non-chemotherapy | 4.8 ± 3.5 | 0.7 ± 1.2 | 1.2 ± 1.4 | 82.7 ± 109.9 |
| P value | 0.0002 | 0.6782 | 0.0509 | 0.0042 |
Occlusion time: The UCSEMS group exhibited the longest mean occlusion time at 122.5 days. In the mixed approach group (84.4 days), the occlusion time was significantly longer compared to the bilateral plastic group (P = 0.0333). In contrast, in the bilateral plastic group (80.0 days), the occlusion time was significantly shorter compared to the UCSEMS group (P < 0.0001) and the mixed approach group (P = 0.0248; Table 2).
Adverse events: The mean number of cholangitis episodes was 2.1 ± 2.0, 0.8 ± 0.9 and 1.7 ± 1.5 in the UCSEMS, bilateral plastic and mixed approach group (P < 0.0001) respectively. Post-hoc tests showed significantly more episodes of cholangitis in the UCSEMS group relative to the mixed approach group (P = 0.0085). The incidence of acute pancreatitis was observed in 27.7% of patients in the UCSEMS group, 24.2% in the plastic bilateral group, and 21.8% in the mixed approach group, with no statistically significant difference between the groups (P = 0.7902). Bleeding complications occurred in 10.6% of patients in the UCSEMS group, 9.1% in the mixed approach group and 6.6% in the plastic group, with no statistically significant difference between the groups (P = 0.745). Liver abscesses occurred in 14.9% of patients in the UCSEMS group, 16.4% in the mixed approach group and 6.6% in the bilateral plastic group, with no statistically significant difference between the groups (P = 0.222).
For this analysis, study participants were categorized into two groups based on the type of stent used: Plastic (n = 116) and UCSEMS (n = 47).
OS: The log-rank test demonstrated significantly higher OS in the UCSEMS stent group compared to the plastic stent group (P < 0.0001). The median survival time was 183 and 445 days for the plastic and UCSEMS groups, respectively (P < 0.0001; Figure 1B).
Total number of interventions: Statistical analysis revealed a significantly higher number of ERCP procedures in the metal stent group compared to the plastic stent group, with mean values of 5.4 and 3.4, respectively (P < 0,0001; Table 2). When adjusted for survival time, the mean number of ERCP procedures per patient-year was lowest in the UCSEMS group (4.43), compared to the mixed approach (6.71) and plastic stent group (8.26).
Occlusion time: The UCSEMS group showed a statistically significantly longer stent occlusion time of 122.5 days compared to the plastic stent group, which had an occlusion time of 82.1 days (P = 0.0032).
Adverse events: The UCSEMS group showed a significantly higher rate of postprocedural cholangitis (2.1%) compared to the plastic stent group (1.2%; P = 0.0076). Acute pancreatitis occurred in 23.1% of patients in the plastic stent group and in 27.7% of patients in the UCSEMS group (P = 0.5366). Bleeding occurred in 10.64% of cases in the UCSEMS group and 5.17% in the plastic stent group (P = 0.299). Liver abscesses were observed in 14.89% of patients in the UCSEMS group and 9.48% in the plastic stent group (P = 0.408).
For this subanalysis patients were divided into two groups based on the drainage approach: Unilateral (n = 10) and bilateral (n = 112).
OS: The log-rank test did not show statistically significant differences in OS time between bilateral and unilateral stent
Total number of interventions: Statistical analysis revealed a significantly higher number of ERCP procedures in the bilateral group compared to the unilateral group, with mean values of 4.0 and 1.9, respectively (P = 0.0276). Additionally, the unilateral group had a statistically significantly higher rate of unsuccessful procedures, with mean values of 1.5 and 0.4 for the unilateral and bilateral groups, respectively (P < 0.0001; Table 2).
Occlusion time: The mean stent occlusion time was longer in the unilateral group (115.6 days vs 99.4 days), but the difference was not statistically significant (P = 0.2748).
Adverse events: Cholangitis episodes were more frequent in the bilateral group than in the unilateral group (1.5 vs 0.7; P = 0.2412). Acute pancreatitis occurred in 50% of unilateral and 24.8% of bilateral group patients (P = 0.53). Bleeding rates were 20% (unilateral) and 8.93% (bilateral; P = 0.18). Liver abscesses were observed in 20% (unilateral) and 14.29% (bilateral; P = 0.64).
RFA: The log-rank test demonstrated a statistically significant higher survival in the group of patients who were treated with RFA (P < 0.0001). The mean number of procedures in the group of patients who underwent RFA was 7.6, which was significantly higher compared to the non-RFA group, with a mean of 3.3 (P < 0.0001). However, RFA did not have an impact on the rate of unsuccessful procedures (P = 0.4589). The mean time to stent occlusion was 79.6 days in the RFA group compared to 96.3 days in the non-RFA group (P = 0.54). Except for transient abdominal pain in three patients (11.5%), no RFA-specific complications were observed (Figure 1D).
Chemotherapy: The log-rank test demonstrated significantly higher survival in patients who underwent chemotherapy (P < 0.0001). The mean number of ERCP procedures in patients receiving chemotherapy was 4.8, which was significantly higher compared to those who did not receive chemotherapy (P < 0.0002). Chemotherapy had no significant impact on the rate of unsuccessful procedures (P = 0.06782). The mean stent occlusion time was significantly longer in the group of patients receiving chemotherapy (103.6 days) compared to those not receiving chemotherapy (82.4 days; P = 0.0042; Figure 1E).
Endoscopic stenting remains a fundamental component of palliative therapy for patients with MHBO, particularly for those ineligible for curative surgery. Unlike distal biliary obstruction, MHBO poses distinct anatomical and technical challenges due to its proximity to the hepatic duct bifurcation. This requires meticulous planning to achieve effective biliary drainage while minimizing complications. Current guidelines recommend that these complex procedures be performed in specialized centers with significant procedural volumes and access to multidisciplinary hepatobiliary teams to ensure optimal patient outcomes. Nevertheless, despite advancements in stent technology and endoscopic techniques, considerable debate persists regarding the optimal stenting approach, including the type of stent (metal vs plastic) and the drainage strategy (unilateral vs bilateral).
Metal stents are consistently favored over plastic stents for MHBO due to their superior performance in terms of stent patency, reduced intervention rates, and lower risk of post-procedural cholangitis[14]. This aligns with prior research indicating that metal stents provide more effective and durable biliary decompression. In our study, the use of UCSEMS was associated with the longest median stent patency (122.5 days) and the best OS. However, these advantages were accompanied by the highest rates of adverse events, including cholangitis (2.2 episodes per patient) and procedural failure (0.7 per patient).
Interestingly, the UCSEMS group, which had the longest survival, also exhibited the highest rate of cholangitis episodes (Table 3). While this correlation is likely confounded by prolonged exposure time due to longer survival, it raises questions about possible immune-modulatory effects that warrant further investigation. One hypothesis is that recurrent inflammation may trigger localized immune responses, though there is no direct evidence of an antitumor effect. The association likely reflects confounding factors and should be interpreted with caution. While this hypothesis requires further validation, it aligns with emerging data suggesting that tumor-immune interactions play a critical role in cancer progression and treatment response[15].
| r value | P value | |
| Age | -0.13 | 0.1004 |
| Time to progression to the next Bismuth stage | 0.56 | 0.0035 |
| Number of ERCP procedures before diagnosis | 0.28 | 0.0003 |
| Number of ERCP procedures after placement of UCSEMS stents | 0.55 | 0.0004 |
| Total number of ERCP procedures | 0.69 | 0.0001 |
| Number of cholangitis episodes in patients with UCSEMS | 0.39 | 0.0467 |
| Number of cholangitis episodes in patients with unilateral plastic | -0.24 | 0.1666 |
| Number of cholangitis episodes in patients with bilateral plastic | 0.19 | 0.1060 |
| Number of cholangitis episodes in patients with mixed approach group | 0.04 | 0.7837 |
| Number of failed ERCP | 0.01 | 0.9236 |
We investigated mixed stenting strategies, including FCSEMS combined with plastic stents or sequential switching. Although these approaches lowered cholangitis rates, they were linked to poorer survival compared to consistent UCSEMS use. This paradox may reflect underlying disease severity or anatomical complexity in patients selected for mixed stenting, which could have negatively influenced survival outcomes. This underscores the need for individualized decisions. Mixed strategies may be considered in select high-risk patients but should not replace standard protocols.
The optimal drainage strategy for MHBO—unilateral or bilateral—remains a topic of ongoing debate[5,16-20]. Prior randomized trials have reported inconsistent findings, with some studies favoring unilateral stenting due to its technical simplicity and lower complication rates, while others advocate bilateral stenting for its superior biliary decompression. It is well established that effective drainage of more than 50% of the liver volume is associated with better clinical outcomes, including reduced cholangitis rates, improved liver function, and prolonged stent patency. In our study, bilateral stenting did not yield a statistically significant improvement in OS compared to unilateral stenting. However, bilateral stenting was associated with fewer procedural failures and improved biliary decompression, supporting its use when technically feasible. These findings suggest that bilateral drainage, when technically feasible, may offer advantages in terms of reducing reintervention rates and maintaining better liver function, even if its impact on survival is less pronounced.
In addition to stenting, we evaluated adjunctive therapies such as RFA and chemotherapy, both of which significantly prolonged survival in MHBO patients. Chemotherapy is already standard, and our findings support incorporating RFA into routine care, as it improves biliary decompression and stent patency. These therapies may complement each other and should be considered as part of a comprehensive treatment strategy. It is important to acknowledge that patients who received RFA or chemotherapy may have had better baseline liver function or lower tumor burden, which could have introduced selection bias and influenced the observed survival benefit.
Unlike most previous studies, we included all ERCP procedures in our analysis, not just the first intervention. We focused on OS as the primary endpoint, aligning with the desirability of outcome ranking framework. We did not emphasize technical success, recognizing that it does not always translate into clinical benefit. Limitations include the observational design, extended recruitment period, and potential confounding factors such as operator technique and stent variability.
Our findings confirm the superior efficacy of metal stents, especially UCSEMS, in managing MHBO, despite their higher rates of complications. Bilateral drainage enhances biliary decompression and procedural success, although its impact on OS remains uncertain. RFA and chemotherapy should be considered standard components of care, given their complementary benefits in prolonging survival and maintaining stent patency. Future research should focus on optimizing stent designs and investigating the synergistic effects of endoscopic, ablative, and systemic therapies. Well-designed prospective trials are essential to define the most effective drainage strategies and to better clarify the role of RFA. A personalized, multimodal treatment strategy appears most promising for improving outcomes in this complex patient population.
| 1. | Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, Khor CJ, Ponnudurai R, Moon JH, Seo DW, Pantongrag-Brown L, Sangchan A, Pisespongsa P, Akaraviputh T, Reddy ND, Maydeo A, Itoi T, Pausawasdi N, Punamiya S, Attasaranya S, Devereaux B, Ramchandani M, Goh KL; Asia-Pacific Working Group on Hepatobiliary Cancers. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 193] [Article Influence: 14.8] [Reference Citation Analysis (1)] |
| 2. | Vienne A, Hobeika E, Gouya H, Lapidus N, Fritsch J, Choury AD, Chryssostalis A, Gaudric M, Pelletier G, Buffet C, Chaussade S, Prat F. Prediction of drainage effectiveness during endoscopic stenting of malignant hilar strictures: the role of liver volume assessment. Gastrointest Endosc. 2010;72:728-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 228] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Lee TH, Moon JH, Park SH. Biliary stenting for hilar malignant biliary obstruction. Dig Endosc. 2020;32:275-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Testoni PA, Mariani A, Aabakken L, Arvanitakis M, Bories E, Costamagna G, Devière J, Dinis-Ribeiro M, Dumonceau JM, Giovannini M, Gyokeres T, Hafner M, Halttunen J, Hassan C, Lopes L, Papanikolaou IS, Tham TC, Tringali A, van Hooft J, Williams EJ. Papillary cannulation and sphincterotomy techniques at ERCP: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2016;48:657-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 434] [Article Influence: 43.4] [Reference Citation Analysis (1)] |
| 5. | Qumseya BJ, Jamil LH, Elmunzer BJ, Riaz A, Ceppa EP, Thosani NC, Buxbaum JL, Storm AC, Sawhney MS, Pawa S, Naveed M, Lee JK, Law JK, Kwon RS, Jue TL, Fujii-Lau LL, Fishman DS, Calderwood AH, Amateau SK, Al-Haddad M, Wani S. ASGE guideline on the role of endoscopy in the management of malignant hilar obstruction. Gastrointest Endosc. 2021;94:222-234.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 6. | Dumonceau JM, Tringali A, Papanikolaou IS, Blero D, Mangiavillano B, Schmidt A, Vanbiervliet G, Costamagna G, Devière J, García-Cano J, Gyökeres T, Hassan C, Prat F, Siersema PD, van Hooft JE. Endoscopic biliary stenting: indications, choice of stents, and results: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline - Updated October 2017. Endoscopy. 2018;50:910-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 539] [Article Influence: 67.4] [Reference Citation Analysis (0)] |
| 7. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3338] [Article Influence: 208.6] [Reference Citation Analysis (15)] |
| 8. | Xia MX, Cai XB, Pan YL, Wu J, Gao DJ, Ye X, Wang TT, Hu B. Optimal stent placement strategy for malignant hilar biliary obstruction: a large multicenter parallel study. Gastrointest Endosc. 2020;91:1117-1128.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 9. | Inoue T, Okumura F, Naitoh I, Fukusada S, Kachi K, Ozeki T, Anbe K, Iwasaki H, Mizushima T, Kobayashi Y, Ishii N, Ito K, Kondo H, Hayashi K, Yoneda M, Sano H. Feasibility of the placement of a novel 6-mm diameter threaded fully covered self-expandable metal stent for malignant hilar biliary obstructions (with videos). Gastrointest Endosc. 2016;84:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Yoshida T, Hara K, Imaoka H, Hijioka S, Mizuno N, Ishihara M, Tanaka T, Tajika M, Niwa Y, Yamao K. Benefits of side-by-side deployment of 6-mm covered self-expandable metal stents for hilar malignant biliary obstructions. J Hepatobiliary Pancreat Sci. 2016;23:548-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Kitamura K, Yamamiya A, Ishii Y, Mitsui Y, Nomoto T, Yoshida H. Side-by-side partially covered self-expandable metal stent placement for malignant hilar biliary obstruction. Endosc Int Open. 2017;5:E1211-E1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Takahashi S, Fujisawa T, Ushio M, Fukuma T, Suzuki A, Takasaki Y, Ito K, Tomishima K, Ishii S, Isayama H. Retrospective evaluation of slim fully covered self-expandable metallic stent for unresectable malignant hilar biliary obstruction. J Hepatobiliary Pancreat Sci. 2023;30:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 13. | Matsubara S, Nakagawa K, Suda K, Otsuka T, Oka M, Nagoshi S. The Feasibility of Whole-Liver Drainage with a Novel 8 mm Fully Covered Self-Expandable Metal Stent Possessing an Ultra-Slim Introducer for Malignant Hilar Biliary Obstructions. J Clin Med. 2022;11:6110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Sawas T, Al Halabi S, Parsi MA, Vargo JJ. Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc. 2015;82:256-267.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Huang R, Kang T, Chen S. The role of tumor-associated macrophages in tumor immune evasion. J Cancer Res Clin Oncol. 2024;150:238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 128] [Reference Citation Analysis (0)] |
| 16. | Chen ZK, Zhang W, Xu YS, Li Y. Unilateral Versus Side-By-Side Metal Stenting for Malignant Hilar Biliary Obstruction: A Meta-Analysis. J Laparoendosc Adv Surg Tech A. 2021;31:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Naitoh I, Ohara H, Nakazawa T, Ando T, Hayashi K, Okumura F, Okayama Y, Sano H, Kitajima Y, Hirai M, Ban T, Miyabe K, Ueno K, Yamashita H, Joh T. Unilateral versus bilateral endoscopic metal stenting for malignant hilar biliary obstruction. J Gastroenterol Hepatol. 2009;24:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Lee TH, Kim TH, Moon JH, Lee SH, Choi HJ, Hwangbo Y, Hyun JJ, Choi JH, Jeong S, Kim JH, Park DH, Han JH, Park SH. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: a multicenter, prospective, randomized study (with video). Gastrointest Endosc. 2017;86:817-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Teng F, Xian YT, Lin J, Li Y, Wu AL. Comparison of Unilateral With Bilateral Metal Stenting for Malignant Hilar Biliary Obstruction. Surg Laparosc Endosc Percutan Tech. 2019;29:43-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Staub J, Siddiqui A, Murphy M, Lam R, Parikh M, Pleskow D, Papachristou G, Sharaiha R, Iqbal U, Loren D, Kowalski T, Noor A, Mumtaz T, Yasuda I, Thomas S, Hsaeeb A, Herrick J, Greene T, Adler DG. Unilateral versus bilateral hilar stents for the treatment of cholangiocarcinoma: a multicenter international study. Ann Gastroenterol. 2020;33:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/