Published online Dec 28, 2016. doi: 10.4254/wjh.v8.i36.1623
Peer-review started: July 3, 2016
First decision: August 5, 2016
Revised: September 16, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: December 28, 2016
Processing time: 179 Days and 10.1 Hours
To identify significant liver disease [including nodular regenerative hyperplasia (NRH)] in asymptomatic Didanosine (DDI) exposed human immunodeficiency virus (HIV) positive patients.
Patients without known liver disease and with > 6 mo previous DDI use had liver stiffness assessed by transient elastography (TE). Those with alanine transaminase (ALT) above upper limit normal and/or TE > 7.65 kPa underwent ultrasound scan (U/S). Patients with: (1) abnormal U/S; or (2) elevated ALT plus TE > 7.65 kPa; or (3) TE > 9.4 kPa were offered trans-jugular liver biopsy (TJLB) with hepatic venous pressure gradient (HVPG) assessment.
Ninety-nine patients were recruited, median age 50 years (range 31-70), 81% male and 70% men who have sex with men. Ninety-five percent with VL < 50 copies on antiretroviral therapy with median CD4 count 639 IU/L. Median DDI exposure was 3.4 years (range 0.5-14.6). Eighty-one had a valid TE readings (interquartile range/score ratio < 0.3): 71 (88%) < 7.65 kPa, 6 (7%) 7.65-9.4 kPa and 4 (6%) > 9.4 kPa. Seventeen (17%) met criteria for TJLB, of whom 12 accepted. All had HVPG < 6 mmHg. Commonest histological findings were steatosis (n = 6), normal architecture (n = 4) and NRH (n = 2), giving a prevalence of previously undiagnosed NRH of 2% (95%CI: 0.55%, 7.0%).
A screening strategy based on TE, liver enzymes and U/S scan found a low prevalence of previously undiagnosed NRH in DDI exposed, asymptomatic HIV positive patients. Patients were more likely to have steatosis highlighting the increased risk of multifactorial liver disease in this population.
Core tip: Human immunodeficiency virus positive patients are at increased risk of liver disease. The aetiology is often multifactorial and includes exposure to antiretroviral therapy. We used a simple screening strategy based on transient elastography, liver enzymes and ultrasound scan to identify that 2% of asymptomatic patients exposed to Didanosine in a clinical cohort had undiagnosed nodular regenerative hyperplasia. A further 6% had undiagnosed steatosis. Implementation of a screening strategy enables identification of liver disease and initiation of earlier targeted interventions in this high-risk group.
- Citation: Logan S, Rodger A, Maynard-Smith L, O’Beirne J, Fernandez T, Ferro F, Smith C, Bhagani S. Prevalence of significant liver disease in human immunodeficiency virus-infected patients exposed to Didanosine: A cross sectional study. World J Hepatol 2016; 8(36): 1623-1628
- URL: https://www.wjgnet.com/1948-5182/full/v8/i36/1623.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i36.1623
Nodular regenerative hyperplasia (NRH) - the development of micronodules in liver parenchyma without intervening fibrosis[1] - has been reported in human immunodeficiency virus (HIV) positive patients who often present late in the course of the condition with complications associated with non-cirrhotic portal hypertension (NCPH). A strong association with NRH is current or previous use of Didanosine (DDI)[1-6]. Although DDI is no longer used as first line antiretroviral therapy (ART), many HIV positive patients have significant previous exposure with a reported prevalence of NRH of between 0.5%-35%[1,2,6]. This wide range is indicative of the unreliability of screening strategies and the fact that studies largely included individuals diagnosed with NRH or NCPH as a result of liver biopsy for other conditions or as a result of late presentation with complications of portal hypertension[1-6]. However prior to this the disease is largely sub clinical and a screening strategy may be useful to identify patients with DDI associated liver disease earlier in the disease process.
Other studies have reported raised transaminases in NRH, but in many cases transaminases are only mildly elevated or not at all this indicating the unreliability of this alone as a screening tool[1]. Hepatic transient elastography (TE) is a validated non-invasive tool with good correlation for identifying hepatic and peri-portal fibrosis[2,7]. TE is also associated with hepatic venous pressure gradients (HVPG) in cirrhosis[8]. The co-relation with NRH is less well delineated, however increased liver stiffness readings have been reported in patients with both NRH and NCPH[9-14], although one study found that liver stiffness readings did not predict the presence of portal hypertension in NRH[10]. Many studies also report reduced platelet levels and the presence of splenomegaly in individuals with NRH[1].
Our aim was to develop and implement a screening strategy incorporating 3 separate measures; TE, platelet and alanine transaminase (ALT) levels, with subsequent ultrasound and trans-jugular liver biopsy (TJLB) in those who met criteria to identify DDI related liver disease in HIV positive patients with previous significant DDI exposure, but who were currently asymptomatic.
This study is a cross-sectional study in HIV outpatients at The Royal Free London NHS Foundation Trust from 2010 to 2011. Ethical approval was obtained (REC 10 /H0720/54). Study subjects were identified from the HIV clinical database. HIV positive patients currently under active follow-up and previously exposed to DDI therapy for longer than 6 mo were eligible to take part. Exclusions were viral hepatitis co-infection, age < 18 years, a body mass index (BMI) > 40, pregnancy or ascites. Patients were sequentially recruited as they attended for routine clinic follow-up. Statistical review of the study was performed by a biomedical statistician (Dr. Colette Smith).
Patients completed a study specific questionnaire on sociodemographic factors, medical history, lifestyle including smoking, alcohol [Michigan Alcoholism Screening test (MAST)] and drug use. Clinical data (HIV viral load, CD4 count, whether on/off treatment, date of diagnosis, date of ART start, lipids, liver panel bloods, blood glucose, BMI) were also collected.
Liver TE was measured using FibroScan (FS) (Echosens, Paris). A median stiffness reading was measured using at least ten readings with a valid reading recording 60% accuracy and an interquartile range (IQR) of less than 30% of the median. Subjects were offered further evaluation with ultrasound of liver and spleen with doppler waveforms of the hepatic vasculature if they had either: (1) an ALT level above 19 IU/mL for women and 31 IU/mL for men; or (2) a platelet count (PLT) less than 120 × 109/L; or (3) TE reading of > 7.65 kPa (IQR < 0.3).
Individuals with evidence on ultrasound of splenomegaly or fatty liver or coarse echotexture or abnormalities of hepatic doppler waveforms in conjunction with a raised ALT or TE reading as above were offered a TJLB with HVPG measurements. Patients with a raised ALT or platelets < 120 × 109/L for whom an elastography score was unobtainable (centripetal obesity) or uninterpretable (less than 10 valid readings or IQR/LSM > 30%) were offered ultrasound and TJLB.
All TJLB procedures were performed in the interventional radiology suite by an experienced operator (O’Beirne J) after a 6-h fast, under local anaesthesia. Biopsies were taken using a 19G Tru-cut type biopsy needle (Quick core; Cook, William Cook Europe, Denmark). Three or 4 passes were performed through the same hepatic vein wall (right or middle) to ensure that sufficient liver tissue was obtained. Wedge hepatic vein pressures (WHVP) were measured using a 5-F Berenstein balloon catheter (Boston Scientific, Natick, MA) using the technique described by Groszmann and Wongcharatrawee[15]. Three sets of measurements were taken. WHVP was measured for at least 1 min each time. HVPG was calculated as the mean of the 3 gradients (the difference between WHVP minus free hepatic pressure). Groups were compared using the Mann Whitney U test for non-parametric continuous variables and using the χ2 test for categorical variables.
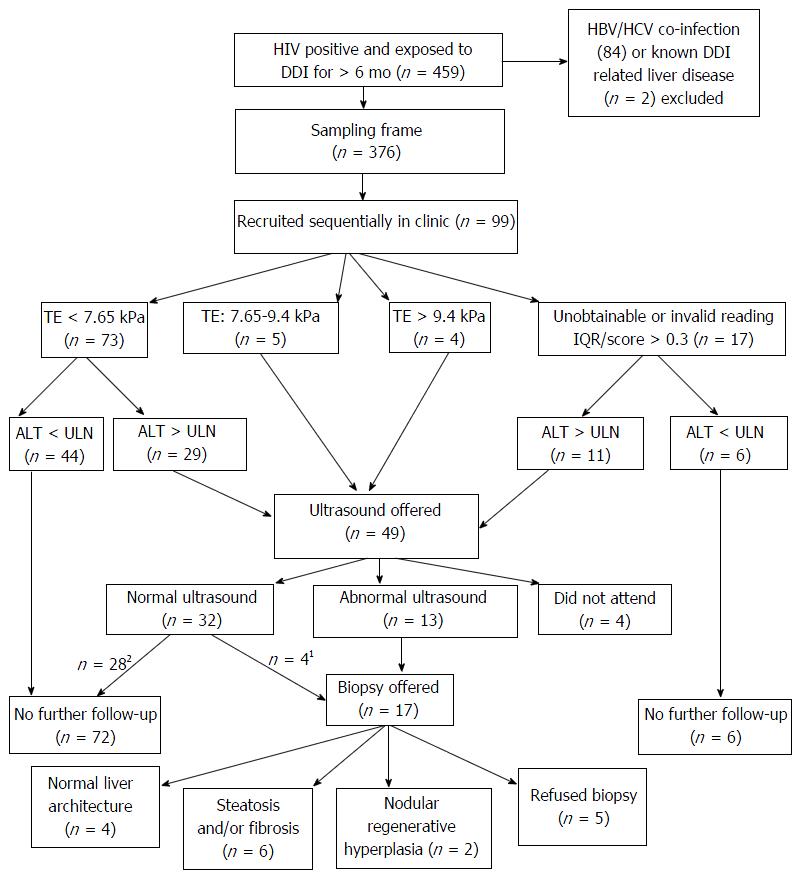
Four hundred and fifty-nine patients exposed to DDI for longer than 6 mo were identified from the clinic database. Eighty-four patients known to have co-infection with hepatitis B or C were excluded. No patients were excluded due to BMI > 40 or presence of ascites. Of the remaining 376 patients, 99 patients were recruited sequentially as they attended HIV clinic during the study time period and response rates in those approached to take part in the study were > 95%.
Characteristics of patients recruited (n = 99) were compared to those not approached to take part (n = 274) to assess potential for recruitment bias. There were no differences in those recruited by sex: 80.8% (80/99 recruited) vs 78.5% (215/274) not recruited were male; P = 0.41, total length of DDI exposure (mean 4.1 years recruited vs 4.1 years not recruited; P = 0.89), most recent median ALT (34 μ/L recruited vs 35 μ/L not recruited; P = 0.85) or most recent median platelets (210 recruited vs 210 not recruited; P = 0.09). However a larger proportion of those of white ethnicity were recruited (77% vs 64% P = 0.02) and of those they had a slightly older mean age (50 years vs 48 years; P = 0.001).
Of the 99 who took part in the study, 75 (75%) were men who have sex with men (MSM). Mean age was 50 years (range: 30 to 70), 76% were White, 19% Black and 5% were of another ethnicity. The majority had well-controlled HIV infection with a median CD4 count of 637 mm3 (IQR: 254 to 1378) and 92% had a suppressed HIV VL at < 40 copies. All were on ART and had been for a median of 15 years (IQR: 13 to 16 years). None were currently on a DDI containing ART regime. Median cumulative time previously on DDI was 43 mo (IQR: 22 mo to 68 mo). Overall, 43 (43%) patients reported never drinking alcohol or consuming less than 2 units monthly. Only 2 patients scored > 6 on the MAST score indicating hazardous drinking.
The screening algorithm is shown in Figure 1. ALT above the upper limit normal (ULN) (19 IU/mL in women and 31 IU/mL in men) was found in 37% (n = 7/19) of women and 50% of men (n = 40/80). Median ALT in men was 32 μ/L (IQR 23-44) and 18 μ/L in women (IQR 15-22). Eight-two (82%) had a valid TE reading (IQR/score ratio < 0.3 and success rate > 60%). Of these, 73 (73%) were < 7.65 kPa, 5 (6%) between 7.65-9.4 kPa and 4 (4%) > 9.4 kPa (Figure 1). Only one subject had platelets < 120 and they were known to have cirrhosis of the liver at study entry.
Ultrasound assessment was offered to 49 patients (49%) based on TE reading and/or ALT result. All those that met the criteria for ultrasound were screened for autoimmune liver disease and thrombophilia with a coagulation screen. Four patients did not attend for ultrasound. The most common abnormality was increased reflectivity indicating fatty filtration in 8 patients (18%). A further 4 patients had a normal liver ultrasound but were offered a TJLB on the basis of their ALT and TE score. In total 17 met criteria for TJLB of whom 12 accepted. The characteristics of these patients are described in Table 1. There were no complications observed from the TJB procedures. In the 5 who did not accept liver biopsy, ultrasound appearances were normal in 2 subjects, indicative of fatty infiltration of the liver in 2 and demonstrated splenomegaly in one. Two had abnormal FS readings > 7.76 kPa.
| Subject number | Gender | Age (yr) | Ethnicity | Prior DDI exposure (mo) | Time since DDI (yr) | BMI (kg/m2) | MAST score | ALT (IU/mL) | PLTs (106/L) | PTT | Alk Phos (U/L) | FS (kPa) | Ultrasound results | Biopsy results | HVPG | Fib 4 |
| Participants who accepted to undergo TJLB as a result of study screening | ||||||||||||||||
| 1 | Male | 43 | White British | 81 | 7 | 25.58 | 0 | 77 | 175 | 14.0 | 83 | 10 | Splenomegaly | NRH with mild steatosis | 5 | 1.29 |
| 2 | Male | 59 | White British | 40 | 6 | 24.42 | 0 | 36 | 175 | 14.8 | 93 | 8 | Normal | NRH | 4 | 2.39 |
| 3 | Male | 48 | White British | 9 | 6 | 29.76 | 0 | 32 | 184 | 14.3 | 108 | NR | Fatty liver, Splenomegaly | Moderate steatosis | 3 | 1.08 |
| 4 | Male | 52 | White British | 70 | 7 | 24.69 | 0 | 50 | 242 | 11.7 | 44 | 5.6 | Fatty liver | Mild steatosis | 3 | 0.79 |
| 5 | Male | 49 | White Other | 34 | 4 | 28.54 | 1 | 60 | 215 | 14.0 | 83 | 12.6 | Fatty liver | Moderate steatosis, moderate fibrosis | 3 | 1.38 |
| 6 | Male | 52 | White British | 21 | 11 | 28.67 | 1 | 58 | 257 | 13.4 | 43 | NR | Fatty liver | Moderate steatosis | Not done | 1.02 |
| 7 | Male | 62 | White Other | 13 | 13 | 32.56 | 2 | 56 | 175 | 11.1 | 45 | NR | Fatty liver | Moderate steatosis | 2 | 2.25 |
| 8 | Male | 50 | White British | 117 | 3 | 31.18 | 0 | 125 | 204 | 10.7 | 58 | 8.7 | Fatty liver | Mild fibrosis with mild steatosis | 2 | 1.71 |
| 9 | Female | 56 | Black African | 24 | 11 | 36.96 | 0 | 27 | 208 | 12.3 | 67 | 4.4 | Coarse echotexture | Normal Architecture | 5 | 1.07 |
| 10 | Male | 57 | White British | 61 | 4 | 0 | 40 | 190 | 13.9 | 71 | NR | Splenomegaly | Normal Architecture | 3 | 1.74 | |
| 11 | Male | 51 | White Irish | 103 | 4 | 20.30 | 0 | 56 | 228 | 10.8 | 154 | 4.8 | Coarse echotexture | Normal Architecture | 3 | 1.04 |
| 12 | Male | 52 | White Other | 44 | 5 | 21.07 | 6 | 24 | 224 | 11.4 | 62 | 9.1 | Normal | Normal Architecture | 2 | 1.16 |
| Participants who refused TJLB offered as a result of study screening | ||||||||||||||||
| 13 | Male | 46 | White British | 74 | 2 | 21.46 | 0 | 18 | 177 | 60 | 10 | Normal | Declined | 1.41 | ||
| 14 | Male | 41 | White British | 97 | 3 | 25.65 | 0 | 63 | 188 | 101 | 7.6 | Splenomegaly | Declined | 1.30 | ||
| 15 | Male | 47 | White British | 33 | 5 | 24.39 | 0 | 34 | 238 | 58 | 7.1 | Fatty liver, dampened waveform | Declined | 0.99 | ||
| 16 | Male | 61 | White British | 110 | 2 | 20.75 | 1 | 35 | 246 | 71 | 9.2 | Normal | Declined | 1.39 | ||
| 17 | Male | 54 | White British | 28 | 5 | 28.93 | 1 | 32 | 333 | 58 | 4.3 | Fatty liver | Declined | 0.67 | ||
Overall, the commonest histological finding on liver biopsy was steatosis (n = 5) or normal architecture (n = 4). All subjects had HVPG < 6 mmHg (n = 11) including the 2 patients with previously undiagnosed NRH on biopsy in-keeping with a pre-sinusoidal component. This gives a prevalence of previously undiagnosed NRH in our cohort of 2% (95%CI: 0.55%, 6.8%).
As a sensitivity analyses we applied our study algorithm to two other patients attending the HIV clinic with previously identified DDI related liver disease. One case had been identified due to complications of portal hypertension and the other from liver biopsy undertaken due to abnormal ultrasound scan (U/S) appearances. Both cases met our study screening criteria to proceed to TJLB indicating they would have been detected using our screening process.
NRH largely presents with complications associated with NCPH after a prolonged asymptomatic period[1-6,9,12]. Although the aetiology may be multi-factorial, an over-riding association is use of DDI. A recent study also identified an association between single-nucleotide polymorphisms in the 5’-nucleotidase and xanthine oxidase genes and development of NCPH after DDI exposure[13] suggesting an element of genetic predisposition via the purine metabolism pathway.
Our study is the first to use a screening strategy to identify asymptomatic individuals with a previous DDI exposure but with no known liver disease at study entry. Such a strategy is important to identify liver disease at an earlier stage so that preventative measures and risk minimisation strategies may be instituted. Using our screening strategy we found 2 cases of previously undiagnosed NRH, but no cases of NCPH. Our strategy was based on TE, platelets and ALT levels, with ultrasound and subsequent TJLB in those who met criteria. We used a combination of methods to improve sensitivity of the screening strategy. Elevated transaminases, low platelets and moderate elevations in TE readings have all been described in known cases of NRH or NCPH. We chose a low TE cut off of 7.65 kPa as one study reported a median FS value of 7.9 kPa in subjects with biopsy confirmed NRH[10]. In addition in order to identify individuals with very low-level transaminase elevations we used a ULN cut off of 19 IU/mL in women and 31 IU/mL in men, contrasting with the ULN of 41 μL/L used in previous studies[2].
A recent multi-centre cohort of DDI-associated NCPH in HIV-infected adults identified thrombocytopenia, splenomegaly and elevated aminotransferases and alkaline phosphatase as significantly associated with NCPH[14]. The authors suggest a screening algorithm for NCPH consisting of DDI exposure or splenomegaly plus either a raised serum aminotransferase or thrombocytopenia or raised alkaline phosphatase as a trigger for further assessment. Using this study’s algorithm only one third of our cohort would have been offered further investigation and one of the two cases of NRH identified in this study prior to development of NCPH would have been missed. Furthermore, splenomegaly is not uncommon in HIV positive patients[16].
We opted to use TE together with ALT and platelet counts on the basis of ready availability of blood tests and the ease of use of FS in the outpatient ambulatory setting. The most common histological abnormality we found on liver biopsy was steatosis, in association with fibrosis in 2 cases. We are unlikely to have missed cases though cannot exclude NRH in those who declined biopsy, but is unlikely that they had underlying NRH in a greater frequency that that seen in patients who did agree to undergo biopsy. Whilst DDI-associated NRH and NCPH is a serious condition with potentially life-threatening complications, this prospective study suggests a relatively low prevalence in treated cohorts. A previous study has identified non-alcoholic steatohepatitis (NAFLD) as a significant cause of unexplained serum aminotransferase elevation[12] in HIV positive people on ART and we also showed a significant rate of hepatic steatosis in association with hepatic fibrosis in our patients.
In this cross sectional study, we found a low prevalence of previously undiagnosed DDI-associated NRH using a screening strategy that combines TE, serum aminotransferase and platelet measurements followed by an U/S. We did, however, demonstrate a higher prevalence of NAFLD, which requires active management to address risk factors and prevent progression to fibrosis in HIV-positive patients.
The authors would like to thank all the patients that took part in this observational study.
Human immunodeficiency virus positive patients are at increased risk of liver disease. This is multifactorial and includes co-infection with hepatitis viruses, prescribed and recreational drug use and alcohol. The anti-retroviral drug Didanosine (DDI) has been implicated in the aetiology in some patients, particularly if the type of liver damage is nodular regenerative hyperplasia (NRH) or a patient has non-cirrhotic portal hypertension.
The authors used a combination of liver enzyme level (with a lower upper limit of normal) and transient elastography (TE) (which measures the liver stiffness) as an initial screen of patients exposed to DDI. This highly sensitive approach identified 42% who required further investigation with an ultrasound scan and 17% who subsequently were offered a transjugular liver biopsy. The authors, therefore, believe that the prevalence rate of 2% NRH in this DDI exposed asymptomatic cohort is accurate.
The prevalence study is the first to systematically screen asymptomatic patients exposed to DDI. Other groups have looked at the association between the drug and liver disease but have not screened a large cohort of exposed but asymptomatic patients to establish a prevalence of disease.
Use of a simple screening strategy in patients previously exposed to DDI will allow clinicians to identify liver disease which if left undiagnosed may present with the complications of portal hypertension such as variceal bleeding.
TE: A technique combining ultrasound waves and a pressure transducer to assess the stiffness of the liver. This has been validated as a tool to measure liver fibrosis and steatosis; NRH is characterized by small (less than 3 mm) regenerative nodules in the absence of fibrous septa on biopsy. The nodules cause obliteration of the portal veins which leads to portal hypertension.
It is an interesting study.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Koubaa M, McQuillan GM S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Sood A, Castrejón M, Saab S. Human immunodeficiency virus and nodular regenerative hyperplasia of liver: A systematic review. World J Hepatol. 2014;6:55-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Maida I, Núñez M, Ríos MJ, Martín-Carbonero L, Sotgiu G, Toro C, Rivas P, Barreiro P, Mura MS, Babudieri S. Severe liver disease associated with prolonged exposure to antiretroviral drugs. J Acquir Immune Defic Syndr. 2006;42:177-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Mallet V, Blanchard P, Verkarre V, Vallet-Pichard A, Fontaine H, Lascoux-Combe C, Pol S. Nodular regenerative hyperplasia is a new cause of chronic liver disease in HIV-infected patients. AIDS. 2007;21:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Maida I, Garcia-Gasco P, Sotgiu G, Rios MJ, Vispo ME, Martin-Carbonero L, Barreiro P, Mura MS, Babudieri S, Albertos S. Antiretroviral-associated portal hypertension: a new clinical condition? Prevalence, predictors and outcome. Antivir Ther. 2008;13:103-107. [PubMed] |
| 5. | Saifee S, Joelson D, Braude J, Shrestha R, Johnson M, Sellers M, Galambos MR, Rubin RA. Noncirrhotic portal hypertension in patients with human immunodeficiency virus-1 infection. Clin Gastroenterol Hepatol. 2008;6:1167-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Stebbing J, Wong N, Tan L, Scourfield A, Jiao LR, Shousha S, Grover D, Bower M, Nelson M. The relationship between prolonged antiretroviral therapy and cryptogenic liver disease. J Acquir Immune Defic Syndr. 2009;50:554-556. [PubMed] |
| 7. | Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, Herrmann E. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1090] [Article Influence: 60.6] [Reference Citation Analysis (1)] |
| 8. | Vizzutti F, Arena U, Rega L, Romanelli RG, Colagrande S, Cuofano S, Moscarella S, Belli G, Marra F, Laffi G. Performance of Doppler ultrasound in the prediction of severe portal hypertension in hepatitis C virus-related chronic liver disease. Liver Int. 2007;27:1379-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Scourfield A, Waters L, Holmes P, Panos G, Randell P, Jackson A, Mandalia S, Gazzard B, Nelson M. Non-cirrhotic portal hypertension in HIV-infected individuals. Int J STD AIDS. 2011;22:324-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Laharie D, Vergniol J, Bioulac-Sage P, Diris B, Poli J, Foucher J, Couzigou P, Drouillard J, de Lédinghen V. Usefulness of noninvasive tests in nodular regenerative hyperplasia of the liver. Eur J Gastroenterol Hepatol. 2010;22:487-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Cotte L, Bénet T, Billioud C, Miailhes P, Scoazec JY, Ferry T, Brochier C, Boibieux A, Vanhems P, Chevallier M. The role of nucleoside and nucleotide analogues in nodular regenerative hyperplasia in HIV-infected patients: a case control study. J Hepatol. 2011;54:489-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ingiliz P, Valantin MA, Duvivier C, Medja F, Dominguez S, Charlotte F, Tubiana R, Poynard T, Katlama C, Lombès A. Liver damage underlying unexplained transaminase elevation in human immunodeficiency virus-1 mono-infected patients on antiretroviral therapy. Hepatology. 2009;49:436-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Vispo E, Cevik M, Rockstroh JK, Barreiro P, Nelson M, Scourfield A, Boesecke C, Wasmuth JC, Soriano V. Genetic determinants of idiopathic noncirrhotic portal hypertension in HIV-infected patients. Clin Infect Dis. 2013;56:1117-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 14. | Parikh ND, Martel-Laferriere V, Kushner T, Childs K, Vachon ML, Dronamraju D, Taylor C, Fiel MI, Schiano T, Nelson M. Clinical factors that predict noncirrhotic portal hypertension in HIV-infected patients: a proposed diagnostic algorithm. J Infect Dis. 2014;209:734-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 397] [Article Influence: 18.0] [Reference Citation Analysis (1)] |
| 16. | Zambetti EF, Haramati LB, Jenny-Avital ER, Borczuk AC. Detection and significance of splenomegaly on chest radiographs of HIV-infected outpatients. Clin Radiol. 1999;54:34-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |