Published online Dec 28, 2016. doi: 10.4254/wjh.v8.i36.1629
Peer-review started: July 1, 2016
First decision: September 5, 2016
Revised: October 2, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: December 28, 2016
Processing time: 181 Days and 4.8 Hours
To evaluate the diagnostic value of serial biochemical blood tests in the diagnosis of biliary colic.
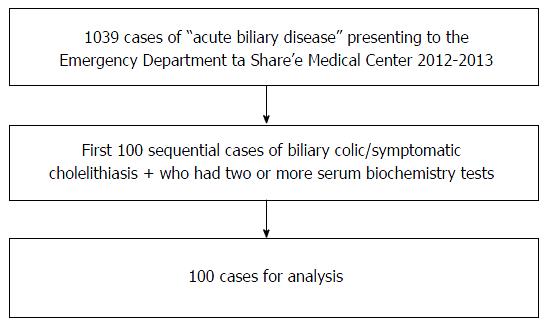
Files were reviewed of 1039 patients who were admitted to the Share’e Zedek Medical Center emergency department between the years 2012-2013, and received the coding of acute biliary disease. Of these, the first 100 cases were selected that met the following criteria: (1) a diagnosis of biliary colic or symptomatic cholelithiasis; (2) at least two biochemical blood tests performed; and (3) 18 years of age or older. Patients with other acute biliary diseases were excluded. The biochemical profile of the patients was analyzed as were their clinical and radiological findings.
Three-quarters of the patients were women, whose average age of 37 years was younger than the average of the men, at 50 years. According to their histories, 47% of the patients had previously known cholelithiasis. Pain in either the right upper quadrant or the epigastrium was the presenting symptom in 93% cases. The greatest change in serum biochemical results was seen during the first day of the patients’ admissions. Alanine aminotransferase (ALT) showed the highest initial rise above the reference range, followed by aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), bilirubin and alkaline phosphatase (ALKP) - all these increases were statistically significant (P < 0.05). AST showed the sharpest decline followed by bilirubin and ALT. GGT and ALKP did not fall. A sharp rise and fall in liver enzymes, especially during the first day, most prominently in AST and ALT, was seen in 70% percent of cases. In 65% of cases trans-abdominal sonography did not give diagnostic findings.
Serial serum liver enzyme measurements are helpful in the initial diagnosis of acute biliary colic.
Core tip: Gallstones are prevalent in affluent countries, more so in women than in men, and their prevalence increases with age. A large proportion of patients presenting to the emergency department with epigastric or right upper quadrant (RUQ) pain present a diagnostic challenge, especially when they belong to the older age group. We found that serial liver and biliary enzyme measurements reveal a characteristic pattern that helps the clinician determine quickly, cheaply and safely that the cause of RUQ/epigastric pain is biliary colic, in 71% of the patients. Serial enzyme testing is a useful adjunct to other diagnostic tools, for the diagnosis of acute upper abdominal pain.
- Citation: Resnick E, Shteingart S, Melamud B, Bdolah-Abram T, Zalut T, Reuben A, Lurie Y. Enzyme pattern of biliary colic: A counterintuitive picture. World J Hepatol 2016; 8(36): 1629-1636
- URL: https://www.wjgnet.com/1948-5182/full/v8/i36/1629.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i36.1629
Gallstones are prevalent in affluent countries, more so in women than in men, and their prevalence increases with age[1]. Gallstones usually produce symptoms when they migrate into the cystic duct or common bile duct (CBD), causing obstruction that increases intraluminal pressure and distends the viscus. The most characteristic symptom of gallstone disease is biliary colic[2], i.e., pain arising from the cystic duct or CBD.
A patient presenting to the emergency department (ED) complaining of epigastric or right upper quadrant (RUQ) pain, with a classic history and physical examination compatible with biliary colic does not present a great diagnostic challenge. A large proportion of patients presenting to the ED with epigastric or RUQ pain do present a diagnostic challenge, especially when they belong to the older age group (in which both a wide array of underlying diseases and polypharmacy are prevalent) which has become more common in Western society over recent decades[1]. Unfortunately, “classic” cases are the exception rather than the rule[3,4]. The differential diagnosis in an older patient with many co-morbidities includes: An acute coronary syndrome, pericarditis, an aortic dissection, peptic ulcer disease including a perforated ulcer, pulmonary embolism, lower lobe pneumonia, renal colic, pyelonephritis, partial colonic obstruction, diverticulitis, appendicitis, pancreatitis, acute cholecystitis, cholangitis, diabetic ketoacidosis, porphyria and biliary colic[2]. The diagnostic pathways are not straightforward, consume time and resources and are subject to pitfalls and misleading findings.
The confirmatory diagnostic test for biliary colic in the ED is the demonstration of gallstones in the cystic duct or CBD by trans-abdominal sonography[4-6]. However the demonstration of cholelithiasis alone is not diagnostic[1], and unfortunately, the demonstration of gallstones in the cystic duct or CBD is very difficult even for an experienced radiologist under optimal conditions, namely in a lean, cooperative and fasted patient, when time is not limited. In practice, it is often very difficult to demonstrate gallstones in the cystic duct or CBD[7]. Due to these limitations, and in the majority of cases, ultrasonography is usually not diagnostic in the first hours or days of the patient’s admission, and a negative ultrasound scan may be misleading[1,5,7-9]. A simple, rapid, cheap, non-invasive, reproducible and reliable test is needed to diagnose biliary colic quickly and efficiently.
Laboratory testing of serum “hepatocellular” and cholestatic liver enzymes [namely: Aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and alkaline phosphatase (ALKP) and gamma-glutamyl transferase (GGT), respectively] is performed almost routinely in patients presenting with epigastric or RUQ pain, as the clinician searches for diagnostic patterns. For example: A patient with or without pain who has elevated “cholestatic” enzymes, ALKP and GGT, is considered a potentially “surgical” patient who is suffering from a chronic mechanical obstruction of the biliary tract. And indeed, it is rare to find such an obstruction without elevation of these enzymes[10]. In this type of obstruction, the hepatocellular enzymes, AST and ALT, will be only mildly elevated. Conversely, a patient with RUQ fullness, malaise, and AST and ALT in the hundreds and even thousands of international units per liter (IU/L), with or without jaundice, and with only mildly elevated ALKP and GGT is considered a “medical” patient, who is suffering from viral, autoimmune or drug-induced hepatitis. These patterns that have served so well for so many years represent entrenched dogma.
We and others[11-13] have also observed another distinct pattern of a sharp (up to 100-fold above the upper limit of the normal reference range), short-lived (usually less than a week) rise in AST and ALT, and only a mild rise in ALKP, bilirubin and GGT, which we think is characteristic of acute “biliary colic”. Our clinical impression is that this pattern has high specificity for the diagnosis of “biliary colic” especially in the first hours of the patient’s admission, which is paradoxically counterintuitive in a patient, who has “surgical” pain with a “medical” enzyme pattern.
Our observations are mentioned (unreferenced) in the two latest editions of the leading textbooks in internal medicine, the leading textbook in gastroenterology as well as in a respected textbook on laboratory tests[2,8,14,15]. It is noteworthy that this pattern is not mentioned in the latest versions of two leading textbooks in general surgery[4,6].
Our current study will examine the existence and the utility of this paradoxical “biliary colic” enzyme pattern in our own patient population.
This was an observational retrospective chart review, which was approved by the Medical Center’s Helsinki committee (number: p 92/13). We reviewed the medical records of 1039 patients, who were admitted to the Share’e Zedek Medical Center ED between December 1st 2013 and January 1st 2012, and who were assigned the coded diagnosis of “acute biliary disease”. Inclusion criteria were: (1) 18 years of age and older; (2) two or more blood tests including a liver profile; and (3) a diagnosis of biliary colic or symptomatic cholelithiasis. Symptomatic cholelithiasis was also included as it was clear that this term was used interchangeably with biliary colic. It was used to differentiate this entity from acute cholecystitis. Exclusion criteria were: (1) acute cholecystitis; (2) ascending cholangitis; (3) ultrasound-confirmed choledocholithiasis; and (4) sepsis. The search for cases meeting the inclusion and exclusion criteria was closed once the first sequential 100 suitable patients (out of 1039 during 23 mo) were identified. The case acquisition flow chart is shown in Figure 1.
Data were collected from the Medical Center computerized database. Clinical data were derived from the either the ED file (if the patient was not hospitalized) or the hospital chart. Laboratory data were collected from the computerized laboratory records. Laboratory tests were performed using the standard techniques used for all biochemical tests at the hospital. Ultrasound interpretations were retrieved from the diagnostic imaging computerized database in written or dictated format.
The primary variables we examined were the serum biochemical laboratory results, including: Bilirubin, ALKP, AST, ALT and GGT, throughout the patient’s stay in the ED or hospital, relating to the particular ED visit with biliary colic.
The secondary variables examined were the location of the patient’s pain, radiation of the pain, whether it was post-prandial, and associated symptoms such as anorexia, nausea, vomiting, fever and chills, and physical examination findings. The interpretation of the radiologic tests performed was also evaluated.
Statistical analysis was performed by Tali Bdolah-Abram from The Hebrew University, Jerusalem, Israel. Paired t-tests as well as non-parametric Wilcoxon signed-rank tests were used to assess the differences between pairs of quantitative variables. The one-sample t-test was used to test the significance of percent changes between two measurements. Repeated measures ANOVA models were applied to quantitative variables in order to simultaneously test trends over time, the difference between subgroups of patients and the interaction between time and group. The significance of the trends and the interactions were tested using the Greenhouse-Geisser test. The Friedman non-parametric test was used for testing a trend over time for quantitative variables when the data was not normally distributed.
All tests applied were two-tailed, and a P-value of 5% or less was considered statistically significant.
The basic demographic characteristics of the 100 cases studied are presented in Table 1. The mean age of the patients in our study was 40 years, 76% of cases were women. On average, the women were younger than the men with mean ages of 37.4 years and 50.0 years respectively. In 47% of cases, cholelithiasis was already known from the patient’s history.
| Age (yr) | |
| Mean ± SD | 40.5 ± 19.1 |
| Median | 37 |
| Gender (#) | |
| Men | 24 |
| Women | 76 |
| Age by gender (yr) | |
| Men | |
| Mean ± SD | 50.0 ± 17.0 |
| Median | 49 |
| Women | |
| Mean ± SD | 37.4 ± 18.8 |
| Median | 31.5 |
| Cholelithiasis known previously (from history) | |
| Yes | 47 |
| No | 53 |
Clinical presentations were extracted from the patients’ files and are reported in Table 2. The clinical variables analyzed were: Location of pain and tenderness, presence of peritoneal irritation, Murphy sign, fever, chills, nausea or vomiting and whether the pain was post-prandial. The location of the pain was noted to be in the RUQ or epigastrium in the large majority of cases (92.8%).
| Pain location | |
| RUQ | 41 (42.3) |
| Epigastric | 43 (44.3) |
| RUQ and/or epigastric + other region | 6 (6.2) |
| Not RUQ or epigastric | 7 (7.3) |
| No data | 3 |
| Fever | |
| Yes | 2 (2.1) |
| No | 95 (97.9) |
| No data | 3 |
| Nausea | |
| Yes | 28 (60.9) |
| No | 18 (39.1) |
| No data | 54 |
| Vomiting | |
| Yes | 20 (31.7) |
| No | 43 (68.3) |
| No data | 37 |
| Tenderness to palpation | |
| RUQ | 47 (49.0) |
| Epigastric | 20 (20.8) |
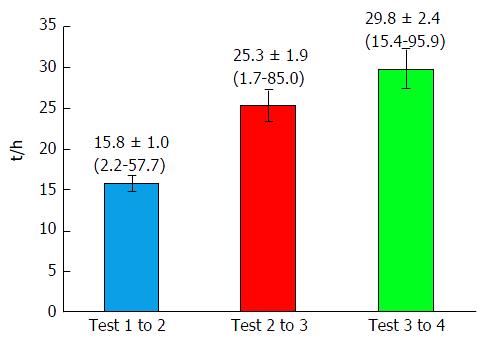
The primary endpoint of our study was the result of the analysis of the serial blood biochemistry laboratory tests, notably: Bilirubin, AST, ALT, ALKP and GGT. All tests were done according to the standard routine of the hospital laboratory. We retrieved the sequential laboratory results for the 100 cases of interest, and analyzed the results from up to four sequential tests in each patient. Some patients had more than four tests done, but as this subset was small, we limited our analysis to four tests. The goal of the study was to differentiate which enzyme variable was most significantly indicative of the clinical event, namely biliary colic. To facilitate comparison between the four enzyme patterns, we normalized the data as percent changes per hour. For example, a change in AST from 50 to 150 units over a 10 h interval, would calculate to a change of 10 units per hour, representing a 20% per hour increase from 50 units. Figure 2 shows the time intervals between the sequential measurements tests, which allows a visual appreciation of the time course of the changes in enzyme levels. The average time between tests grew longer from the first to the last test, at 15.8, 25.3 and 29.8 h, respectively.
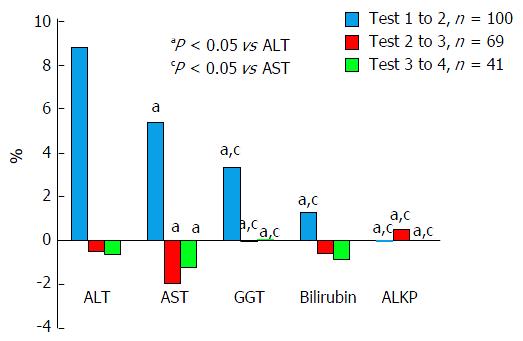
We compared changes in all five primary variables to one another at three different points, i.e., percent changes per hour between tests 1 and 2, 2 and 3, and 3 and 4, as is shown in Figure 3.
It can be readily seen that the largest percent changes in enzyme levels, which were also statistically significant, were seen between the first and second tests, and that ALT changed the most followed by AST, GGT, bilirubin and ALKP. Between tests two and three, the effects were far smaller with AST being the only variable with greater than one percent change. Between tests three and four effect percent changes were of similar magnitude to the changes between test two and three. AST still showed the largest absolute change, but this was smaller than between the previous tests, at 1.2%.
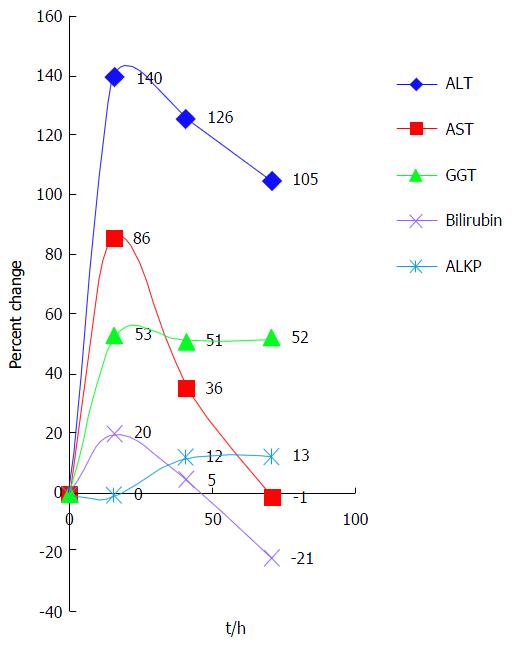
In order to demonstrate the enzyme pattern we plotted the percent change over time as seen in Figure 4. As this is a retrospective study and not protocol-driven, blood was drawn for testing at different intervals in each case. Therefore, the time between tests is represented as the average time interval between tests. To permit a graphic presentation of the different enzyme patterns, the hourly percent changes are multiplied by the corresponding average time intervals.
As shown above, the largest initial change was in ALT, at 140% between the first and second tests. AST rose less dramatically (86%) but showed the most dramatic average fall (50%). GGT rose initially by 53% but then hardly changed at all, while bilirubin and ALKP showed only minor fluctuations.
To determine how many of the 100 patients studied presented with the aforementioned enzyme pattern that is characteristic for biliary colic, we devised two criteria to separate patients into those either positive or negative for the pattern; positivity for either criterion was considered a characteristic pattern.
The first criterion was that a patient had at least doubled the levels of AST or ALT between the first two tests, compared to any subsequent test. The second criterion was that a patient had at least halved the first test results for AST or ALT in any one of the following three tests. Additionally, to be counted positive for the second criterion, only patients who had a first test result of more than double the upper limit of normal (ULN) for AST or ALT were counted. We used more than twice the ULN of normal as a cut-off as this has been used for many years in the field of chronic hepatitis B surveillance and in treatment algorithms (between 1-2 times the ULN is considered minimally raised)[16,17]. ULN for AST and ALT in our medical center are 36 and 52 units, respectively.
We found 33 (33%) patients positive for the first criterion (of a doubling in aminotransferases) all of whom showed a rise in ALT and 18 (54.5%) of whom had a rise in AST. Fifty-two (52%) patients were positive for the second criterion (of a significant subsequent fall in enzymes) - all had a fall in AST and 14 (26.9%) of whom also showed a fall in ALT. All told, 71 (71%) patients were positive for either one or the other of the criteria and 14 (57.6%) out of the 71 were positive for both criteria.
Trans-abdominal ultrasonography was performed in all but one case, a woman who had had sonography performed the previous day, when she was found to have gallbladder stones. The sonography reports given by the hospital radiologists, in either written or dictated form, were reviewed. We divided the findings into two categories, namely (1) highly suggestive or compatible with a cause for biliary colic; and (2) questionable or possibly non-contributory to the diagnosis of biliary colic. The results and the categorization of the different findings are displayed in Table 3. As can be seen, in only a third of the cases were the sonographic findings highly suggestive or compatible with a cause for biliary colic. In other words there was definite, albeit somewhat indirect, evidence of CBD disease. In the remaining 64 cases, whereas there was no evidence of CBD disease, it is conceivable, nonetheless, that gallstones could have migrated from the gallbladder in 62 cases without causing visible CBD injury.
| Frequency | |
| Non contributory | 64 |
| Contracted gallbladder | 2 |
| Cholelithiasis | 58 |
| Contracted gallbladder + cholelithiasis | 4 |
| Compatible or highly suggestive | 35 |
| Dilated CBD | 5 |
| Dilated CBD + filling defect | 1 |
| Thickened gallbladder wall | 6 |
| Distended gallbladder + cholelithiasis | 13 |
| Distended gallbladder | 2 |
| Stone in cystic duct | 4 |
| Distended gallbladder + pericystic fluid | 2 |
| Thickened gallbladder wall + cholelithiasis | 2 |
| Total valid | 99 |
| Missing | 1 |
| Total | 100 |
Biliary colic is a common symptom that is defined as pain caused by spasm of the cystic duct or CBD, usually caused by intraluminal calculi that have migrated from the gallbladder, or occasionally that form in situ[1]. Patients diagnosed with biliary colic present with symptoms of RUQ and/or epigastric pain. In some cases, diagnosis is straightforward but in some the differential diagnosis is wide and the evaluation is time and resource-consuming and occasionally potentially harmful.
Trans-abdominal sonography is readily available, but has low sensitivity and a low negative predictive value for choledocholithiasis. Indeed, in our study all but one of the patients were finally diagnosed as having biliary colic due to the passage of a stone through the CBD despite, the fact that choledocholithiasis was not proven directly in 99% of them. Ultrasonic findings, i.e., CBD abnormalities, were compatible or highly suggestive of biliary colic, in only 35% of the patients. But even in this minority, sonography did not clinch the diagnosis, since there seemingly was enough uncertainty to prevent the clinician from discharging the patient or referring him/her for laparoscopic cholecystectomy without additional tests. The same is truer for those 62 patients with typical biliary colic in whom cholelithiasis was present, but there was no visible CBD abnormality. The ED staff in Galveston, Texas, made similar observations on the utility of ultrasound scans, and this led to overuse of computed tomography scanning, especially at night[18].
Jafari et al[7] also point out that passage of stones through the CBD can be fast, so that by the time the patient is transported to the radiology department the diagnostic picture - a dilated CBD containing a stone, can be missed. They advocate that ultrasound scans should be performed in the ED, and for CBD diameter to be measured routinely. It is in this situation that the characteristic enzyme elevation and fall pattern comes into its own.
As opposed to these limitations of ultrasound scanning, we found that serial liver and biliary enzyme tests (2-4 tests performed during the first 80 h from admission) increase and decrease with statistical significance, and reveal a characteristic liver enzyme pattern that helps the ED clinician determine quickly, cheaply and safely, in concert with simultaneous other diagnostic maneuvers, that the cause of RUQ/epigastric pain is biliary colic. Thus, our findings confirm and extend what is already known. We assume that in most of the 29% of patients in whom the characteristic temporal pattern was not seen, the first blood sample was taken too late for the sharp rise and fall to be fully appreciated.
The most statistically significant changes in the variables we examined were seen between the 1st and the 2nd test. The most prominent effect is seen with ALT followed by AST. However, GGT and ALKP, the classic “obstructive” biliary enzymes, and bilirubin, contribute little, if at all, to the diagnosis. This finding is compatible with our assumption that ALT and AST, the “medical” hepatocellular enzymes, are deranged early in biliary colic, for which the most plausible hypothesis is that high biliary pressures lead to impairment of bile secretion and retention of bile acids with accompanying hepatocyte apoptosis, necrosis or leakage of enzymes[19]. The classic rise in the “biliary” enzymes, alkaline phosphatase and GGT, in prolonged obstruction is thought to reflect increased enzyme synthesis rather than cholangiocyte damage and leakage[20]. Moreover, the greatest changes in enzyme levels occur early and up to the first 24 hours of the patient’s admission, which makes this a diagnostic tool particularly useful in the ED setting.
In our study many patients were seen to have both the rise and the fall of hepatocellular enzymes, unlike the findings in two earlier studies that showed mainly the down-sloping phase of the temporal enzyme pattern[12,21]. This can be explained by lesser availability of liver enzyme testing in the late 1980s, when these studies were published. And indeed, in a later study, published in 2010, an enzyme pattern almost identical to ours was found, in which both the ascending and descending phases were detected. This latter study was performed on subjects in whom choledocholithiasis was proven invasively, which, admittedly, is a different and far less common situation[22].
The pattern of a short term (less than a week), sharp rise and sharp (but to a lesser degree) fall in ALT and AST is classically recognized in two clinical scenarios, namely ischemic liver injury - so-called Hypoxic Hepatitis - and acetaminophen intoxication[23-25]. It is our opinion that this pattern is also typical of a third clinical scenario - biliary colic due to passage of a biliary stone that causes transient biliary obstruction.
Our impression is that this pattern is not widely appreciated by general clinicians although it is mentioned in several articles and in some leading textbooks[2,8,12-15,21-22,26]. One explanation for this pattern’s relative anonymity is the dogma that elevated cholestatic enzymes mean biliary obstruction has served so well for prolonged biliary obstruction. The corollary has been assumed, that all biliary obstruction is accompanied by a cholestatic enzyme pattern. This is clearly not the case, as transient biliary obstruction due to passage of a stone through the CBD causes elevation of ALT and AST - the medical or hepatocellular enzymes.
Limitations of this enzyme temporal analysis include the fact that this is a retrospective study, with its unavoidable selection bias, and that the exact timing of the clinical event, i.e., the passage of the CBD stone, is unknown. Hence, the appropriateness of the time of first blood drawing is also uncertain. A third limitation of this study is that we only included patients who had two or more blood tests performed, which introduces further selection bias of patients who had initial enzyme elevations. Irrespective, the more prominent abnormal enzymes were the hepatocellular. Another bias is that our subjects were already diagnosed as suffering from biliary colic. It remains to be tested, therefore, whether the characteristic “biliary colic” pattern will be useful in the more real life scenario of a patient presenting to the ED with abdominal pains. We hope to be able to resolve this question in a prospective study, in which all patients presenting with abdominal pain will undergo serial liver enzyme measurement at protocol-defined intervals, along with a precise history of the time of onset of the abdominal pain. Finally, although we were careful to exclude cases of cholecystitis, it must still be acknowledged that aminotransferase elevation occurs in active gallbladder inflammation, usually associated with fever and leukocytosis (both absent in our 100 cases) and of slower resolution than seen in our cases. In gallbladder inflammation, the mechanism of aminotransferase rise is usually attributed to cytokine release and other mediators of the Systemic Inflammatory Response Syndrome[27].
Based on a retrospective statistical analysis of 100 patients who were diagnosed as having biliary colic - which is defined as upper abdominal pain due to passage of a gallstone through the cystic duct or CBD, we found that a sharp, short term rise and fall of ALT and AST, typically thought to be indicative of ischemic hepatic injury and acetaminophen intoxication, is also typical of biliary colic. Whereas the current observations are not entirely novel, they are worth emphasizing and bringing to general attention as they do not appear to be widely appreciated. Thus, in the ED, when a patient presents with an appropriate history and physical exam, we recommend adding liver enzymes to the list of blood tests already ordered. If liver enzymes are found to be elevated, we recommend repeating these tests twice or thrice at intervals over the next 24 h. If the pattern that is characteristic of biliary colic is seen, concomitant with the resolution or even only marked improvement of the pain, and no other diagnosis is suspected (e.g., an acute coronary event, pancreatitis, cholecystitis, etc.), then the patient can be discharged home or admitted for laparoscopic cholecystectomy according to local practice[28].
We would like to thank Tzina Lindenberg, director of Share’e Zedek Medical Center’s archive, for her support of this project.
Patients complaining of abdominal pain are frequently seen in the emergency department. The differential diagnosis is vast and therefore the clinical workup is resource and time consuming. However, some clinical observations led us to the thought that a few cheap and routine blood tests could greatly aid the clinician in this diagnostic challenge.
A few studies, mainly from the 1980’s and 1990’s described the enzymatic profile of biliary colic and reached. However this very useful profile did not become common knowledge.
In recent years more patients were tested for liver enzymes as part of the routine workup of acute abdominal pain. Additionally many patients had more than one blood sample taken. This has allowed us to follow the level of liver enzymes and observe a unique pattern.
This pattern helps clinicians in the clinical scenario of acute abdominal pain diagnosis. It is cheap, rapid, readily available, accurate and non-invasive.
Biliary colic is a term used to describe pain arising from stones obstructing the cystic duct or the passage of a stone through the common bile duct.
This manuscript provides the updated evidence to the readers. The topic is an important one and deserves a practical value.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Israel
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Liao KF, Shih SC, Zimmer V S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Duncan CB, Riall TS. Evidence-based current surgical practice: calculous gallbladder disease. J Gastrointest Surg. 2012;16:2011-2025. [PubMed] [DOI] [Full Text] |
| 2. | Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J. Harrison’s principles of internal medicine. 18th edition, USA: Mc Graw Hill 2012; 2615-2628. |
| 3. | Gunn A, Keddie N. Some clinical observations on patients with gallstones. Lancet. 1972;2:239-241. [PubMed] [DOI] [Full Text] |
| 4. | Brunicardi F, Andersen D, Billiar T, Dunn D, Hunter JG, Matthews JB, Pollock RE. Schwartz’s Principles of Surgery. 10th edition, USA: McGraw-Hill 2014; 1309-1340. |
| 5. | Bar-Meir S. Gallstones: prevalence, diagnosis and treatment. Isr Med Assoc J. 2001;3:111-113. [PubMed] |
| 6. | Townsend CM Jr, Beauchamp RD, Evers BM, Mattox KL. Sabiston Textbook of Surgery. 19th edition, Canada: Elsevier Saunders 2012; 1476-1514. |
| 7. | Jafari D, Cheng AB, Dean AJ. Dynamic changes of common bile duct diameter during an episode of biliary colic, documented by ultrasonography. Ann Emerg Med. 2013;62:176-179. [PubMed] [DOI] [Full Text] |
| 8. | Feldman M, Friedman LS, Brandt LJ. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th edition, Canada: Elsevier Saunders 2010; 1089-1120. |
| 9. | Einstein DM, Lapin SA, Ralls PW, Halls JM. The insensitivity of sonography in the detection of choledocholithiasis. AJR Am J Roentgenol. 1984;142:725-728. [PubMed] [DOI] [Full Text] |
| 10. | Freitas ML, Bell RL, Duffy AJ. Choledocholithiasis: evolving standards for diagnosis and management. World J Gastroenterol. 2006;12:3162-3167. [PubMed] [DOI] [Full Text] |
| 11. | Isogai M, Hachisuka K, Yamaguchi A, Nakano S. Etiology and pathogenesis of marked elevation of serum transaminase in patients with acute gallstone disease. HPB Surg. 1991;4:95-105; discussion 106-107. [PubMed] [DOI] [Full Text] |
| 12. | Halvorsen FA, Ritland S. [Biliary duct obstruction presenting with laboratory levels indicating liver cell damage]. Tidsskr Nor Laegeforen. 1989;109:1779-1781. [PubMed] |
| 13. | Hayat JO, Loew CJ, Asrress KN, McIntyre AS, Gorard DA. Contrasting liver function test patterns in obstructive jaundice due to biliary strictures [corrected] and stones. QJM. 2005;98:35-40. [PubMed] [DOI] [Full Text] |
| 14. | Goldman L, Schafer AI. Goldman’s Cecil Medicine. 24th edition, USA: Elsevier Saunders 2011; 1011-1021. |
| 15. | Williamson MA, Snyder LM. Wallach’s Interpretation of Diagnostic Tests. 9th edition, China: Lippincott, Williams & Wilkins 2011; 38-40. |
| 16. | Liaw YF, Leung N, Kao JH, Piratvisuth T, Gane E, Han KH, Guan R, Lau GK, Locarnini S. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2:263-283. [PubMed] [DOI] [Full Text] |
| 17. | Liaw YF, Kao JH, Piratvisuth T, Chan HL, Chien RN, Liu CJ, Gane E, Locarnini S, Lim SG, Han KH. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2012 update. Hepatol Int. 2012;6:531-561. [PubMed] [DOI] [Full Text] |
| 18. | Benarroch-Gampel J, Boyd CA, Sheffield KM, Townsend CM, Riall TS. Overuse of CT in patients with complicated gallstone disease. J Am Coll Surg. 2011;213:524-530. [PubMed] [DOI] [Full Text] |
| 19. | Nathwani RA, Kumar SR, Reynolds TB, Kaplowitz N. Marked elevation in serum transaminases: an atypical presentation of choledocholithiasis. Am J Gastroenterol. 2005;100:295-298. [PubMed] [DOI] [Full Text] |
| 20. | Kaplan MM, Righetti A. Induction of rat liver alkaline phosphatase: the mechanism of the serum elevation in bile duct obstruction. J Clin Invest. 1970;49:508-516. [PubMed] [DOI] [Full Text] |
| 21. | Patwardhan RV, Smith OJ, Farmelant MH. Serum transaminase levels and cholescintigraphic abnormalities in acute biliary tract obstruction. Arch Intern Med. 1987;147:1249-1253. [PubMed] [DOI] [Full Text] |
| 22. | Sharara AI, Mansour NM, El-Hakam M, Ghaith O, El Halabi M. Duration of pain is correlated with elevation in liver function tests in patients with symptomatic choledocholithiasis. Clin Gastroenterol Hepatol. 2010;8:1077-1082. [PubMed] [DOI] [Full Text] |
| 23. | Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063-1070. [PubMed] [DOI] [Full Text] |
| 24. | Ebert EC. Hypoxic liver injury. Mayo Clin Proc. 2006;81:1232-1236. [PubMed] [DOI] [Full Text] |
| 25. | Singer AJ, Carracio TR, Mofenson HC. The temporal profile of increased transaminase levels in patients with acetaminophen-induced liver dysfunction. Ann Emerg Med. 1995;26:49-53. [PubMed] [DOI] [Full Text] |
| 26. | Grau F, Almela P, Aparisi L, Bautista D, Pascual I, Peña A, Rodrigo JM. Usefulness of alanine and aspartate aminotransferases in the diagnosis of microlithiasis in idiopathic acute pancreatitis. Int J Pancreatol. 1999;25:107-111. [PubMed] [DOI] [Full Text] |
| 27. | Chang CW, Chang WH, Lin CC, Chu CH, Wang TE, Shih SC. Acute transient hepatocellular injury in cholelithiasis and cholecystitis without evidence of choledocholithiasis. World J Gastroenterol. 2009;15:3788-3792. [PubMed] [DOI] [Full Text] |