Published online Dec 18, 2016. doi: 10.4254/wjh.v8.i35.1564
Peer-review started: August 23, 2016
First decision: September 28, 2016
Revised: October 1, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: December 18, 2016
Processing time: 116 Days and 7.2 Hours
To determine the association between cirrhosis and ischemic stroke in a large nationally representative sample.
A retrospective cross-sectional study of all hospitalized patients during 2012 and 2013 in the United States was performed using the National Inpatient Sample database. Hospitalizations with acute stroke, cirrhosis and other risk factors were identified using ICD-9-CM codes.
There were a total of 72082638 hospitalizations in the United States during the years 2012 and 2013. After excluding hospitalizations with missing demographic variables, that there were a total of 1175210 (1.6%) out of these were for acute ischemic stroke. Cirrhosis was present among 5605 (0.4%) cases of ischemic stroke. Mean age among the cirrhotic and non-cirrhotic groups with ischemic stroke were 66.4 and 70.5 years, respectively. Prevalence of risk factors among the two groups was also calculated. After adjusting for various known risk factors the odds of having an ischemic stroke (OR = 0.28, P < 0.001) were 72% lower in cirrhotics compared to non-cirrhotics.
Our study suggests that in a large, nationally representative sample of the United States population, cirrhosis is associated with a lower likelihood of stroke.
Core tip: Our study demonstrates that in a large, nationally representative sample, cirrhosis is associated with a lower likelihood of having an ischemic stroke, after adjusting for known risk factors. Although the odds of having a stroke are lower in cirrhotics, the mortality is significantly higher in them compared to non-cirrhotics.
- Citation: Goyal A, Chatterjee K, Shah N, Singh S. Is cirrhosis associated with lower odds of ischemic stroke: A nationwide analysis? World J Hepatol 2016; 8(35): 1564-1568
- URL: https://www.wjgnet.com/1948-5182/full/v8/i35/1564.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i35.1564
Cirrhosis is among the top ten leading causes of death in the United States[1,2]. With recent advances in the management of various complications of cirrhosis, it has become one of the most prevalent chronic conditions, that patients live with for a considerable duration of time[3]. For instance, there were 633323 patients living with cirrhosis in 2010[4].
Due to altered homeostasis and hemodynamics in cirrhosis it is reasonable to assume that the risk of an ischemic cerebrovascular event [acute ischemic stroke (AIS)] in cirrhotics would be different from that of the general population[5-8]. The question whether cirrhosis is associated with a reduced risk of stroke has been a source of controversy for a long time. There have been various studies reporting increased incidence of carotid plaques and atherosclerosis in patients with advanced liver disease, both known risk factors for ischemic stroke[9-11]. On the other hand, it is also well known that liver disease causes thrombocytopenia and coagulopathy which should in turn be protective against an ischemic cerebrovascular accident (CVA)[12]. Recently, Chen et al[12] and Berzigotti et al[13] showed that patients with liver cirrhosis may be at a lower risk of experiencing an ischemic CVA[12-14]. However, due to predominance of one ethnic group in the former and the relatively small sample size in the latter, the impact of cirrhosis on risk of stroke still remains inconclusive.
We therefore aim to define the impact of cirrhosis and extent of its association with ischemic stroke by using the largest national database for hospitalized patients in the United States.
The National Inpatient Sample (NIS) formerly known as Nationwide Inpatient Sample database is an administrative database developed by the Agency of Healthcare Research and Quality for Healthcare Cost and Utilization Project (HCUP). It is the largest all-payer database of hospitalized patients in the United States. NIS is a 20% stratified sample of all discharges from United States community hospitals[15]. Thus, manufacturer provided sampling weights were used to produce national estimates. We used NIS databases for the years 2012 and 2013 in this study. The NIS database provides de-identified information regarding the demographic characteristics (age, gender, race), mortality, principal and secondary diagnoses, etc., for each hospitalization. It however does not contain any lab values, imaging or other advanced diagnostic information.
This is a retrospective cross-sectional study using a national inpatient database. We used International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes 571.2 (Alcoholic cirrhosis of liver), 571.5 (biliary cirrhosis) and 571.6 (cirrhosis without mention of alcohol) to identify the patients with cirrhosis[3,16]. ICD-9-CM codes 433.x1, 434.x1, 435 and 436 listed as principal diagnoses were used to identify hospitalizations for acute ischemic cerebrovascular events. These ICD-9-CM codes with modifiers have been previously validated and used to identify AIS in administrative databases with good accuracy[17-20]. All the patients with missing age, gender or race information were excluded. The patients with missing age and gender information constituted < 1% of the included population. The hospitalization with missing race were more prevalent, however they were due to non-participation of some states in reporting ethnic information and thus did not result in under-representation of any particular ethnic group. The basic demographic characteristics for different sub-groups have been described in Table 1. We used ICD-9-CM codes to identify the known risk factors for ischemic stroke[21-25]. Prevalence of these risk factors was also calculated for different subgroups (Table 1). Since, this study was conducted using a de-identified commercially available database Institutional Review Board approval was not required.
| Cirrhotic | Non-cirrhotic | P value | |
| Mean age (SD) | 66.4 (11.9) | 70.5 (14.3) | < 0.001 |
| Age categories | |||
| Age < 40 | 0.8 | 2.3 | < 0.001 |
| Age 40-64 | 46.6 | 30.6 | |
| Age > 65 | 52.6 | 67.1 | |
| Gender | |||
| Male | 55.1 | 46.5 | < 0.001 |
| Female | 44.9 | 53.5 | |
| Race | |||
| Caucasian | 66.6 | 70.8 | < 0.001 |
| African-American | 14.9 | 15.9 | |
| Other races | 18.5 | 13.3 | |
| Hospital characteristics | |||
| Teaching status | |||
| Teaching | 51.3 | 48.3 | 0.13 |
| Non-teaching | 48.7 | 51.7 | |
| Location | |||
| Rural | 9.3 | 10.9 | 0.04 |
| Urban | 90.7 | 89.1 | |
| Bed size | |||
| Small | 10.3 | 12 | 0.46 |
| Medium | 26.8 | 26.7 | |
| Large | 62.9 | 61.3 | |
| Risk factors | |||
| Hypertension | 52.2 | 62.5 | < 0.001 |
| Diabetes | 45.1 | 37.8 | < 0.001 |
| Tobacco use | 37.2 | 28.8 | < 0.001 |
| CHF | 18.9 | 13.1 | < 0.001 |
| Personal history of stroke | 13.1 | 14.8 | < 0.001 |
| CAD | 25.3 | 27.7 | < 0.001 |
| Peripheral artery disease | 8.7 | 6.9 | < 0.001 |
| Atrial fibrillation | 23.8 | 21.9 | 0.001 |
| Anticoagulation use | 4.4 | 6.8 | < 0.001 |
| Dyslipidemia | 34.4 | 56.5 | < 0.001 |
| Alcohol abuse | 28.8 | 3.8 | < 0.001 |
| Family history of stroke | 1.5 | 2.3 | < 0.001 |
Stata 13.1 (Stata Corp, College Station TX) and SPSS 23.0 (SPSS Inc., Chicago, Ill) were used for statistical analysis. National estimates were produced by using the sampling weights provided by HCUP. χ2 test and Independent-samples t-test for means were used to determine statistical significance of differences in the prevalence of risk factors and demographic variables among the two groups. Due to the binary nature of the outcome/dependent variable, i.e., presence of ischemic stroke, multivariate logistic regression model was used to assess the association between cirrhosis and ischemic stroke while controlling for known risk factors (as listed in Table 1) of ischemic stroke. Wald’s test was used to determine statistical significance of association between the factors used in the regression model and the outcome, i.e., stroke. P-value less than 0.05 was considered statistically significant. The coefficient obtained as a result of the regression model was converted to OR for ease of understanding and is being reported here along with 95%CI. Since the prevalence of our outcome (Stroke) was less than 10%, the OR provides a good estimate of the relative risk[26]. The biostatistical methods and tests used in this study were reviewed by a biomedical statistician.
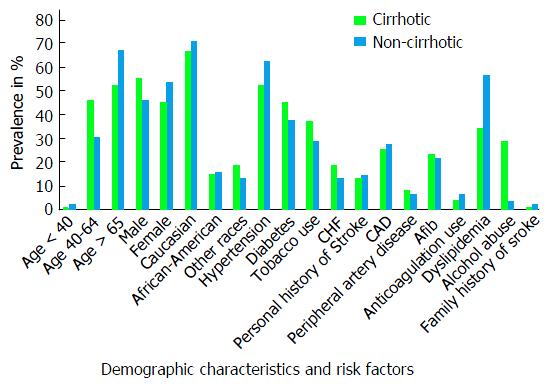
There were a total of 72082638 hospitalizations in the United States during the year 2012 and 2013. After excluding hospitalizations with missing demographic variables (age, gender, and race), a total of 1175210 (1.6%) hospitalizations were for AIS. Out of these, 5605 (0.4%) were identified to have co-existing cirrhosis of liver. Decompensated cirrhosis which was defined by presence of variceal hemorrhage, ascites, spontaneous bacterial peritonitis or hepatic encephalopathy, constituted 14.3% of the cirrhotic group[27]. Mean age among cirrhotic and non-cirrhotic patients were 66.4 (SD = 11.9) and 70.5 (SD = 14.3) years, respectively. Proportion of males among the two groups was 55.1% and 46.5% respectively. The racial distribution was similar among the two groups with 66.6% and 70.8% Caucasians; and 14.9% and 15.9% African-Americans in cirrhotic and non-cirrhotics respectively. The prevalence of some risk factors (Figure 1) for AIS like - diabetes (45.1% vs 37.8%), hypertension (52.2% vs 62.5%), congestive heart failure (18.9% vs 13.1%), smoking (37.2% vs 28.8%), atrial fibrillation (23.8% vs 21.9%), alcohol use (28.8% vs 3.8%) and peripheral vascular disease (8.7% vs 6.9%), were all higher among cirrhotics compared to non-cirrhotics (Table 1). Whereas, others like Coronary atherosclerosis (25.3% vs 27.7%), previous history of stroke (13.1% vs 14.8%), hypercholesterolemia (34.4% vs 56.5%) were found to be higher among the non-cirrhotic group (Table 1). Anticoagulation use, which is considered to be associated with a lower risk of ischemic stroke was more prevalent among the non-cirrhotics at 6.8% compared to 4.4% in cirrhotics.
The overall P-value of the logistical regression model was statistically significant at < 0.001. The odds of having an AIS for patients with cirrhosis were 72% lower than patients without cirrhosis (OR = 0.28, 95%CI: 0.26 to 0.29) and was statistically significant with a P-value < 0.001. The all cause in-hospital mortality among the cirrhotic group (5%) with AIS was significantly higher than non-cirrhotic group (3.3%) (P < 0.001). Even after adjusting (using logistic regression) for various co-morbidities using Charlson comorbidity index (modified to exclude liver disease)[28,29], patient demographics, and hospital characteristics; the mortality remained higher among cirrhotics with stroke (OR = 1.6, 95%CI: 1.22-2.10, P = 0.001).
The impact of cirrhosis on stroke has been controversial for a long time. Our study demonstrates that the odds of having an AIS for cirrhotics are significantly lower (72%) than non-cirrhotics. This is consistent with some of the other smaller non-United States based studies done previously. The magnitude of association however, is different[12,13]. The study by Chen et al[12] showed that risk of having an AIS was lower in non-alcoholic cirrhosis. But, it was conducted in Taiwan, and has limited generalizability due to predominance of only one kind of ethnic population. Since, ethnicity is itself an independent risk factor of AIS, our results have a more generalized applicability. Our study also had a much larger sample size and adjusted for the most number of risk factors of stroke in any study so far. The reduced likelihood of AIS in patients with cirrhosis represents a very important clinical finding. It may aid a clinician in determining the optimal management of often complicated cirrhotic patients with co-morbidities, that put them at a higher than usual risk of AIS, such as atrial fibrillation. The mechanism of this “protective effect of cirrhosis” is unclear but could be related to the underlying coagulopathy, thrombocytopenia or the altered hemodynamic flow patterns[12,13]. This study demonstrates the need for a prospective study to further explore this “protective effect of cirrhosis” on AIS.
Our study is to date the largest of its kind, and likely represents the true association between cirrhosis and strokes after adjusting for several known risk factors. Despite patients with cirrhosis being less likely to have a stroke, the mortality was significantly higher in them compared to non-cirrhotics. This is likely due to complications arising from the cirrhosis which may interfere with the usual management of stroke, for example, the coagulopathy due to cirrhosis may pose problems for planned interventions, if needed.
Our study findings, although important, need to be interpreted in light of some limitations. Firstly, NIS being an administrative database is not free from coding errors, especially related to liver diseases and acute strokes. However, we have used either the previously validated or commonly used codes for cirrhosis and AIS which have been shown to have good accuracy[3,30-32]. Secondly, the OR provides a close estimate of Relative Risk due to relatively low prevalence of Stroke in our population, but its not a replacement for the true relative risk which can only be obtained from a prospective cohort study.
Our study demonstrates that in a large, nationally representative sample, cirrhosis is associated with a lower likelihood of having an ischemic stroke, after adjusting for known risk factors. Although the odds of having a stroke are lower in cirrhotics, the mortality is significantly higher in them compared to non-cirrhotics. Prospective studies are needed to establish the causal relationship and better define this association in future.
Cirrhosis is one of the leading causes of morbidity and mortality in the United States. Cirrhotic patients usually suffer from coagulopathy while simultaneously being at an increased risk of deep venous thrombosis. These problems along with the usually encountered thrombocytopenia imply that the risk of an ischemic cerebrovascular accident (CVA) in a cirrhotic would be different from that of general population. This impact of cirrhosis on the risk of ischemic cerebrovascular events (ischemic stroke) has been controversial.
The relationship between cirrhosis and ischemic CVA has not been studied in detail.
The study is the first study with such a large sample size that controls for so many known risk factors of stroke to explore the true relationship between ischedmic stroke and cirrhosis.
The reduced likelihood of acute ischemic stroke (AIS) in patients with cirrhosis represents a very important clinical finding. It may aid a clinician in determining the optimal management of often complicated cirrhotic patients with co-morbidities, that put them at a higher than usual risk of AIS, such as atrial fibrillation (Afib).
Charlson comorbidity index is a tool to adjust for the impact of co-morbidites developed for use with administrative databases utilizing ICD-9 codes.
This paper is described in detail, which, as valuable information, could help the readers that have better understand the first-hand knowledge of this topic to start novel studies.
| 1. | Vong S, Bell BP. Chronic liver disease mortality in the United States, 1990-1998. Hepatology. 2004;39:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 104] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 2. | Murray CJL, Atkinson C, Bhalla K, Birbeck G, Burstein R, Chou D, Dellavalle R, Danaei G, Ezzati M, Fahimi A. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310:591-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1893] [Article Influence: 145.6] [Reference Citation Analysis (1)] |
| 3. | Fc I, States U. Exam 2: Decreasing Mortality Among Patients Hospitalized With Cirrhosis in the United States From 2002 Through 2010. Gastroenterology. 2015;148:e15-16. [DOI] [Full Text] |
| 4. | Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, Volk ML. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 519] [Article Influence: 47.2] [Reference Citation Analysis (0)] |
| 5. | Frøkjaer VG, Strauss GI, Mehlsen J, Knudsen GM, Rasmussen V, Larsen FS. Autonomic dysfunction and impaired cerebral autoregulation in cirrhosis. Clin Auton Res. 2006;16:208-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Lagi A, La Villa G, Barletta G, Cencetti S, Bacalli S, Cipriani M, Foschi M, Lazzeri C, Del Bene R, Gentilini P. Cerebral autoregulation in patients with cirrhosis and ascites. A transcranial Doppler study. J Hepatol. 1997;27:114-120. [PubMed] |
| 7. | Strauss GI, Hansen BA, Herzog T, Larsen FS. Cerebral autoregulation in patients with end-stage liver disease. Eur J Gastroenterol Hepatol. 2000;12:767-771. [PubMed] |
| 8. | Larsen FS, Olsen KS, Ejlersen E, Hansen BA, Paulson OB, Knudsen GM. Cerebral blood flow autoregulation and transcranial Doppler sonography in patients with cirrhosis. Hepatology. 1995;22:730-736. [PubMed] |
| 9. | O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14-22. [PubMed] |
| 10. | Targher G, Bertolini L, Padovani R, Rodella S, Zoppini G, Zenari L, Cigolini M, Falezza G, Arcaro G. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29:1325-1330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 11. | Nahandi MZ, Khoshbaten M, Ramazanzadeh E, Abbaszadeh L, Javadrashid R, Shirazi KM, Gholami N. Effect of non-alcoholic fatty liver disease on carotid artery intima-media thickness as a risk factor for atherosclerosis. Gastroenterol Hepatol Bed Bench. 2014;7:55-62. [PubMed] |
| 12. | Chen YH, Chen KY, Lin HC. Non-alcoholic cirrhosis and the risk of stroke: a 5-year follow-up study. Liver Int. 2011;31:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Berzigotti A, Bonfiglioli A, Muscari A, Bianchi G, Libassi S, Bernardi M, Zoli M. Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver Int. 2005;25:331-336. [PubMed] |
| 14. | Lee HJ, Hinrichs CR. Is coagulopathic liver disease a factor in spontaneous cerebral hemorrhage? J Comput Assist Tomogr. 2002;26:69-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | HCUP-US NIS Overview [Internet]. 2015. [accessed 2016 Apr 23]. Available from: http://www.hcup-us.ahrq.gov/nisoverview.jsp. |
| 16. | Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 17. | Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 607] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 18. | Roumie CL, Mitchel E, Gideon PS, Varas-Lorenzo C, Castellsague J, Griffin MR. Validation of ICD-9 codes with a high positive predictive value for incident strokes resulting in hospitalization using Medicaid health data. Pharmacoepidemiol Drug Saf. 2008;17:20-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Moradiya Y, Crystal H, Valsamis H, Levine SR. Thrombolytic utilization for ischemic stroke in US hospitals with neurology residency program. Neurology. 2013;81:1986-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 20. | Goldstein LB. Accuracy of ICD-9-CM coding for the identification of patients with acute ischemic stroke: effect of modifier codes. Stroke. 1998;29:1602-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 301] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Teunissen LL, Rinkel GJ, Algra A, van Gijn J. Risk factors for subarachnoid hemorrhage: a systematic review. Stroke. 1996;27:544-549. [PubMed] |
| 22. | Juvela S, Hillbom M, Palomäki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke. 1995;26:1558-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 100] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Martin-Schild S, Albright KC, Hallevi H, Barreto AD, Philip M, Misra V, Grotta JC, Savitz SI. Intracerebral hemorrhage in cocaine users. Stroke. 2010;41:680-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, Goldstein LB, Gorelick PB, Howard G, Kittner SJ. American Heart Association Prevention Conference. IV. Prevention and Rehabilitation of Stroke. Risk factors. Stroke. 1997;28:1507-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 351] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 25. | McEvoy AW, Kitchen ND, Thomas DG. Lesson of the week: intracerebral haemorrhage in young adults: the emerging importance of drug misuse. BMJ. 2000;320:1322-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 38] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Viera AJ. Odds ratios and risk ratios: what’s the difference and why does it matter? South Med J. 2008;101:730-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 223] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 27. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1609] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 28. | Bajaj JS, Ananthakrishnan AN, Hafeezullah M, Zadvornova Y, Dye A, McGinley EL, Saeian K, Heuman D, Sanyal AJ, Hoffmann RG. Clostridium difficile is associated with poor outcomes in patients with cirrhosis: A national and tertiary center perspective. Am J Gastroenterol. 2010;105:106-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 29. | Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7532] [Cited by in RCA: 8786] [Article Influence: 258.4] [Reference Citation Analysis (0)] |
| 30. | Lo Re V, Lim JK, Goetz MB, Tate J, Bathulapalli H, Klein MB, Rimland D, Rodriguez-Barradas MC, Butt AA, Gibert CL. Validity of diagnostic codes and liver-related laboratory abnormalities to identify hepatic decompensation events in the Veterans Aging Cohort Study. Pharmacoepidemiol Drug Saf. 2011;20:689-699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 31. | Lo Re V, Haynes K, Goldberg D, Forde KA, Carbonari DM, Leidl KB, Hennessy S, Reddy KR, Pawloski PA, Daniel GW. Validity of diagnostic codes to identify cases of severe acute liver injury in the US Food and Drug Administration’s Mini-Sentinel Distributed Database. Pharmacoepidemiol Drug Saf. 2013;22:861-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Singla A, Hart JL, Li Y, Tseng JF, Shah SA. Hospitalization for complications of cirrhosis: does volume matter? J Gastrointest Surg. 2011;15:330-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Gong ZJ, Kai K S- Editor: Ji FF L- Editor: A E- Editor: Li D