©The Author(s) 2026.
World J Gastrointest Endosc. Jan 16, 2026; 18(1): 112943
Published online Jan 16, 2026. doi: 10.4253/wjge.v18.i1.112943
Published online Jan 16, 2026. doi: 10.4253/wjge.v18.i1.112943
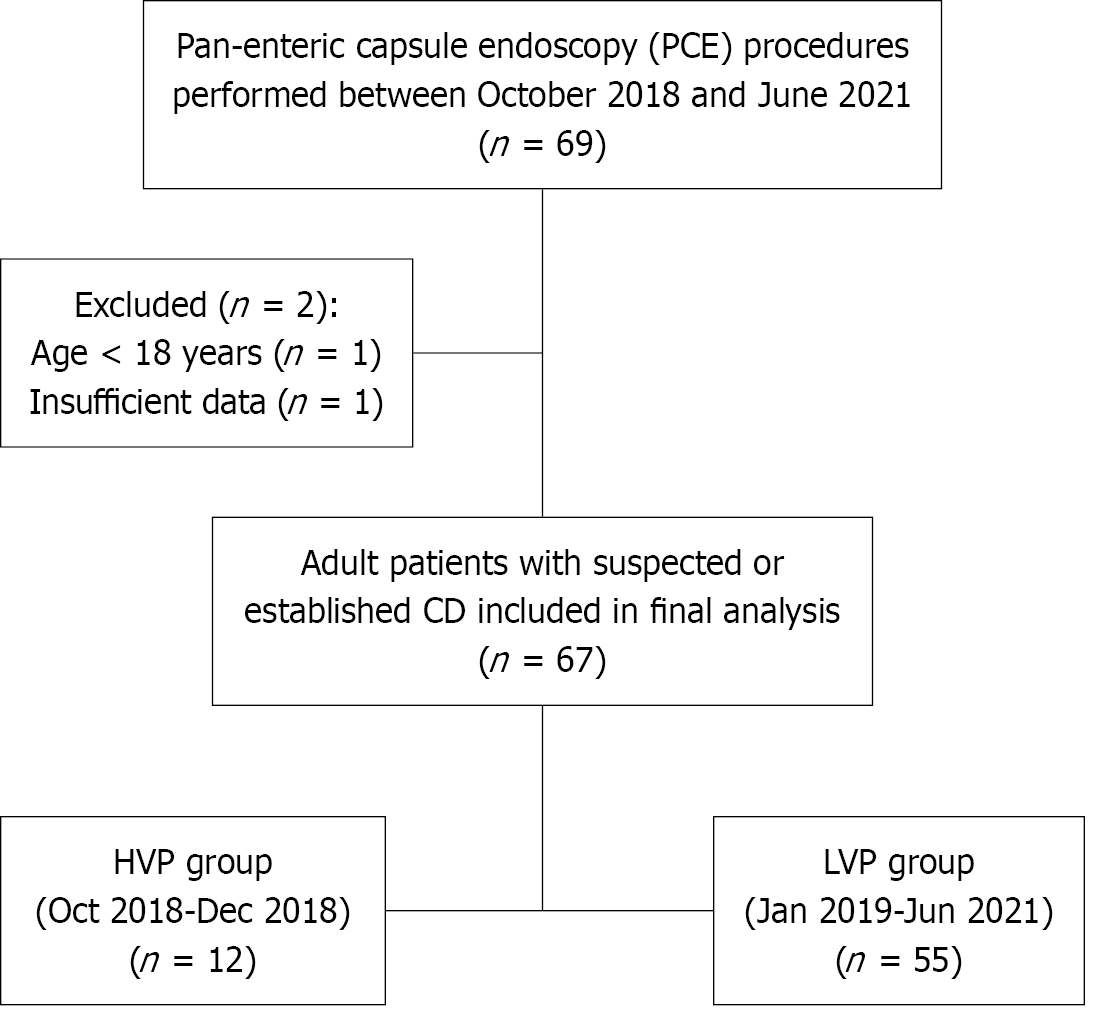
Figure 1 Study flow diagram.
Between October 2018 and June 2021, 69 patients underwent pan-enteric capsule endoscopy. Two were excluded, leaving 67 adults with suspected or established Crohn’s disease. Initially, 12 patients were examined using a high-volume preparation (October 2018 to December 2018). Thereafter, the protocol was replaced by a low-volume preparation, applied in 55 patients (January 2019 to June 2021). PCE: Pan-enteric capsule endoscopy; CD: Crohn’s disease; LVP: Low-volume protocol; HVP: High-volume protocol.
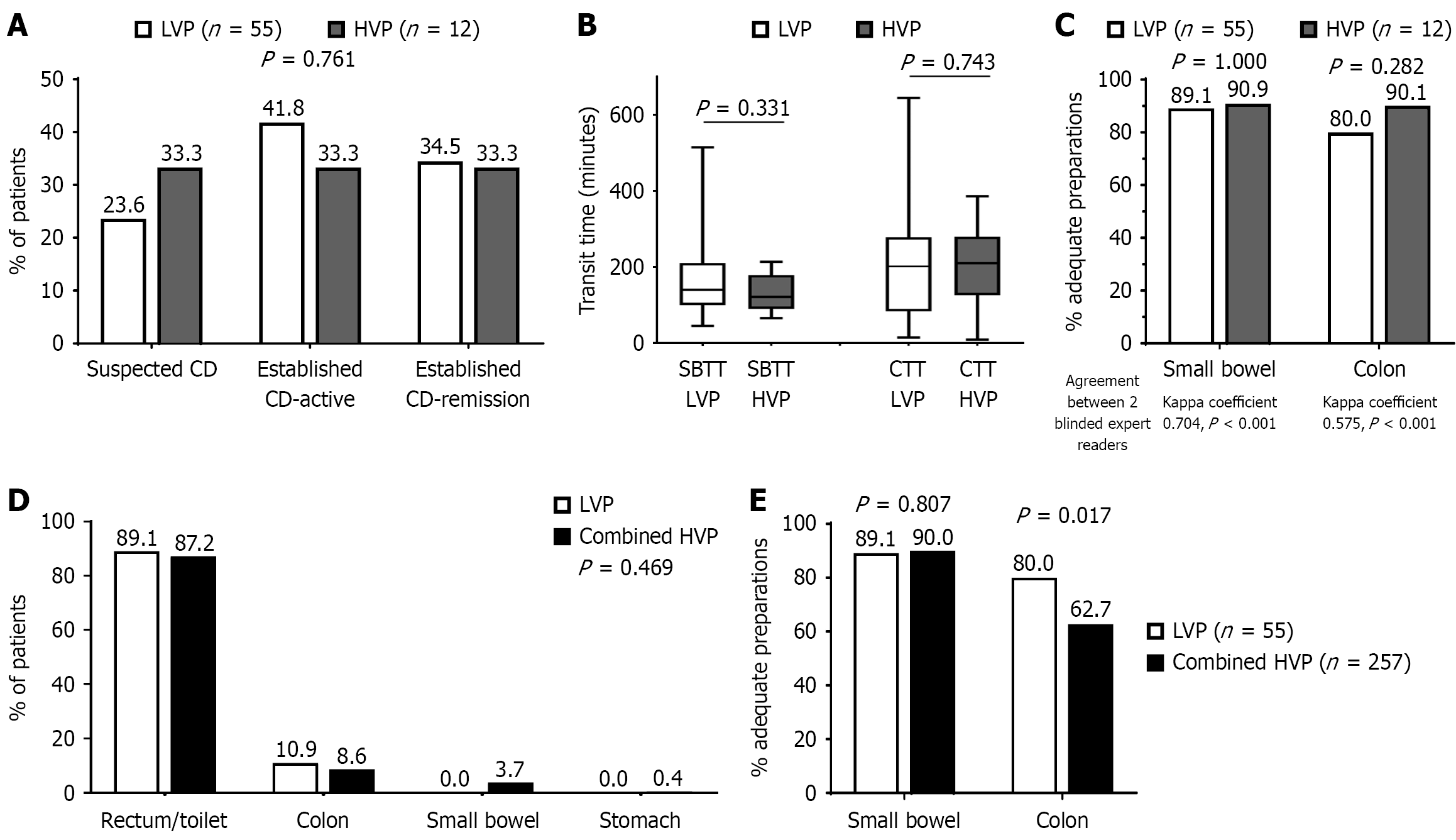
Figure 2 Comparison of study outcomes.
A-C: Comparison of study outcomes between the low-volume protocol group and the high-volume protocol group. Distribution of the three possible indications for pan-enteric capsule endoscopy referral within each group (A); small bowel transit time and colonic transit time among the low-volume protocol group (LVP) and the high-volume protocol (HVP) group (B); comparable adequate preparation rates between the LVP and the HVP group; agreement between 2 blinded expert readers (C); D and E: Comparison of study outcomes between the LVP and the combined HVP group. Location of the capsule at the end of the study (maximal study duration was approximately 12 hours) (D); adequate preparation rates between the LVP and the combined HVP group (E). P < 0.05 vs combined high-volume protocol. LVP: Low-volume protocol; HVP: High-volume protocol; CD: Crohn’s disease; CTT: Colonic transit time; SBTT: Small bowel transit time.
- Citation: Livne S, Cohen NA, Fliss-Isakov N, Leshno M, Maharshak N, Niv E, Deutsch L. Low-volume bowel preparation provides safe and effective pan-enteric capsule endoscopy in suspected or established Crohn’s disease. World J Gastrointest Endosc 2026; 18(1): 112943
- URL: https://www.wjgnet.com/1948-5190/full/v18/i1/112943.htm
- DOI: https://dx.doi.org/10.4253/wjge.v18.i1.112943