Published online Dec 28, 2017. doi: 10.4254/wjh.v9.i36.1378
Peer-review started: June 19, 2017
First decision: July 20, 2017
Revised: November 8, 2017
Accepted: November 19, 2017
Article in press: November 20, 2017
Published online: December 28, 2017
Processing time: 192 Days and 12 Hours
Primary biliary cholangitis (PBC) is a progressive cholestatic liver disease characterized by the presence of highly specific antimitochondrial antibodies, portal inflammation and lymphocyte-dominated destruction of the intrahepatic bile ducts, which leads to cirrhosis. While its pathogenesis remains unclear, PBC that shows histological progression to fibrosis carries a high risk of carcinogenesis; the same is true of viral liver diseases. In patients with PBC, the development of hepatocellular carcinoma (HCC) is rare; the development of combined hepatocellular carcinoma and cholangiocellular carcinoma (cHCC-CCC) is extraordinary. Herein, we report a rare case of PBC metachronously complicated by cHCC-CCC and HCC, which, to the best of our knowledge, has never been reported. We present a case report of a 74-year-old Japanese woman who was diagnosed as PBC in her 40’s by using blood tests and was admitted to our department for further management of an asymptomatic liver mass. She had a tumor of 15 mm in size in segment 8 of the liver and underwent a partial resection of the liver. Subsequent pathological findings resulted in the diagnosis of cHCC-CCC, arising from stage 3 PBC. One year after the initial hepatectomy, a second tumor of 10 mm in diameter was found in segment 5 of the liver; a partial resection of the liver was performed. Subsequent pathological findings led to HCC diagnosis. The component of HCC in the initial tumor displayed a trabecular growth pattern while the second HCC showed a pseudoglandular growth pattern, suggesting that metachronous tumors that arise from PBC are multicentric.
Core tip: Primary biliary cholangitis (PBC) is a progressive cholestatic liver disease characterized by the presence of highly specific antimitochondrial antibodies, portal inflammation and lymphocyte-dominated destruction of the intrahepatic bile ducts, which leads to cirrhosis. While its pathogenesis remains unclear, PBC that shows histological progression to fibrosis carries a high risk of carcinogenesis; the same is true of viral liver diseases. In patients with PBC, the development of hepatocellular carcinoma is rare; the development of combined hepatocellular carcinoma and cholangiocellular carcinoma (cHCC-CCC) is extraordinary. Herein, we report a rare case of PBC metachronously complicated by cHCC-CCC and HCC, which, to the best of our knowledge, has never been reported.
- Citation: Ide R, Oshita A, Nishisaka T, Nakahara H, Aimitsu S, Itamoto T. Primary biliary cholangitis metachronously complicated with combined hepatocellular carcinoma-cholangiocellular carcinoma and hepatocellular carcinoma. World J Hepatol 2017; 9(36): 1378-1384
- URL: https://www.wjgnet.com/1948-5182/full/v9/i36/1378.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i36.1378
Primary biliary cholangitis (PBC)[1] is a progressive cholestatic liver disease characterized by the presence of a highly specific antimitochondrial antibody, portal inflammation, and lymphocyte-dominated destruction of the intralobular bile ducts, which lead to cirrhosis. According to recent and relatively large cohort studies conducted in European countries, the United States and Japan, the development of hepatocellular carcinoma (HCC) is estimated to be 0.7%-3.6%; this frequency increases as histological stages progress[2]. While its pathogenesis remains unclear, PBC cases that display histological progression to fibrosis are at a high risk of carcinogenesis; the same is true of viral liver diseases[3,4]. Although some cases of PBC complicated by HCC have been reported[5-8], to our knowledge, a case of PBC with cholangiocellular carcinoma (CCC) has never been described. In patients with PBC, the development of combined hepatocellular carcinoma and cholangio cellular carcinoma (cHCC-CCC) is extremely rare[9]. Herein, we report a case of PBC metachronously complicated by cHCC-CCC and HCC.
A 74-year-old Japanese woman was diagnosed as PBC in her 40’s by using blood tests. Imaging studies, including abdominal ultrasonography (US) and computed tomography (CT), and tumor markers consisting of alpha fetoprotein (AFP) and protein induced by vitamin K absence (PIVKA-II) were checked up every 6 mo to 12 mo[4]. She was admitted to our department for further management of an asymptomatic liver mass. The patient denied alcohol consumption. Hepatitis B virus antigen and anti-hepatitis C virus antibody tests were negative. Liver function test results, with daily intake of 600 mg of ursodeoxycholic acid, were stable. Serum levels of AFP, PIVKA-II, carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9 and the L3 fraction of AFP were all within normal limits (Table 1).
| WBC | 5800/μL | ALP | 228 U/L | PIVKA-II | 18 mAU/mL |
| RBC | 432 × 104/μL | γ-GTP | 65 U/L | AFP | 3 ng/mL |
| Hb | 13.0 g/dL | ChE | 280 IU/L | AFP-L3 | 0.5% |
| Ht | 38% | BUN | 14.5 mg/dL | CEA | 1.2 ng/mL |
| Plt | 22.6 × 104/μL | Cr | 0.54 mg/dL | CA 19-9 | 7 U/mL |
| PT | 77.3% | T-Chol | 203 mg/dL | ANA | × 40 |
| PT-INR | 1.04 | TG | 77 mg/dL | AMA | × 640 |
| TP | 7.9 g/dL | ICG-R15 | 8.3% | AMA-M2 | 158 Index |
| Alb | 4.2 g/dL | Glucose | 109 mg/dL | HBs Ag | (-) |
| TBil | 0.5 mg/dL | CRP | 0.2 mg/dL | HBs Ab | (-) |
| AST | 19 U/L | IgG | 1760 mg/dL | HBc Ab | (-) |
| ALT | 14 U/L | IgM | 305 mg/dL | HCV Ab | (-) |
| LDH | 183 U/L |
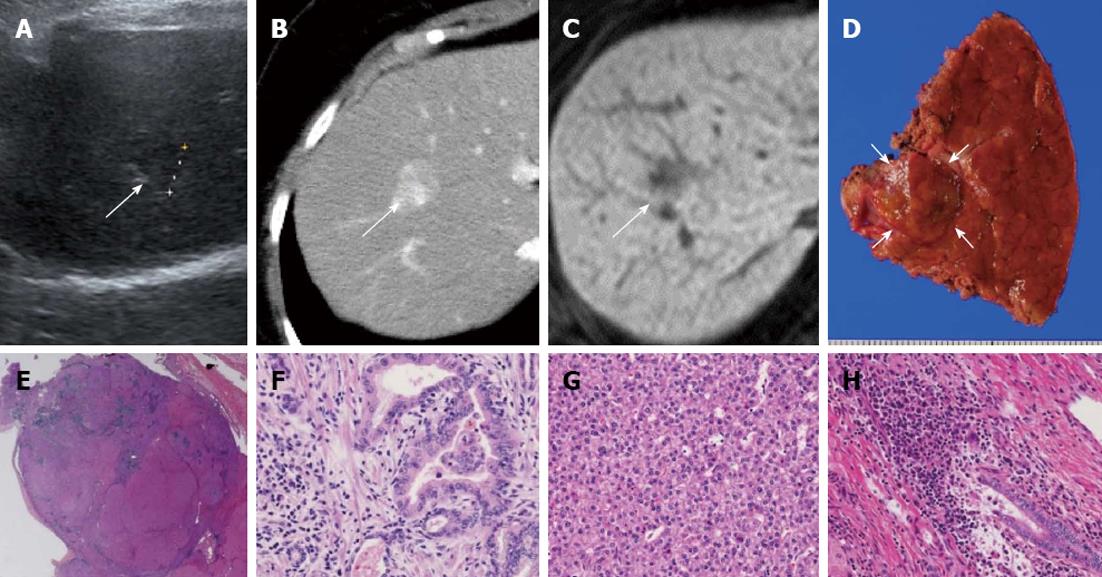
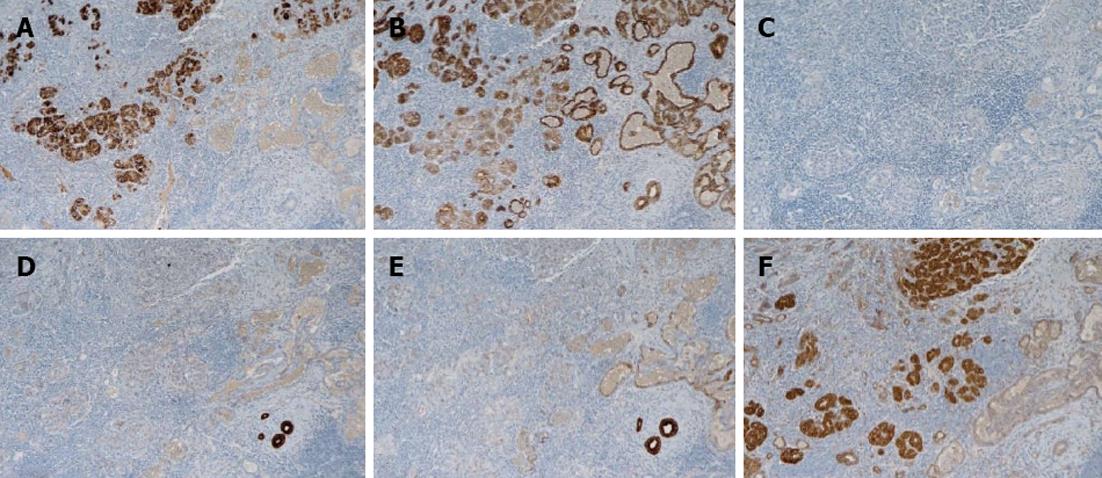
Abdominal US, dynamic CT, and magnetic resonance imaging (MRI) showed a liver tumor of 15 mm in size in segment 8 of the liver. Since the tumor was located in the peripheral lesion and was in contact with the middle hepatic vein (MHV), we performed partial resection of the liver in segment 8 including partial resection of MHV. Hematoxylin-eosin (HE) staining revealed two components consisting of the trabecular type of HCC and CCC, resulting in the definitive diagnosis of cHCC-CCC. According to the classification for the severity of PBC[10,11], the hepatic parenchyma, excluding carcinomatous tissue, showed stage 3 PBC (Figure 1). In the immunohistochemistry, the component of HCC was negative for AFP but positive for cytokeratin (CK) 18 and hepatocyte, while that of CCC was positive for CK7 and CK19. The components of both HCC and CCC are positive for the epithelial cell adhesion molecule (EpCAM) (Figure 2).
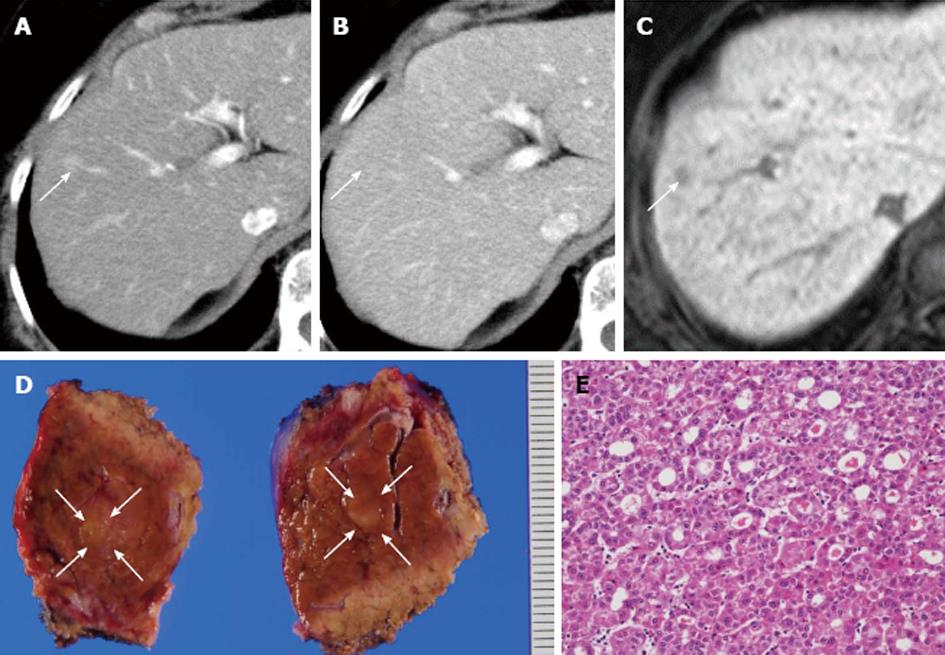
One year after the initial hepatectomy, tumor marker levels for AFP, PIVKA-II, CEA and CA 19-9 were within normal limits; only AFP-L3 isoform level was elevated (Table 2). Dynamic CT and MRI showed a peripheral tumor of 10 mm in diameter in segment 5 of the liver. Since it was not possible to detect the tumor with intraoperative US, partial resection of the liver on the basis of the anatomical structure, including the Glissonean sheath and the hepatic vein, was performed. HE staining revealed a pseudoglandular pattern of HCC (Figure 3). In the immunohistochemistry, recurrent HCC was negative for AFP and EpCAM but positive for CK18 and hepatocyte (data not shown). There was no recurrence and/or metastasis 10 mo after re-hepatectomy.
| WBC | 3600/μL | AST | 29 U/L | PIVKA-II | 28 mAU/mL |
| RBC | 397 × 104/μL | ALT | 18 U/L | AFP | 5 ng/mL |
| Hb | 12.0 g/dL | LDH | 186 U/L | AFP-L3 | 11.7% |
| Ht | 35.9% | ALP | 300 U/L | CEA | 1.0 ng/mL |
| Plt | 22.3 × 104/μL | γ-GTP | 79 U/L | CA 19-9 | 29 U/mL |
| PT | 77.3% | ChE | 211 IU/L | ICG-R15 | 7.4% |
| PT-INR | 1.12 | BUN | 16.1 mg/dL | Glucose | 138 mg/dL |
| TP | 7.3 g/dL | Cr | 0.6 mg/dL | CRP | 0.2 mg/dL |
| Alb | 3.8 g/dL | T-Bil | 0.4 mg/dL |
While some cases of PBC complicated by HCC have been reported[5-8], only 1 case of PBC with cHCC-CCC has been reported[9]. The present case of PBC was metachronously complicated by both cHCC-CCC and HCC; to the best of our knowledge, such a case has never been reported.
While the etiology of PBC remains unknown, it is well known that the intrahepatic bile ducts are to be destructed slowly and progressively, leading to cirrhosis[12]. PBC occurs more often in middle-aged women and is often asymptomatic in its early stage[13,14]. The frequency of HCC development in patients with PBC is estimated to be 0.7%-3.6%. While this frequency increases as the histological stages progress[2,5,6,9,11,15-20], the carcinogenic mechanism of primary liver cancer in PBC remains unclear. Although our patient’s PBC progressed to stage 3 of 4, when primary liver cancer was found, she had no liver cirrhosis symptoms.
Few studies have evaluated the imaging characteristics of cHCC-CCC, and no studies have evaluated the ability of preoperative imaging to determine diagnosis. The appearance of HCC and CCC is well known on contrast-enhanced MRI and CT. The histological composition and relative ratio of CCC and HCC components within cHCC-CCC appear to dictate the imaging appearance. Tumors may show features typical of HCC, such as arterial enhancement, washout and pseudocapsule, whereas other regions within the tumor show progressive or delayed enhancement, necrosis and possible ductal dilation more akin to CCC[21]. The cHCC-CCC display enhancement patterns resembling CCC or HCC in comparable proportion on both contrast-enhanced US and CT[22]. Some suggest that the combination of imaging features and tumor markers may be helpful in preoperative diagnosis of cHCC-CCC[23]. In our case, since dynamic CT showed arterial enhancement and washout imaging, we performed initial hepatectomy expected for HCC.
Allen et al[24] classified cHCC-CCC into three subtypes: type A, “double cancer” representing cases in which HCC and CCC exist separately; type B, “combined” type, HCC and CCC components existing contiguously, but independently; and type C, “mixed” type, consisting of truly combined HCC and CCC components originating from the same tumor. Based on the morphological findings from HE staining, the present case was classified as mixed type cHCC-CCC.
In recent years, the ability of hepatic precursor cells to differentiate into hepatocytes and bile duct cells, and of hepatic stem cells to proliferate and differentiate have been proposed. As candidate stem cells, cells derived from the Herring duct or small oval cells may be able to differentiate into hepatocytes and bile duct cells[25-27]. Carcinogenesis of the precursor cells has been suggested as a developmental mechanism for cHCC-CCC with tissue components of HCC and CCC. In the present case, as Theise et al[28] indicated, the result of EpCAM immunohistochemistry (a stem cell marker), might be consistent with that of mixed type cHCC-CCC.
The pathological results of the initial tumor showed the trabecular pattern in the component of HCC, while that of the second tumor showed the pseudoglandular pattern in HCC. Immunohistochemistry also revealed the different pattern, which led the authors to speculate that the second tumor did not recur from the HCC component of cHCC-CCC, but the multicentric development of PBC-derived metachronous tumors.
In conclusion, we herein report a rare case of PBC metachronously complicated by both cHCC-CCC and HCC. In patients with PBC, it is necessary to check up not only liver function but also carcinogeneses, including HCC, CCC and cHCC-CCC.
A 74-year-old Japanese woman was diagnosed as primary biliary cholangitis (PBC) in her 40’s by using blood tests. Imaging studies, including abdominal ultrasonography (US) and computed tomography (CT), and tumor markers consisting of alpha fetoprotein (AFP) and protein induced by vitamin K absence (PIVKA-II) were checked up every 6-12 mo. She was admitted to the authors’ department for further management of an asymptomatic liver mass.
Combined hepatocellular carcinoma and cholangiocellular carcinoma (cHCC-CCC), hepatocellular carcinoma (HCC) and cholangiocellular carcinoma (CCC) were considered from imaging tests.
In the initial surgery, serum levels of AFP, PIVKA-II, carcinoembryonic antigen (CEA), carbohydrate antigen (CA) 19-9, and the L3 fraction of AFP were all within normal limits. One year after the initial hepatectomy, tumor marker levels for AFP, PIVKA-II, CEA, and CA 19-9 were within normal limits; only AFP-L3 isoform level was elevated.
The authors diagnosed both the first and second tumors as HCC from the imaging findings.
First, hematoxylin-eosin (HE) staining revealed two components, consisting of the trabecular type of HCC and CCC, resulting in the definitive diagnosis of cHCC-CCC. Second, HE staining revealed a pseudoglandular pattern of HCC.
The first one was that the tumor was involved in middle hepatic vein (MHV). If radiofrequency ablation was performed, the cooling effect around the MHV would have occurred, leading to the insufficient ablation. The second one was that the tumor was not detected using US preoperatively. Moreover, the tumor was not detected even with intraoperative contrast-enhanced US. Therefore, the authors performed partial resection on the basis of the anatomical structure, including the Glissonean sheath and the hepatic vein.
This report relates to this reference: Kobayashi M, Furuta K, Kitamura H, Oguchi K, Arai M, Koike S, Nakazawa K. A case of primary biliary cirrhosis that complicated with combined hepatocellular and cholangiocellular carcinoma. Clin J Gastroenterol 2011; 4: 236-241.
PBC: Primary biliary cholangitis, is marked by slow progressive destruction of the intrahepatic bile ducts, which leads to cirrhosis.
In patients with PBC, it is necessary to check up not only liver function but also carcinogenesis including HCC, CCC and cHCC-CCC.
| 1. | Beuers U, Gershwin ME, Gish RG, Invernizzi P, Jones DE, Lindor K, Ma X, Mackay IR, Parés A, Tanaka A. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Clin Res Hepatol Gastroenterol. 2015;39:e57-e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 2. | Abe M, Onji M. Natural history of primary biliary cirrhosis. Hepatol Res. 2008;38:639-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4333] [Cited by in RCA: 4520] [Article Influence: 215.2] [Reference Citation Analysis (4)] |
| 4. | Silveira MG, Suzuki A, Lindor KD. Surveillance for hepatocellular carcinoma in patients with primary biliary cirrhosis. Hepatology. 2008;48:1149-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Nijhawan PK, Therneau TM, Dickson ER, Boynton J, Lindor KD. Incidence of cancer in primary biliary cirrhosis: the Mayo experience. Hepatology. 1999;29:1396-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Jones DE, Metcalf JV, Collier JD, Bassendine MF, James OF. Hepatocellular carcinoma in primary biliary cirrhosis and its impact on outcomes. Hepatology. 1997;26:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 102] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Lööf L, Adami HO, Sparén P, Danielsson A, Eriksson LS, Hultcrantz R, Lindgren S, Olsson R, Prytz H, Ryden BO. Cancer risk in primary biliary cirrhosis: a population-based study from Sweden. Hepatology. 1994;20:101-104. [PubMed] |
| 8. | Yano Y, Yoon S, Seo Y, Ninomiya T, Nagano H, Nakaji M, Hayashi Y, Kasuga M. A case of well-differentiated hepatocellular carcinoma arising in primary biliary cirrhosis. Kobe J Med Sci. 2003;49:39-43. [PubMed] |
| 9. | Kobayashi M, Furuta K, Kitamura H, Oguchi K, Arai M, Koike S, Nakazawa K. A case of primary biliary cirrhosis that complicated with combined hepatocellular and cholangiocellular carcinoma. Clin J Gastroenterol. 2011;4:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Scheuer P. Primary biliary cirrhosis. Proc R Soc Med. 1967;60:1257-1260. [PubMed] |
| 11. | Nakamura M, Kondo H, Mori T, Komori A, Matsuyama M, Ito M, Takii Y, Koyabu M, Yokoyama T, Migita K. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 2007;45:118-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 12. | Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 940] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 13. | Heathcote EJ. Management of primary biliary cirrhosis. The American Association for the Study of Liver Diseases practice guidelines. Hepatology. 2000;31:1005-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 278] [Article Influence: 10.7] [Reference Citation Analysis (1)] |
| 14. | Kadokawa Y, Omagari K, Ohba K, Kitamura S, Ohara H, Takeshima F, Mizuta Y, Nanashima A, Yamaguchi H, Kohno S. Hepatocellular carcinoma in a male patient with early stage (stage I) primary biliary cirrhosis. Intern Med. 2005;44:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Caballería L, Parés A, Castells A, Ginés A, Bru C, Rodés J. Hepatocellular carcinoma in primary biliary cirrhosis: similar incidence to that in hepatitis C virus-related cirrhosis. Am J Gastroenterol. 2001;96:1160-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Cavazza A, Caballería L, Floreani A, Farinati F, Bruguera M, Caroli D, Parés A. Incidence, risk factors, and survival of hepatocellular carcinoma in primary biliary cirrhosis: comparative analysis from two centers. Hepatology. 2009;50:1162-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Floreani A, Baragiotta A, Baldo V, Menegon T, Farinati F, Naccarato R. Hepatic and extrahepatic malignancies in primary biliary cirrhosis. Hepatology. 1999;29:1425-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Harada K, Hirohara J, Ueno Y, Nakano T, Kakuda Y, Tsubouchi H, Ichida T, Nakanuma Y. Incidence of and risk factors for hepatocellular carcinoma in primary biliary cirrhosis: national data from Japan. Hepatology. 2013;57:1942-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Howel D, Metcalf JV, Gray J, Newman WL, Jones DE, James OF. Cancer risk in primary biliary cirrhosis: a study in northern England. Gut. 1999;45:756-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Shibuya A, Tanaka K, Miyakawa H, Shibata M, Takatori M, Sekiyama K, Hashimoto N, Amaki S, Komatsu T, Morizane T. Hepatocellular carcinoma and survival in patients with primary biliary cirrhosis. Hepatology. 2002;35:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 73] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Fowler KJ, Sheybani A, Parker RA 3rd, Doherty S, M Brunt E, Chapman WC, Menias CO. Combined hepatocellular and cholangiocarcinoma (biphenotypic) tumors: imaging features and diagnostic accuracy of contrast-enhanced CT and MRI. AJR Am J Roentgenol. 2013;201:332-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 22. | Li R, Yang D, Tang CL, Cai P, Ma KS, Ding SY, Zhang XH, Guo DY, Yan XC. Combined hepatocellular carcinoma and cholangiocarcinoma (biphenotypic) tumors: clinical characteristics, imaging features of contrast-enhanced ultrasound and computed tomography. BMC Cancer. 2016;16:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 23. | Jarnagin WR, Weber S, Tickoo SK, Koea JB, Obiekwe S, Fong Y, DeMatteo RP, Blumgart LH, Klimstra D. Combined hepatocellular and cholangiocarcinoma: demographic, clinical, and prognostic factors. Cancer. 2002;94:2040-2046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 260] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 24. | Allen RA, Lisa JR. Combined liver cell and bile duct carcinoma. Am J Pathol. 1949;25:647-655. [PubMed] |
| 25. | Kofman AV, Morgan G, Kirschenbaum A, Osbeck J, Hussain M, Swenson S, Theise ND. Dose- and time-dependent oval cell reaction in acetaminophen-induced murine liver injury. Hepatology. 2005;41:1252-1261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 26. | Tan J, Hytiroglou P, Wieczorek R, Park YN, Thung SN, Arias B, Theise ND. Immunohistochemical evidence for hepatic progenitor cells in liver diseases. Liver. 2002;22:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 100] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Zuckerman E, Misselevich I, Boss JH. Oval cell hyperplasia in asparaginase--induced liver damage. Liver. 2002;22:363-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 28. | Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic ‘stem cell’ malignancies in adults: four cases. Histopathology. 2003;43:263-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 154] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bessone FO, Bhatti ABH, Giorgio A, Yu WB S- Editor: Kong JX L- Editor: Filipodia E- Editor: Wang CH