Published online Oct 16, 2025. doi: 10.4253/wjge.v17.i10.111408
Revised: July 24, 2025
Accepted: September 9, 2025
Published online: October 16, 2025
Processing time: 107 Days and 18.1 Hours
Malignant transformation of an ectopic pancreas is exceptionally rare, posing significant diagnostic challenges. As such, there are currently no established man
A 58-year-old female was admitted to our hospital in August 2024 due to elevated carbohydrate antigen 19-9. Relevant examinations found a huge abdominal tumor that was radiologically adherent to both the pancreatic head and the greater curv
We hope that our findings will facilitate the clinical recognition of this entity and help to increase knowledge regarding its management.
Core Tip: We have described a case of adenocarcinoma arising from gastric heterotopic pancreas. If the cancer has invaded the mucosal layer, gastric endoscopy with biopsy has great utility for preoperative diagnosis. Alternatively, evaluation can be performed with endoscopic ultrasound, and with endoscopic ultrasound-guided fine needle aspiration if necessary. Tumor markers need to be regularly tested in the postoperative follow-up of such patients. Clinicians need to be mindful of the possibility of malignant transformation of heterotopic pancreas, especially with duodenal or gastric submucosal tumor-like lesions with obstructive symptoms.
- Citation: Wang YX, Wang J, Liang SX, Wu Q. Primary adenocarcinoma from a gastric heterotopic pancreas: A case report. World J Gastrointest Endosc 2025; 17(10): 111408
- URL: https://www.wjgnet.com/1948-5190/full/v17/i10/111408.htm
- DOI: https://dx.doi.org/10.4253/wjge.v17.i10.111408
Heterotopic pancreas (HP), also known as ectopic or aberrant pancreas, is defined as histologically confirmed pancreatic tissue located outside the normal pancreatic gland that lacks any anatomic, vascular, or neural connection to the orthotopic pancreas. HP was first described in 1727 within an ileal diverticulum[1]. HP may occur anywhere in the gastrointestinal tract, but is most commonly seen in the duodenum (9%-36%), stomach (24%-38%), jejunum (0.5%-27%), and Meckel’s diverticulum (2%-6.5%)[1]. Based on Heinrich’s original histological classification system, HP can be categorized into three distinct subtypes: Types I-III. However, Fuentes and others have since modified this system to include a fourth subtype, type IV[2].
HP is relatively uncommon and typically asymptomatic. Its malignant transformation is exceptionally rare, with reported incidence rates of 0.7%-1.8% among all HP cases[1]. Morphologically, malignant HP lesions may present as smooth nodules or, less frequently, irregular masses. Combined with the tissue’s ectopic nature, this poses significant diagnostic challenges. Although conventional endoscopic biopsy can identify the adenocarcinoma, it often fails to detect its ectopic pancreatic origin. Given the diagnostic complexity and the scarcity of reported cases, we have supplemented our presentation of this rare instance of gastric adenocarcinoma arising from HP with a comprehensive literature review (Table 1). We aim to facilitate the clinical recognition of this pathology and to consolidate existing knowledge regarding its diagnosis and therapeutic management.
| Ref. | Sex, age | Location | Morphology | Clinical presentation | Tumor markers | Diagnostic methods | Histology | Heinrich type | Initial diagnosis | Outcome | Adjuvant therapy |
| Matsuki et al[5], 2005 | Female, 58 years old | Gastric antrum | SET with stenosis | Gastric outlet obstruction (vomiting) | CEA, CA19-9: Normal | EUS: Diffuse wall thickening in prepyloric region | Adenocarcinoma | II | Suspected malignancy | No recurrence (1.5 years) | None |
| Fujita et al[13], 2008 | Female, 64 years old | Jejunum | Umbilicated mass | Abdominal distension/epigastric pain | Not tested | DBE: Umbilicated mass (intraoperative confirmation) | Adenocarcinoma | I | Jejunal cancer | Death (5 months after surgery; metastasis) | Gemcitabine |
| Bini et al[14], 2010 | Male, 56 years old | Duodenum (D1) | SET | Vomiting | CA19-9/CA125/AFP: Normal limits | EGD + EUS: Submucosal hypoechoic lesion and biopsies were performed | Adenocarcinoma | I | Adenocarcinoma of unknown origin | Not reported | Referred for chemotherapy |
| Song et al[15], 2012 | Male, 74 years old | Jejunum | Perforating tumor | Acute abdominal pain, stool problems | CA19-9 elevated | Not performed | Adenocarcinoma | II | Peritonitis from perforation of sigmoid colon cancer | Not reported | Not reported |
| Stock et al[16], 2011 | Female, 79 years old | Duodenum (D4) | Not described | Early satiety (2 years) | Not tested | EGD: Duodenal mass | Adenocarcinoma | I | Not reported | Not reported | Adjuvant chemotherapy |
| Fukumori et al[9], 2011 | Female, 76 years old | Gastric pylorus | SET | Not reported | CA19-9: 177.5 to 279.5 U/mL | EUS: SMT | Adenocarcinoma | II | GIST | Not reported | Not reported |
| Okamoto et al[17], 2012 | Female, 75 years old | Stomach | SET | Epigastric pain | CEA, CA19-9 elevated | EGD: Subepithelial tumor | Adenocarcinoma | I | SET | No recurrence (11 years) | Not reported |
| Kinoshita et al[18], 2012 | Female, 62 years old | Duodenum | Stenotic | Vomiting/epigastric pain | CEA, CA19-9 elevated | EGD: Gastric outlet obstruction | Adenocarcinoma | I | Advanced gastric cancer | No recurrence (12 months) | Not reported |
| Ginori et al[19], 2013 | Male, 86 years old | Duodenal bulb | Stenotic | Abdominal pain/dyspepsia for 2 months | Not tested | Not performed | Adenocarcinoma | I | Acute cholecystitis | Gastrectomy, alive with metastasis | Not reported |
| Endo et al[4], 2014 | Male, 75 years old | Duodenum | Stenotic | Epigastric pain/tarry stools | CEA, CA19-9 increased | EUS-FNA: SMT | Adenocarcinoma | I | Duodenal cancer | No recurrence (5 years) | Not reported |
| Endo et al[4], 2014 | Male, 73 years old | Stomach | SET | Epigastric pain | CEA, CA19-9: Normal | EUS: 4th layer hypoechoic mass (EMR-C + biopsy) | Adenocarcinoma | I | Gastric cancer | Lymph node metastasis (2 years follow-up) | Not reported |
| Fukino et al[12], 2015 | Male, 62 years old | Duodenum (D3) | SET | 6 kg weight loss in (4 months) | CEA: Normal limits, CA19-9/DUPAN-2/SPan-1 elevated | Duodenal fibroscopy: SMT | Adenocarcinoma | IV | Duodenal carcinoma | Death (33 months after surgery) | S-1 + cisplatin to radiation + GEM (recurrence) |
| Mehra et al[10], 2015 | Male, 51 years old | Duodenum (D3) | Not described | Abdominal pain/nausea | Not tested | EGD: Mucosal growth in duodenum | Adenocarcinoma | II | Not reported | No recurrence | Not reported |
| Hisanaga et al[20], 2020 | Female, 70 years old | Duodenum | Rough, protuberant lesion | Incidental (diabetes) | Not tested | EUS: Multilocular protuberant lesion | Adenocarcinoma | I | Pancreatic carcinoma | No recurrence (10 months) | Not reported |
| Jung et al[21], 2020 | Male, 75 years old | Stomach | Submucosal lesion with gastric outlet obstruction | Dyspepsia, nausea/3 kg weight loss within 1 month | CEA, CA19-9 increased | GIE and EUS (2008) hypoechoic lesion involving the 2nd/3rd layers; GIE (2018) tumor increased | Adenocarcinoma | Not specified | Gastric outlet obstruction | Follow-up < 6 months | Capecitabine + oxaliplatin |
| Oto et al[22], 2021 | Male, 37 years old | Stomach | Pylorus stenosis | Nausea/upper abdominal pain | Not tested | EGD: Pyloric stenosis | Adenocarcinoma | Not specified | Not reported | Not reported | Not reported |
| Hirokawa et al[11], 2021 | Female, 65 years old | Duodenum (D1) | Ulcer formation | Epigastric discomfort | CEA, CA125 elevated | EGD: Ulcerated tumor (biopsy-confirmed) | Adenocarcinoma | Not specified | Unresectable duodenal cancer | Progression (24 months) | Trastuzumab (HER2+) pre-operation + chemo, post-operation |
| Qian et al[2], 2023 | Female, 59 years old | Stomach | Stenotic | Abdominal discomfort/acid regurgitation | Not tested | EGD: Pyloric area congestion and swelling; pyloric canal stenosis | Adenocarcinoma | III | Pyloric obstruction and adenocarcinoma | Recurrence and metastasis (1-year post-op) | Gemcitabine + tigecycline |
| Woo et al[6], 2023 | Male, 65 years old | Duodenal bulb | Stricture | Dyspepsia/vomiting | Not tested | EGD: Duodenal stricture | Adenocarcinoma | III | Benign stricture | No recurrence (24 months) | FOLFIRINOX |
| Yamaoka et al[8], 2015 | Female, 65 years old | Jejunum | SET | Incidental finding | CA19-9: 635 U/mL, CEA: 22 ng/mL | DBE: SMT | Adenocarcinoma | II | Peritoneal metastasis from colon cancer | Peritoneal recurrence (9 months) | S-1 to gemcitabine + nab-paclitaxel |
| Ji et al[7], 2024 | Female, 75 years old | Stomach (antrum) | SMT | Health screening | CA19-9 elevated | EGD: Submucosal bulge | Adenocarcinoma | Not specified | Primary pyloric malignancy | Not reported | Not reported |
A 58-year-old woman presented to our institution in August 2024 after routine blood tests revealed an elevated carbohydrate antigen 19-9 (CA19-9) level (93 U/mL; normal range 0-37 U/mL). Although this can be indicative of other conditions, high CA19-9 is sufficiently associated with pancreatic cancer to suggest the need for further investigation. The patient’s physical examination and vital signs were unremarkable.
An elevated CA19-9 level.
No other medical conditions.
The patient reported no significant personal or family medical history.
No relevant factors were identified on physical examination.
Routine blood tests revealed an elevated CA19-9 level (93 U/mL; normal range: 0-37 U/mL).
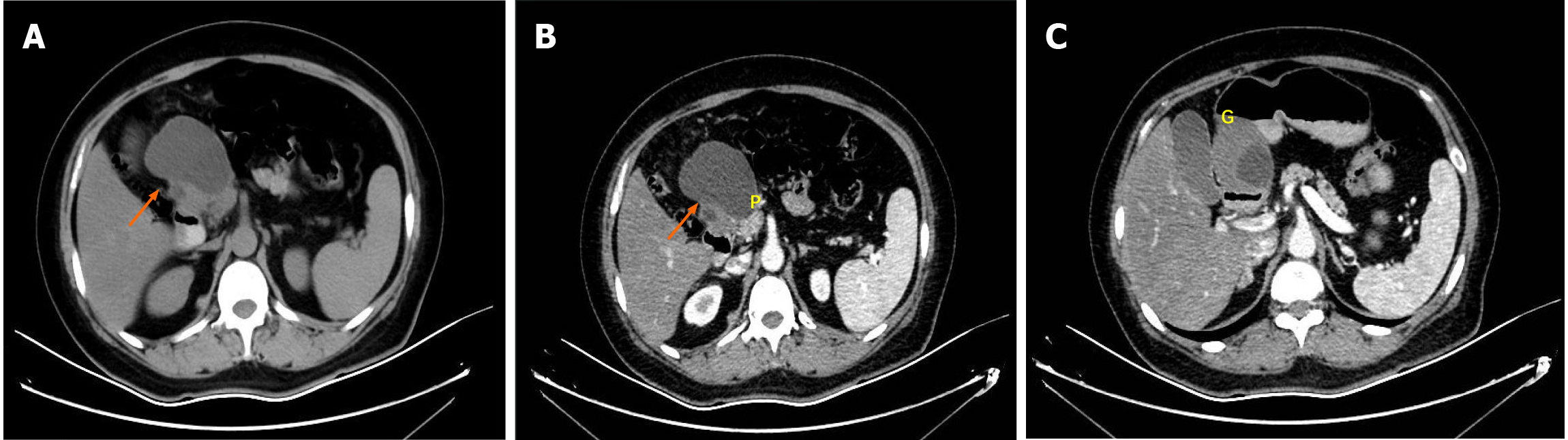
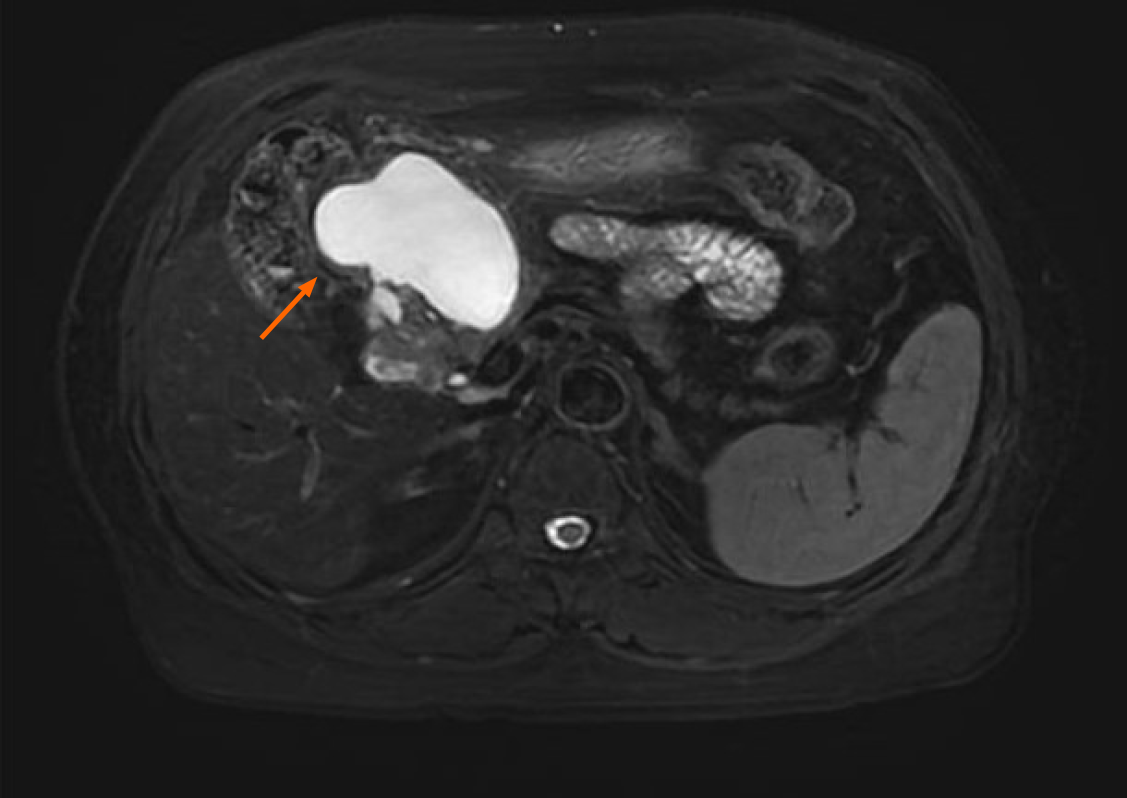
Contrast-enhanced abdominal computed tomography (CT) identified a cystic lesion in the right upper abdomen (Figure 1). This showed close anatomical relationships with both the pancreatic head and the greater curvature of the gastric antrum. An initial radiological assessment favored a benign pathology, with differential diagnoses including intestinal duplication and pancreatic cystadenoma. The subsequent pancreatic protocol magnetic resonance imaging with diffusion-weighted imaging (Figure 2) confirmed these findings but showed no evidence of malignancy.
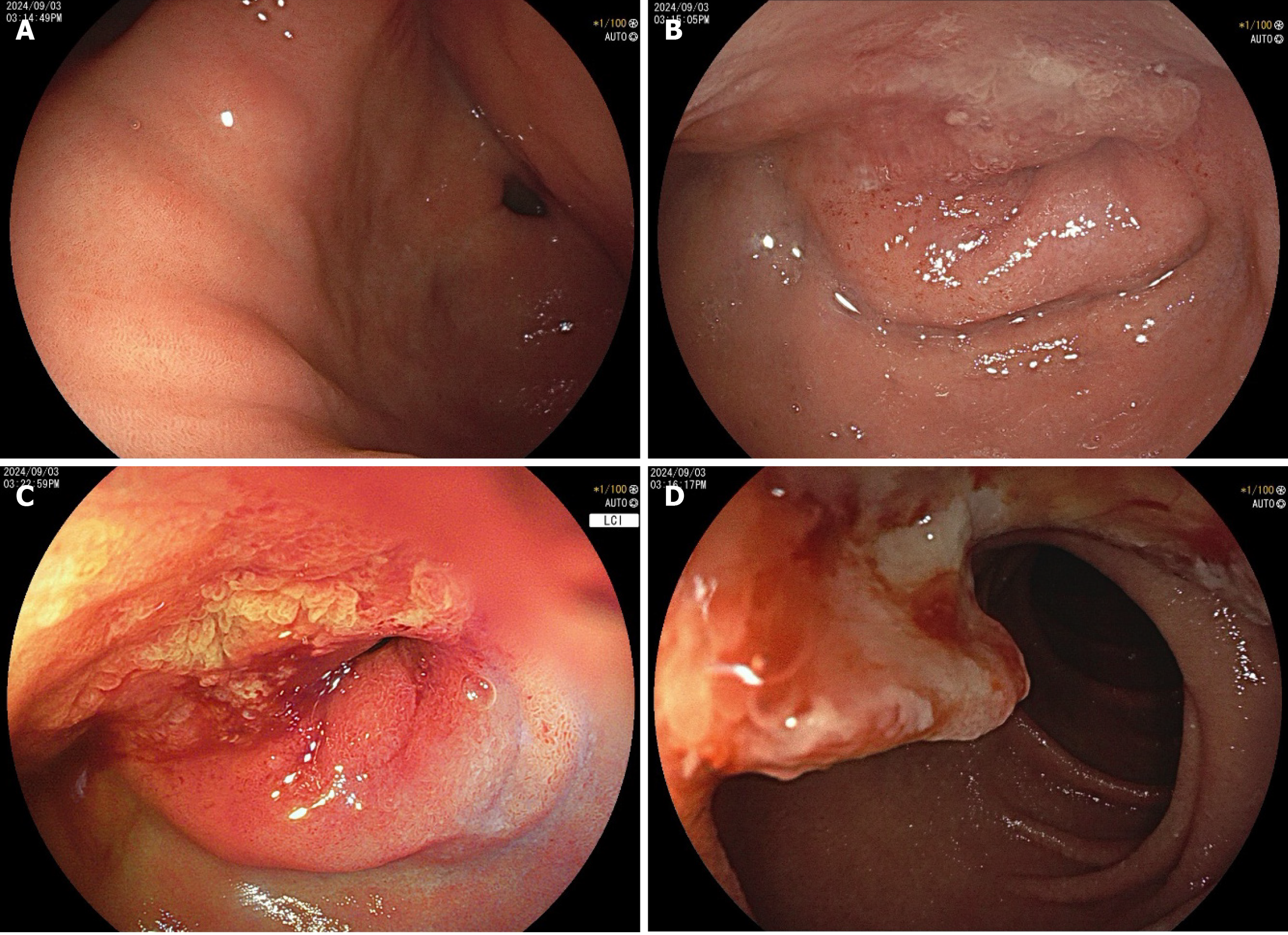
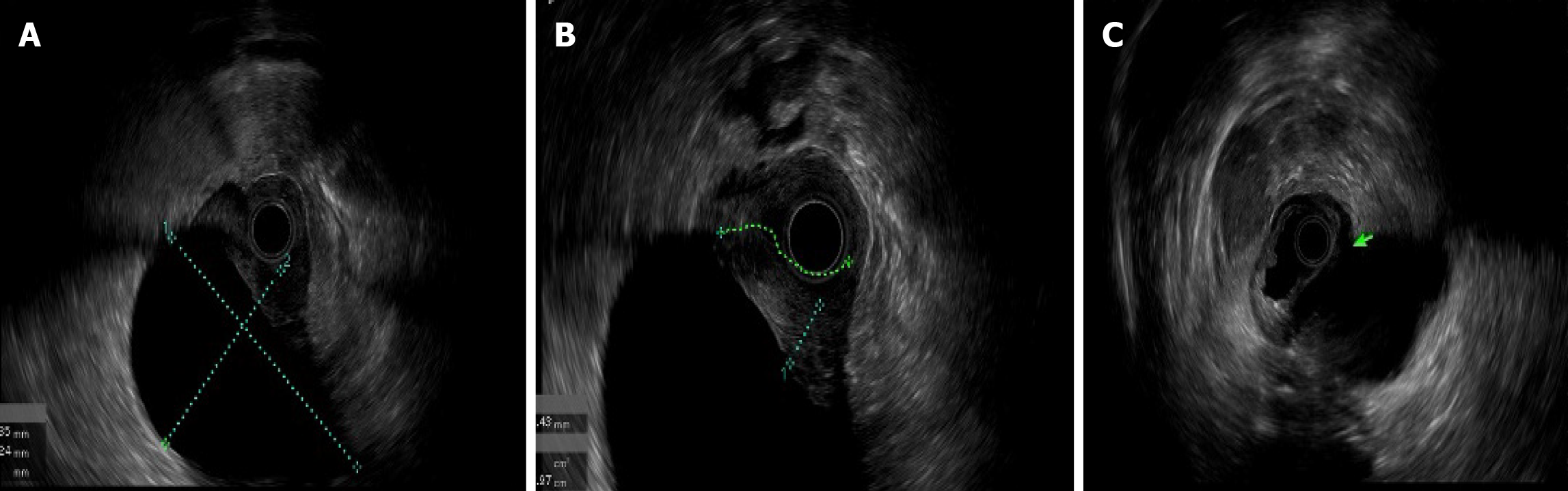
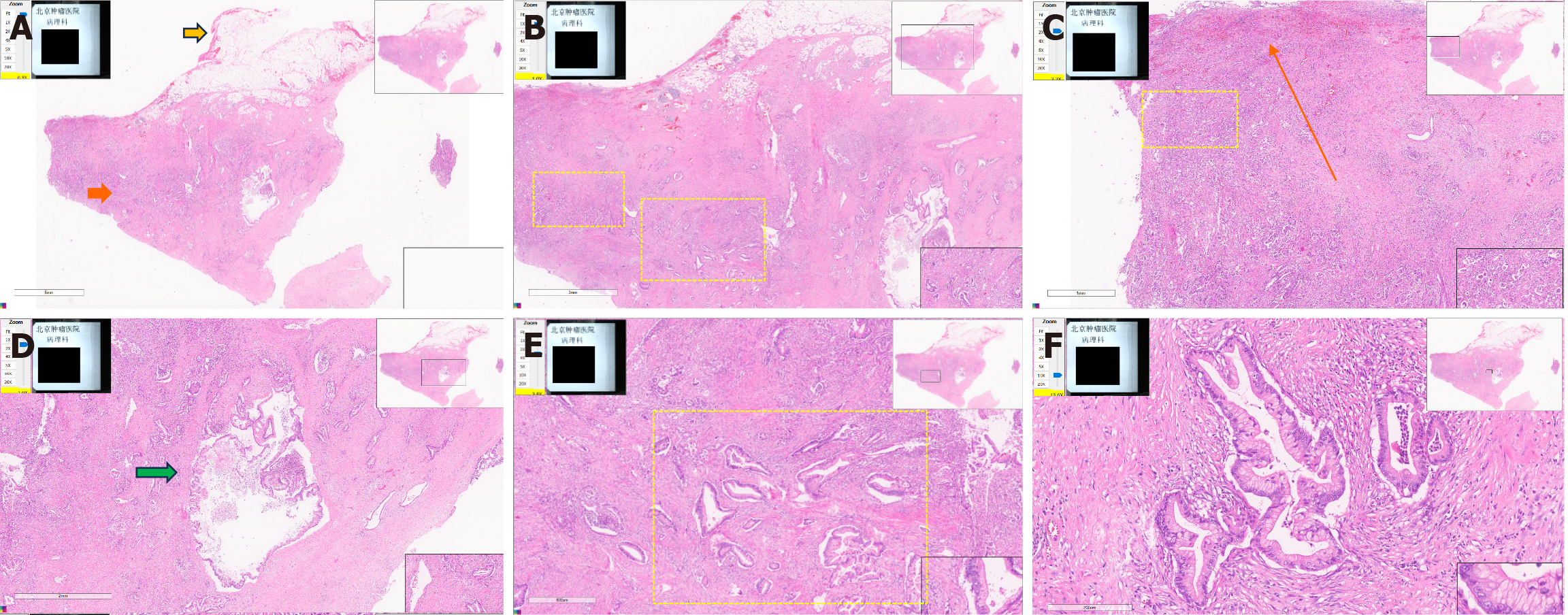
An upper endoscopy revealed the following: (1) A large, soft submucosal lesion involving the pylorus and greater curvature of the gastric antrum, with preserved luminal patency; (2) An ulcerated lesion at the duodenal bulb-descending junction, with luminal narrowing and raised margins (Figure 3); and (3) Endoscopic ultrasonography (EUS) identified a 7 cm × 4.8 cm mixed cystic-solid mass with clear demarcation from the adjacent organs but a loss of interface with the fourth layer of the gastric wall (Figure 4).
Biopsy of the ulcer confirmed moderately differentiated adenocarcinoma, obviating the need for the planned endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Notably, the patient’s CA19-9 level decreased to 43 U/mL pre
Adjuvant chemotherapy (gemcitabine + capecitabine) commenced on November 13, 2024.
After five cycles, surveillance imaging (April 3, 2025) revealed metastatic progression, prompting a transition to second-line therapy (nab-paclitaxel + gemcitabine). The patient’s CA19-9 level had normalized by December 23, 2024, and remained within normal limits at her most recent follow-up.
Our case reported here is from the stomach and also has a close relationship with the first portion of the duodenum. Although pancreatic heterotopia can occur throughout the entire gastrointestinal tract, the most frequent sites are the stomach (25%-60%), duodenum (30%), and jejunum (15%)[3]. When it occurs in the stomach, the ectopic pancreas is usually located within a few centimeters of the gastroduodenal junction[3]. This form is often symptomatic, with patients complaining of dyspepsia, nausea, upper gastric pain, acid reflux, abdominal distension, early satiety, vomiting, and weight loss. HP rarely causes abnormal stools directly, but they may result indirectly through mechanical effects or local inflammation. If the patient has intractable diarrhea or related manifestations, clinicians should look first for other diseases of the digestive system. Only 16.7% (4/22 cases, including our patient) of the patients identified in our review were asymptomatic, and the lesions were found incidentally. This suggests that when malignant transformation of HP occurs, patients are more prone to clinical symptoms or their original symptoms worsen.
Among the reviewed cases, we found that the morphology of adenocarcinomas arising from HP varies considerably. Preoperative diagnosis of a malignant ectopic pancreas with stenotic or submucosal tumor (SMT) morphology and no ulceration can be difficult using imaging studies such as CT and radiography. This is because imaging can only indicate the presence of a mass; it cannot determine its nature. For this reason, most of the reviewed cases of adenocarcinomas arising from an ectopic pancreas were resected as SMTs with tentative diagnoses of gastrointestinal stromal tumor, lymphoma, or other[4]. The mass in our patient had a predominantly SMT-like appearance, with focal ulceration in the duodenum. Malignant transformation of an HP usually occurs intramurally, and involvement of the overlying mucosa occurs late in the course of the disease. While upper endoscopy with conventional biopsy at the location of the tumor rupture can obtain pathological findings, it often fails in SMT-like lesions. The utility of EUS-FNA and endoscopic mucosal resection with cap for histological diagnosis has been demonstrated for many gastrointestinal and pancreatic malignancies. EUS is especially helpful in the diagnosis of SMTs. EUS-FNA combined with immunohistochemistry can achieve a higher histological diagnostic rate. An endoscopic mucosal resection with cap is an endoscopic mucosal resection performed using a panendoscope with a fitted transparent plastic cap. This, followed by a biopsy[4], should be considered before selecting a treatment approach. Matsuki et al[5] have reported a case with no distinct cystic lesion on EUS due to its obscuration by severe stenosis. Therefore, although EUS is generally helpful for the diagnosis of SMTs, the location of the tumor may determine the clarity of the scan. The detection of cystic components inside the tumor is helpful for the diagnosis of HP[5].
Most patients with malignant HP have clinical symptoms. It has been reported that the size of non-neoplastic epithelial tissue is usually < 4 cm, whereas malignant HP is more often > 4 cm[2]. In our case, the mass was about 7 cm × 4.8 cm, which was sufficiently large to suggest a high-risk lesion. Woo et al[6] analyzed 15 cases and reported a size range of malignant duodenal HP of 12-55 mm, with a mean of 29.6 mm. Accordingly, they recommend early treatment of HP to prevent malignant transformation, especially when the lesion is > 1 cm or associated with clinical symptoms of obstruction or weight loss. According to Heinrich’s classification with Fuentes’ modification, HP can be of four types (described above in the classification and demographics subsection of the literature review). In the cases we reviewed, Heinrich types I and II were most common (63.6%)[2]. As the reviewed cases were all malignant, we could not draw a clear conclusion about differences in the probability of malignant transformation between Heinrich types. It was also unclear whether these types were relevant to prognosis and survival. To facilitate the collection of sufficient data to address such questions, a multicenter database needs to be established. The most frequent histological malignancy subtype arising from HP was adenocarcinoma. Other malignancies included anaplastic carcinoma, mucinous cystadenocarcinoma, acinar cell carcinoma, solid pseudopapillary tumor, pancreatoblastoma, perivascular epithelioid cell tumor, and neuroendocrine tumor[7]. Previous studies have found type I HP to be the most common source of malignant tumors. Our review confirmed that type I accounts for the largest proportion of all HP. The biological behavior of type I HP appears to most closely mimic that of a normal pancreas because it contains acinar and islet cells; however, all subtypes have the potential for malignancy, including ductal adenocarcinomas, acinar cell carcinomas, and neuroendocrine tumors. Types II and III are primarily ductal, with similar molecular characteristics to pancreatic ductal adenocarcinoma, such as KRAS and TP53 mutations. Future research investigating the differences in the frequency of driver gene mutations between the Heinrich subtypes is warranted. The interstitial components of HP such as inflammation and fibrosis may affect their malignant transformation owing to differences in their anatomical location (stomach, duodenum, or jejunum) and type. For example, chronic inflammation of an ectopic pancreas in the stomach (mostly type I/II) could be more or less likely to promote malignancy. Whether the immune microenvironment (e.g., T cell infiltration and programmed death-ligand 1 expression) in a malignant ectopic pancreas is affected by the primary site (e.g., stomach vs small intestine) also requires further investigation. We speculate that Heinrich type may indirectly regulate cancer risk and the HP subtypes due through differences in the histological structure, microenvironment, and molecular characteristics. Future research should use multi-omics to analyze pathological cohorts, focusing on exploration of commonalities between the mechanisms of the ductal components of type II/III HP and pancreatic ductal adenocarcinoma.
In our patient, the initial serum CA19-9 level was significantly elevated but decreased to a normal level soon after surgery. Ji et al[7] suggest that elevated serum CA19-9 may be diagnostically helpful, as approximately 57% of patients with HP adenocarcinoma have an increased CA19-9. Woo et al[6] found that 50% of the cases they identified had increased CA19-9 or carcinoembryonic antigen (CEA) levels. In our review, other tumor markers such as DUPAN-2, SPan-1, and CA125 were also found to be increased in some of the cases. In a case reported by Yamaoka et al[8] in 2015, the tumor markers CA19-9 and CEA increased considerably after a colectomy as a result of adenocarcinoma arising from HP in the small intestine. The patient underwent a laparoscopic jejunectomy with regional lymph node dissection. One month after surgery, her CA19-9 level had decreased to a normal level. However, 9 months after surgery, her serum CA19-9 and CEA levels had increased again, and peritoneal metastasis from HP adenocarcinoma was suspected. A case of gastric HP was reported by Fukumori et al[9] in 2011. Initially, a neoplastic lesion was detected in the pyloric region of an upper esophagogastroduodenoscopy of a 76-year-old woman. The lesion required biopsy, but because it was classified as Heinrich type I, it was only observed temporarily. Following blood tests 2 years later found that the patient’s CA19-9 had increased from 177.5 to 279.5. Esophagogastroduodenoscopy, EUS, and a CT scan were performed, and, as the results could not rule out malignancy, the patient underwent gastrectomy on the pylorus side and lymph node dissection. This led to the eventual diagnosis of HP cancer. Thus, tumor markers, especially CA19-9 and CEA, appear to be useful indices of potential HP malignancy and its subsequent metastasis. Therefore, tumor markers should be tested regularly in HP patients and in the postoperative follow-up of such patients. If elevated tumor markers are found in a patient with a history of HP, the possibility of malignant transformation needs to be considered. However, a previous report found the rate of tumor-positive markers, including CA19-9 and CEA, to be lower in patients with HP adenocarcinoma than those with primary pancreatic cancer[4].
Gastric HP is usually asymptomatic and requires no treatment. In high-risk cases, a resection is required, with histological confirmation of non-malignancy. High-risk is indicated by an increasing size and symptoms such as ulceration, bleeding, and pyloric obstruction. A previous study reported surgical resection as the diagnostic approach for malignant ectopic pancreas in 87% of the 54 included cases[1]. In our case, the patient was treated with gemcitabine + capecitabine as adjuvant chemotherapy, but metastatic progression was found at the 6-month follow-up. Because of the small number of reports of adenocarcinoma arising from HP, there is no clear evidence of the efficacy of chemotherapy or the optimal type of adjuvant therapy[6]. The patient described by Mehra et al[10] responded well to gemcitabine + oxaliplatin adjuvant therapy; however, the general prognosis for HP adenocarcinoma is not well known. Further detailed studies may be able to clarify this. In Hirokawa’s case[11], trastuzumab treatment was effective against human epidermal growth factor receptor 2-positive adenocarcinoma originating from HP tissue in the duodenum. It can be seen that targeted therapy has shown potential in the treatment of ectopic pancreatic cancer, and routine detection of molecular markers (such as human epidermal growth factor receptor 2/BRAC) is necessary. In a case reported by Fukino et al[12], gemcitabine was effective for recurrent adenocarcinoma arising from a duodenal ectopic pancreas. At present, immunotherapy for pancreatic cancer has progressed from being almost entirely ineffective to a stage of limited breakthrough. Individualized vaccines and microtargeting strategies are the most promising directions for future research. Therefore, we look forward to corresponding increases in the effectiveness of immunotherapy for ectopic pancreatic cancer. We believe that there will be more breakthroughs in the drug treatment of ectopic pancreatic cancer in the future.
The recurrence rate of HP cancer is low. A few patients have remained alive without recurrence more than 5 years after resection of adenocarcinoma arising from ectopic gastric or duodenal pancreas, which has a 5-year survival rate of 10%. Reports suggest a more favorable prognosis than for ordinary pancreatic cancer because of the earlier presentation of gastrointestinal obstruction and other symptoms, allowing more expeditious control of the disease[8].
We have described a case of adenocarcinoma arising from gastric HP. Although the incidence of gastric adenocarcinoma arising from HP is rare, a careful diagnostic and therapeutic approach should be adopted in suspected cases. If the cancer has invaded the mucosal layer, gastric endoscopy with biopsy has great utility for preoperative diagnosis. Alternatively, evaluation can be performed with EUS, and with EUS-FNA if necessary. Tumor markers need to be regularly tested in the postoperative follow-up of such patients. Until now, there has been no clear evidence for the efficacy of chemotherapy or the optimal type of adjuvant therapy. However, this case found that gemcitabine + capecitabine and gemcitabine + oxaliplatin have proven to be effective in several reports. Despite their rarity, clinicians need to be mindful of the possibility of malignant transformation of HP, especially with duodenal or gastric SMT-like lesions with obstructive symptoms.
| 1. | Cazacu IM, Luzuriaga Chavez AA, Nogueras Gonzalez GM, Saftoiu A, Bhutani MS. Malignant Transformation of Ectopic Pancreas. Dig Dis Sci. 2019;64:655-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 2. | Qian L, Yang J, Zhang J. Gastric ectopic pancreas adenocarcinoma: A case report and literature review. Saudi Med J. 2023;44:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 3. | Goodarzi M, Rashid A, Maru D. Invasive ductal adenocarcinoma arising from pancreatic heterotopia in rectum: case report and review of literature. Hum Pathol. 2010;41:1809-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Endo S, Saito R, Ochi D, Yamada T, Hirose M, Hiroshima Y, Yamamoto Y, Ueno T, Hasegawa N, Moriwaki T, Narasaka T, Kaneko T, Fukuda K, Suzuki H, Mizokami Y, Hyodo I. Effectiveness of an endoscopic biopsy procedure using EUS-FNA and EMR-C for diagnosing adenocarcinoma arising from ectopic pancreas: two case reports and a literature review. Intern Med. 2014;53:1055-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Matsuki M, Gouda Y, Ando T, Matsuoka H, Morita T, Uchida N, Kuriyama S. Adenocarcinoma arising from aberrant pancreas in the stomach. J Gastroenterol. 2005;40:652-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Woo CG, Lee J, Son SM. Adenocarcinoma arising from heterotopic pancreas at the first portion of the duodenum: a case report. J Int Med Res. 2023;51:3000605231194902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Ji X, Dong A, Wang Y. FDG PET/CT in Pancreatic Ductal Adenocarcinoma Arising From a Heterotopic Pancreas of the Pylorus. Clin Nucl Med. 2024;49:e42-e44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Yamaoka Y, Yamaguchi T, Kinugasa Y, Shiomi A, Kagawa H, Yamakawa Y, Numata M, Sugimoto S, Imai K, Hotta K, Sasaki K. Adenocarcinoma arising from jejunal ectopic pancreas mimicking peritoneal metastasis from colon cancer: a case report and literature review. Surg Case Rep. 2015;1:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Fukumori D, Matsuhisa T, Taguchi K, Minato M. Ectopic gastric pancreatic cancer: report of a case. Hepatogastroenterology. 2011;58:740-744. [PubMed] |
| 10. | Mehra R, Pujahari AK, Jaiswal SS. Duodenal heterotopic pancreatic tissue: a case report and literature review. Gastroenterol Rep (Oxf). 2015;3:262-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 11. | Hirokawa YS, Iwata T, Okugawa Y, Tanaka K, Sakurai H, Watanabe M. HER2-positive adenocarcinoma arising from heterotopic pancreas tissue in the duodenum: A case report. World J Gastroenterol. 2021;27:4738-4745. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 2] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Fukino N, Oida T, Mimatsu K, Kuboi Y, Kida K. Adenocarcinoma arising from heterotopic pancreas at the third portion of the duodenum. World J Gastroenterol. 2015;21:4082-4088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Fujita K, Hirakawa K, Matsumoto T, Amano K, Yanai S, Fujioka S, Himeno Y, Motoyama K, Nakashima Y, Iida M. Small-intestinal cancer arising from heterotopic pancreas. Endoscopy. 2008;40 Suppl 2:E240-E241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Bini R, Voghera P, Tapparo A, Nunziata R, Demarchi A, Capocefalo M, Leli R. Malignant transformation of ectopic pancreatic cells in the duodenal wall. World J Gastroenterol. 2010;16:1293-1295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Song JY, Han JY, Choi SK, Kim L, Choi SJ, Park IS, Chu YC, Kim KH, Kim JM. Adenocarcinoma with intraductal papillary mucinous neoplasm arising in jejunal heterotopic pancreas. Korean J Pathol. 2012;46:96-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Stock C, Keutgen XM, Pisapia D, Crawford C, Zarnegar R. Heterotopic pancreatic neoplasm presenting as an obstructing mass at the fourth portion of the duodenum. JOP. 2011;12:241-243. [PubMed] |
| 17. | Okamoto H, Kawaoi A, Ogawara T, Fujii H. Invasive ductal carcinoma arising from an ectopic pancreas in the gastric wall: a long-term survival case. Case Rep Oncol. 2012;5:69-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Kinoshita H, Yamaguchi S, Shimizu A, Sakata Y, Arii K, Mori K, Nasu T. Adenocarcinoma arising from heterotopic pancreas in the duodenum. Int Surg. 2012;97:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Ginori A, Vassallo L, Butorano MA, Bettarini F, Di Mare G, Marrelli D. Pancreatic adenocarcinoma in duodenal ectopic pancreas: a case report and review of the literature. Pathologica. 2013;105:56-58. [PubMed] |
| 20. | Hisanaga E, Sano T, Kubo N, Ishii N, Shirabe K, Takagi H, Hirato J, Ikota H. Adenocarcinoma with intraductal papillary mucinous neoplasm arising in a duodenal heterotopic pancreas: a case report. Clin J Gastroenterol. 2020;13:1373-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 21. | Jung HS, Lee J, Nam KH, Jeong SJ, Oh EH, Park YE, Park J, Kim TO. Gastric Adenocarcinoma Arising from Heterotopic Pancreas Presenting as Gastric Outlet Obstruction 10 Years after the First Diagnosis. Korean J Gastroenterol. 2020;76:37-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Oto I, Yabe N, Yajima K, Iwama N, Takenoya T, Yoshikawa T, Osumi K, Murai S, Yamada T. [A Case of Adenocarcinoma Arising from an Ectopic Pancreas of the Stomach Presenting as Pyloric Stenosis]. Gan To Kagaku Ryoho. 2021;48:1613-1615. [PubMed] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/