Published online Dec 18, 2016. doi: 10.4254/wjh.v8.i35.1569
Peer-review started: July 14, 2016
First decision: September 7, 2016
Revised: October 6, 2016
Accepted: October 22, 2016
Article in press: October 24, 2016
Published online: December 18, 2016
Processing time: 156 Days and 3.1 Hours
To investigate whether a novel immune function biomarker QuantiFERON-Monitor (QFM) can identify cirrhotic patients at greatest risk of infection.
Adult cirrhotic patients on the liver transplant waiting list were recruited for this observational cohort study from a tertiary liver transplant referral unit. The immune function biomarker, QFM was performed using the same method as the widely available Quantiferon-gold assay, and measures output in interferon gamma in IU/mL after dual stimulation of the innate and adaptive immune systems. Ninety-one cirrhotic patients were recruited, with 47 (52%) transplanted on the day of their QFM. The remaining 44 (48%) were monitored for infections until transplant, death, or census date of 1st February 2014.
Cirrhotic patients express a median QFM significantly lower than healthy controls (94.5 IU/mL vs 423 IU/mL), demonstrating that they are severely immunosuppressed. Several factors including model for end stage liver disease, presence of hepatocellular carcinoma, bilirubin, international normalized ratio and haemoglobin were associated with QFM on univariate analysis. Disease aetiology did not appear to impact QFM. On multivariate analysis, only Child-Pugh score and urea were significantly associated with a patient’s immune function as objectively measured by QFM. In the 44 patients who were not transplanted immediately after their blood test and could be monitored for subsequent infection risk, 13 (29.5%) experienced a pre-transplant infection a median 20 d (range 2-182) post-test. QFM < 214 IU/mL was associated with HR = 4.1 (P = 0.01) for infection. A very low QFM < 30 IU/mL was significantly associated (P = 0.003) with death in three patients who died while awaiting transplantation (HR = 56.6).
QFM is lower in cirrhotics, allowing objective determinations of an individual’s unique level of immune dysfunction. Low QFM was associated with increased susceptibility to infection.
Core tip: QuantiFERON-Monitor (QFM) is a net immune function biomarker that measures interferon-γ after stimulation of the innate and adaptive immune systems and is based on a readily available pathology platform. Measuring QFM in cirrhotic patients provides an objective marker of their immune dysfunction, which has otherwise been difficult to quantify. Low QFM is significantly associated with the risk of pre-transplant infection, and very low QFM may be associated with increased risk of mortality.
- Citation: Sood S, Yu L, Visvanathan K, Angus PW, Gow PJ, Testro AG. Immune function biomarker QuantiFERON-monitor is associated with infection risk in cirrhotic patients. World J Hepatol 2016; 8(35): 1569-1575
- URL: https://www.wjgnet.com/1948-5182/full/v8/i35/1569.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i35.1569
QuantiFERON-Monitor (QFM, Qiagen Ltd, United States) was developed as an immune function monitoring tool in the post-transplant setting and provides a general biomarker of immune function based on stimulation of both the innate and adaptive immune systems[1]. It was developed based on the same diagnostic platform as the widely available QuantiFERON-Gold assay (QFN-gold, Qiagen Ltd, United States) and requires minimal laboratory processing. A high QFM result suggests a robust immune response, whilst a low result implies impaired immunity. Initial pilot data showed low QFM compared with age-sex matched controls not just in patients on immunosuppression post-transplant, but also in cirrhotic patients on the waiting list prior to transplant[1].
Patients with decompensated cirrhosis have inherently impaired immune responses, with bacterial infections occurring in 20%-60% of patients hospitalized for cirrhosis[2] and responsible for up to 25% of deaths in patients with liver disease[3]. The immune dysfunction in cirrhosis involves impairments of both quantity and quality of many immune cells that have been individually studied but are not always appreciated in clinical care.
In this study we present data that represents the first well described clinical cohort of patients to be evaluated with the QFM assay. We describe their immune function and investigate whether low QFM is associated with infection risk in this prospective cohort of pre-transplant cirrhotic patients.
We performed a prospective observational cohort study on 91 patients with cirrhosis awaiting liver transplantation at a single centre. Patients were recruited between November 2011 to December 2013 and followed until the census date of 1st February 2014. Approximately half the patients had blood taken immediately prior to their transplant surgery, while the remainder had a period of time in between their blood test and transplantation, death or the census date.
The QFM assay was performed on 1 mL of whole blood. As per manufacturer’s guidelines, blood was stimulated with the QFM immune ligands anti-CD3 and R848 in the form of a single lyophilized ball within 8 h of being taken. Stimulated blood was incubated overnight at 37 °C. Following incubation, the blood underwent centrifugation and plasma harvested. An enzyme-linked immunosorbent assay (ELISA) was performed by a separate investigator who was blinded to clinical data. Clinicians caring for the cirrhotic patients were blinded from the QFM assay results. QFM output was measured as interferon-γ (IFN-γ) production measured as IU/mL, in a process similar to that applied to perform a QFN-gold assay: Samples were brought to room temperature and given 60 min to equilibrate. The lyophilized IFN-γ standard was reconstituted with deionized water. This was gently mixed to minimize frothing and ensure complete solubilisation. Dilutions were prepared to validate the standard curve.
The lyophilized conjugate was reconstituted with 0.3 mL of deionized water and mixed gently to minimize frothing and ensure complete solubilisation. Further dilutions were performed with addition of Green Diluent. Fifty microliters of prepared conjugate was added to each ELISA well, after which 50 μL of each sample were added. Plates were covered and mixed using a microplate shaker for 1 min and then incubated at room temperature for 120 min ± 5 min.
Wells were then washed with 400 μL of working strength wash buffer for at least 6 cycles in a microplate washer. Plates were tapped, while facing down on absorbent, low-lint towels to remove residual wash buffer. One hundred microliters of enzyme substrate solution was then added to each well, and plates covered with a lid. These were mixed using a microplate shaker, and then incubated at room temperature for a further 30 min.
Following this further incubation, 50 μL of enzyme stopping solution was added to each well and mixed thoroughly with the microplate shaker. The optical density was then measured within 5 min of stopping the reaction using a microplate reader fitted with a 450 nm filter, as well as a 620 nm-650 nm reference filter. The optical density values were used to calculate the output result of IFN-γ in IU/mL. Low QFM was suggestive of an immunosuppressed state.
Basic clinical data was collected from participants who were recruited as part of a post-transplant research trial. Collected data included age, gender, disease aetiology and blood biochemistry. Patients were also evaluated for commonly used scoring systems for the severity of liver disease, the Child-Pugh Score and model for end stage liver disease (MELD) score. Patients were monitored prospectively for infection occurring after their QFM sample and up to either liver transplant, infection, death or the census date. Infections were per pre-defined criteria of “probable” or “definite” infection adjusted from The International Sepsis Forum Consensus Conference on Definitions of Infection in the Intensive Care Unit[4]. All patients were admitted to hospital for intravenous antimicrobial treatment.
Logistic regression, Mann-Whitney U test and Kaplan-Meier survival curves were analyzed with GraphPad Prism 6.0 for Mac (IBM, United States). All variables that showed potential predictive capacity of 15% (P < 0.15) were entered into a multivariate logistic regression mode using STATA/SE version 12.0 for Mac (Statacorp, United States). P values under 0.05 were considered significant. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the appropriate institutional review committee. All patients provided written informed consent.
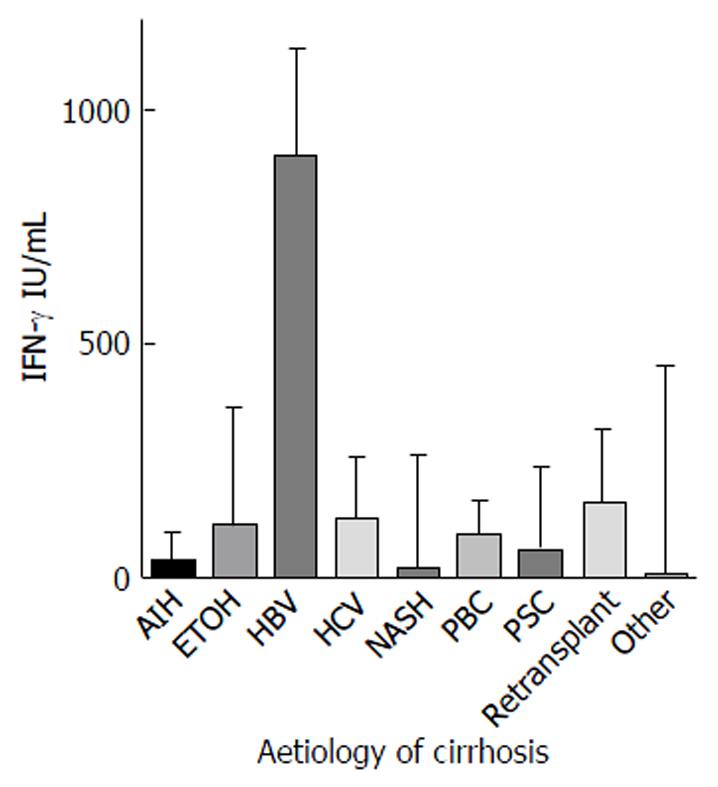
Ninety-one cirrhotic patients were recruited (Table 1). The majority (n = 62, 68.1%) were male. The mean age was 51 years (median 54, range 20-72 years). The most common aetiology of liver disease was hepatitis C virus infection (HCV, 43%), followed by Primary Sclerosing Cholangitis (PSC, 11%), alcoholic liver disease (ETOH, 11%), and non-alcoholic steatohepatitis (NASH, 10%) (Figure 1). ETOH was a significant co-factor in 14/24 (58.3%) of patients with HCV. QFM level did not vary significantly by aetiology (P = 0.08).
| Demographics | Median QFM (95%CI) IU/mL | |
| Age (median, yr) | 54 (20-72) | 94.5 (37.3-158) |
| Male | 62 (68.1%) | 124.5 (37.3-223) |
| Female | 29 (31.9%) | 73.9 (7.50-158) |
| Child-Pugh score | ||
| A | 7 | 381 (12.9-1234) |
| B | 29 | 224 (94.4-506) |
| C | 55 | 37.3 (19.5-128) |
| MELD score | ||
| 0-10 | 10 | 319 (12.9-904) |
| 11-20 | 42 | 155.5 (94.5-240) |
| 21-30 | 34 | 30.0 (9.16-157) |
| ≥ 30 | 5 | 8.81 (0.63-47.6) |
| Primary aetiology of cirrhosis, n (%) | ||
| HCV | 39 (42.9) | 130 (47.6-223) |
| PSC | 10 (11.0) | 61.6 (1.19-279) |
| ETOH | 10 (11.0) | 113.3 (8.81-385) |
| NASH | 9 (9.89) | 20.3 (6.20-375) |
| AIH | 5 (5.49) | 37.3 (0.04-137) |
| PBC | 4 (4.40) | 93.0 (24.1-168) |
| HBV | 3 (3.30) | 904 (799-1132) |
| Retransplant | 3 (3.30) | 163 (2.06-318) |
| Other | 8 (8.79) | 6.59 (0.07-774) |
| HCC | 31 (34.1) | 194 (87.9-425) |
| No HCC | 61 (65.9) | 73.9 (28.0-154) |
The mean QFM in cirrhotics was 214.3 IU/mL, median 94.5 IU/mL compared to a historical cohort of healthy controls (mean 555.2 IU/mL, median 423 IU/mL)[1]. There was no significant difference between QFM in males and females (P = 0.11). Of the patient group as a whole, the median MELD was 20 and Child-Pugh Score was 10. Hepatocellular carcinoma (HCC) was present in 31 patients (34.1%) and associated with a lower median MELD compared with non-HCC patients (15 vs 20, P < 0.0001). Accordingly, HCC patients who expressed a more robust immune response with a median QFM more than double that of non-HCC patients (194 IU/mL vs 73.9 IU/mL, P = 0.03).
Several other factors were associated with QFM on univariate analysis. Along with presence of HCC, haemoglobin level was positively associated with QFM. Alternatively, an inverse association was found with advancing MELD score, Child-Pugh score, urea and international normalized ratio (Table 2). On a multivariate regression model, only Child-Pugh score and urea were independently associated with QFM levels in cirrhotic patients (Table 3).
| Coefficient | P vaule | 95%CI | |
| MELD score | -17.3 | < 0.001 | -25.3:-9.29 |
| Child-Pugh score | -65.6 | < 0.001 | -91.1:-40.2 |
| Alcohol | -69.3 | 0.285 | -197.4:58.7 |
| HCC | 193.9 | 0.002 | 71.6:316.1 |
| Age | 3.18 | 0.252 | -2.30:8.66 |
| WCC | -9.4 | 0.351 | -29.2:10.5 |
| Neutrophils | -19.0 | 0.137 | -44.1:6.17 |
| HCV | -44.4 | 0.476 | -168.0:79.1 |
| Male | 70.1 | 0.288 | -60.1:200.4 |
| Bilirubin | -0.59 | 0.001 | -0.95:-0.24 |
| Urea | -14.5 | 0.023 | -26.9:-2.00 |
| Creatinine | 0.17 | 0.702 | -0.71:1.06 |
| Haemoglobin | 5.04 | < 0.001 | 2.48:7.59 |
| Platelets | 0.59 | 0.164 | -0.24:1.42 |
| Albumin | 9.50 | 0.055 | -0.21:19.2 |
| INR | -230.6 | < 0.001 | -342:-119 |
| Coefficent | P vaule | 95%CI | |
| Child-Pugh score | -51.9 | 0.013 | -92.6:-11.3 |
| MELD score | 16.0 | 0.131 | -4.88:36.9 |
| HCC | 62.9 | 0.366 | -74.8:201 |
| Bilirubin | -0.39 | 0.131 | -0.91:0.120 |
| Urea | -14.3 | 0.046 | -28.3:-0.261 |
| Haemoglobin | 1.77 | 0.250 | -1.27:4.82 |
| Albumin | 7.15 | 0.141 | -2.43:16.7 |
| INR | -114 | 0.168 | -278:49.3 |
Of the 91 cirrhotic patients, approximately half (n = 47, 51.6%) were transplanted on the day of their QFM measurement. The remaining 44 (48.4%) had the QFM assay performed a median 46 d (range 2-591) prior to the date of censor. This sub-group were further investigated for rates of infection prior to transplantation. Most were receiving antibiotic prophylaxis (34/44, 77.3%).
At the census date, 33 patients (75%) had been transplanted, 3 patients had died (6.8%) and 8 (18.2%) were still awaiting transplantation. Advanced MELD (r2 = 0.27, P = 0.002) and Child-Pugh score (r2 = 0.15, P = 0.03) were associated with shorter time to transplant, while QFM was not (r2 = 0.01, P = 0.64).
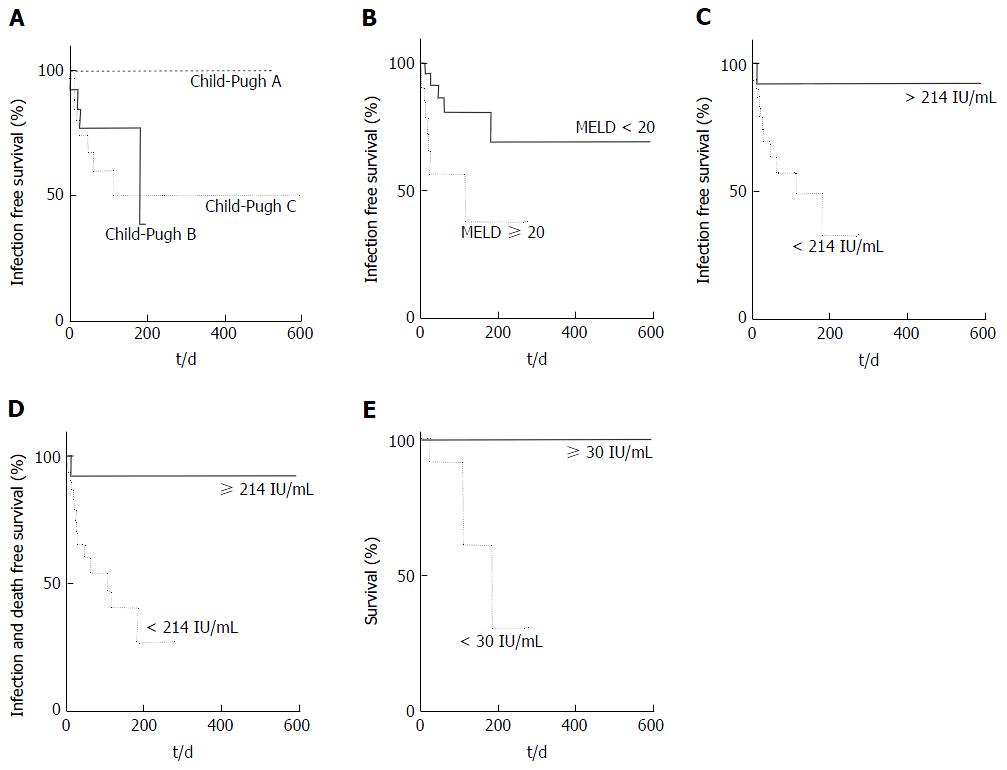
Thirteen of 44 patients (29.5%) experienced a pre-transplant infection at a median of 20 d (range 2-182) after their pre-transplant blood test. Three patients had spontaneous bacterial peritonitis (SBP), 4 pneumonia, 3 bacteraemia, 1 fungaemia, 1 urinary tract infection and 1 cholangitis. Most patients (n = 9, 69%) who experienced an infection had Child-Pugh C cirrhosis but Child-Pugh score was not associated with risk of infection (Figure 2A, P = 0.2), whereas MELD score (≥ 20) was (Figure 2B; HR = 4.7, P = 0.01). Urea above the laboratory reference range of 6.7 mmol/L was not associated with infection risk (P = 0.15).
A QFM under the cohort mean of 214 IU/mL was significantly associated with infection pre-transplant (HR = 4.1, Figure 2C, P = 0.01) and the combined outcome of infection or death on the waiting list (Figure 2D, HR = 4.4, P = 0.006).
Three patients died in this cohort while awaiting transplantation, two from bleeding (one intracranial, one variceal) and one from sepsis and multiorgan failure. The median MELD of these patients was 24 and Child-Pugh score of 12. Patients who died pre-transplant had a significantly lower QFM (AUROC 0.88, P = 0.03), and on survival analysis, a very low QFM (< 30 IU/mL) was most associated with a HR of 56.6 for death (Figure 2E, P = 0.003).
Infections are implicated in up to 25% of deaths of patients with cirrhosis[3], and are the second leading cause of death in patients with end-stage liver disease awaiting liver transplantation[5,6]. Immune dysfunction in cirrhosis is likely multifactorial, with impaired function identified in neutrophils[7-10], monocytes[11] and lymphocytes[12]. Many of which also show impaired numbers, partly as a result of portal hypertension and splenic sequestration. Advanced cirrhosis is also associated with deficiencies in both structure and function of the reticuloendothelial system[13,14], complement production[15], and a chronic immune activation that appears to result in a systemic immune paralysis[16-20]. Although each individual aspect of immune deficiency has been studied in isolation, estimating a patient’s overall level of immune function has been unattainable.
QFM was designed as a net immune function biomarker to manage immunosuppression in the post-transplant setting. Unlike other immune function assays that are predominantly confined to research settings, it has potential clinical utility as it is based on QFN-gold, an assay already in widespread use, and requires only minimal laboratory processing. QFM incorporates both an innate and adaptive stimulant which offers an objective, albeit non-specific overview of a patient’s individual immune response. A perhaps not unexpected finding of the original pilot study was that low QFM was identified in patients awaiting, and not only after liver transplantation[1]. In this study, we confirm and are able to quantify the immunosuppressed status of cirrhotic patients, with a median QFM of 94.5 IU/mL less than 25% that of healthy controls (423 IU/mL)[1]. Most importantly, we not only demonstrate a low QFM in cirrhotic patients (indicative of inherent immunosuppression), but that the most severe immune dysfunction is associated with heightened infection risk. Low QFM in cirrhotic patients had a HR of 4.1 for pre-transplant infection risk. A simple blood test that could highlight a patient’s individual risk of subsequent infection would be of value to treating clinicians.
There are some limitations when performing a study in a transplant-waitlisted population. Firstly, with the sickest patients (based on MELD) often receiving priority organ selection, there was risk of patients with greatest risk of infection (and lowest QFM) being transplanted earlier. This risks a type II error, which potentially underestimates the clinical value of the assay in predicting infections; Secondly, we may have underestimated the infection rate as diagnosing infections in patients with cirrhosis can be difficult, and empirical antibiotics are often used on presentation to hospital with conditions such as variceal bleeding or hepatic encephalopathy; and thirdly, since this data represents the first clinical cohort of non-transplant recipients evaluated with the QFM assay, readily defined set-points for low and very low QFM have not previously been evaluated or described.
Conversely, studying a transplant wait-listed population does offer some advantages since transplant listed patients are more unwell and at greatest susceptibility to infections (reducing the potential sample size and necessary follow-up period). They are closely monitored, with all events being reported to the transplant centre even if occurring at peripheral hospitals, thus allowing all clinical events to be documented.
Early identification and treatment of infections is essential in the management of cirrhotic patients, particularly given the morbidity and mortality often attributed to infections in this vulnerable population. However, infections can be difficult to distinguish from other non-infectious causes of systemic inflammatory response syndrome and symptoms of liver deterioration[21]. Serum biomarkers are therefore being examined, although currently available tests such as C-reactive protein, ferritin and white blood cells lack specificity[21].
To prevent infections, some cirrhotic patients are offered antibiotic prophylaxis, although mainly they have been used to prevent episodes of SBP. SBP has a recurrence rate of 70% within 12 mo[22], and secondary prophylaxis is a part of internationally accepted guidelines[23]. Primary antibiotic prophylaxis has also been recommended in patients with low protein in ascitic fluid as there is an understanding that it improves incidence of infections and short-term survival[24,25]. However, adherence to these guidelines is low, and in part may be due to fears over antimicrobial resistance and reduced effectiveness over time[26,27]. An objective immune function biomarker that could highlight patients with the most severe immune-deficiency could enable the use of more targeted antibiotic prophylaxis to those most at risk of all infections, and not just SBP.
There was no significant difference in QFM based on aetiology of the underlying liver disease. Patients with hepatocellular carcinoma as their primary indication for transplant were often not as unwell as other cirrhotic patients, and therefore had significantly higher QFM results on univariate analysis. This was verified in multivariate analysis where HCC was not independently associated with high QFM. Only Child-Pugh score and urea were individually identified on a logistic multivariate regression model as being associated with low QFM. This suggests that commonly used disease scoring systems such as MELD or biochemical investigations such as white cell count are not truly indicative of a patient’s underlying level of immune dysfunction and further highlights the possible value of an objective immune function biomarker. The significance of urea with QFM is interesting and would need further study. It could be a surrogate marker of renal function which is known to impact mortality in cirrhosis, although interestingly creatinine had no rela–tionship to QFM. An alternate hypothesis may be that the urea level reflects an increased catabolic state associated with nutritional deficiencies that may impact immune function.
A very low QFM was significantly associated with pre-transplant mortality in this cohort. Although two patients subsequently died of bleeding rather than sepsis, this may highlight the severely immunosuppressed state of patients with critical illness, and offer QFM as an alternate overall prognostic marker. However, despite reaching statistical significance, it is difficult with low numbers to make any firm conclusions regarding QFM and mortality risk. Further studies in a non-transplant wait-listed cirrhotic population would be needed to further explore and confirm this association.
In conclusion, patients with cirrhosis are at high risk of infection, but quantification of immune dysfunction has been difficult in clinical practice. Immune functional assays are often isolated to one small component of immunity, associated with significant laboratory processing or confined to limited situations and medical research. This study describes the first clinical assessment of the QFM immune function assay in patients not receiving immunosuppressant medications. We show that patients with cirrhosis are not only significantly immunosuppressed, but that a low level of QFM (suggestive of significant immune dysfunction) is associated with a four-fold increased risk for infection. The ability to employ a clinical assay that can objectively provide a biomarker of an individual’s innate and adaptive immune function offers obvious benefits to patient care, even outside the transplantation setting. This study also serves as a proof of concept that immune function monitoring may be available and have clinical utility in other fields of medicine where patients are either inherently or iatrogenically immunosuppressed.
Patients with cirrhosis are known to be immunosuppressed and infections are a significant cause of morbidity and mortality. Predicting patients at greatest risk of infection is difficult. The QuantiFERON-Monitor assay (QFM) is a novel immune function biomarker designed to assess immune function in a transplant setting. In this first study to employ QFM in a non-transplant setting, the authors aim to identify whether QFM can objectively measure a patient’s immune dysfunction and whether this correlates with the risk of infection.
Identifying cirrhotic patients at greatest risk of infection is difficult. Usual biochemical measures such as C-reactive protein and white cell count are not associated with infection in cirrhotic patients.
QFM is a novel immune function biomarker that provides an objective measure of immune function. In particular, it benefits from measuring interferon gamma production after stimulation of both arms of the immune system (innate and adaptive). In this study, the assay is shown to objectively measure immune dysfunction in cirrhotic patients, and that the patients with lower values had the greatest risk of infection.
QFM may have utility in measuring the level of immune function in patients with cirrhosis. This could then identify patients at greatest risk of infection, and who may benefit from either earlier transplantation or antibiotic prophylaxis. Furthermore, the assay may be useful in other medical conditions where patients are either inherently or iatrogenically immunosuppressed.
This is an questionable topic. First of all, the material method section should be described in a detailed manner. More aspects shous be enlightened. Moreover, discussion part should be enlarged properly and more recent studies should be mentioned and more recent studies should be added to references part.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Tanoglu A S- Editor: Qi Y L- Editor: A E- Editor: Li D
| 1. | Sood S, Cundall D, Yu L, Miyamasu M, Boyle JS, Ong SY, Gow PJ, Jones RM, Angus PW, Visvanathan K. A novel biomarker of immune function and initial experience in a transplant population. Transplantation. 2014;97:e50-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Garcia-Tsao G. Spontaneous bacterial peritonitis: a historical perspective. J Hepatol. 2004;41:522-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 46] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Wyke RJ. Problems of bacterial infection in patients with liver disease. Gut. 1987;28:623-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 132] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med. 2005;33:1538-1548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 554] [Cited by in RCA: 602] [Article Influence: 28.7] [Reference Citation Analysis (1)] |
| 5. | Fernández J, Gustot T. Management of bacterial infections in cirrhosis. J Hepatol. 2012;56 Suppl 1:S1-S12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 252] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 6. | Arvaniti V, D’Amico G, Fede G, Manousou P, Tsochatzis E, Pleguezuelo M, Burroughs AK. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139:1246-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 720] [Cited by in RCA: 851] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 7. | Rajkovic IA, Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 189] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 8. | Fiuza C, Salcedo M, Clemente G, Tellado JM. In vivo neutrophil dysfunction in cirrhotic patients with advanced liver disease. J Infect Dis. 2000;182:526-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Tritto G, Bechlis Z, Stadlbauer V, Davies N, Francés R, Shah N, Mookerjee RP, Such J, Jalan R. Evidence of neutrophil functional defect despite inflammation in stable cirrhosis. J Hepatol. 2011;55:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 157] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Ono Y, Watanabe T, Matsumoto K, Ito T, Kunii O, Goldstein E. Opsonophagocytic dysfunction in patients with liver cirrhosis and low responses to tumor necrosis factor-alpha and lipopolysaccharide in patients’ blood. J Infect Chemother. 2004;10:200-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Lin CY, Tsai IF, Ho YP, Huang CT, Lin YC, Lin CJ, Tseng SC, Lin WP, Chen WT, Sheen IS. Endotoxemia contributes to the immune paralysis in patients with cirrhosis. J Hepatol. 2007;46:816-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Doi H, Iyer TK, Carpenter E, Li H, Chang KM, Vonderheide RH, Kaplan DE. Dysfunctional B-cell activation in cirrhosis resulting from hepatitis C infection associated with disappearance of CD27-positive B-cell population. Hepatology. 2012;55:709-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 806] [Article Influence: 62.0] [Reference Citation Analysis (0)] |
| 14. | Bolognesi M, Merkel C, Bianco S, Angeli P, Sacerdoti D, Amodio P, Gatta A. Clinical significance of the evaluation of hepatic reticuloendothelial removal capacity in patients with cirrhosis. Hepatology. 1994;19:628-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Homann C, Varming K, Høgåsen K, Mollnes TE, Graudal N, Thomsen AC, Garred P. Acquired C3 deficiency in patients with alcoholic cirrhosis predisposes to infection and increased mortality. Gut. 1997;40:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Albillos A, de la Hera A, González M, Moya JL, Calleja JL, Monserrat J, Ruiz-del-Arbol L, Alvarez-Mon M. Increased lipopolysaccharide binding protein in cirrhotic patients with marked immune and hemodynamic derangement. Hepatology. 2003;37:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 346] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 17. | Guarner C, Soriano G, Tomas A, Bulbena O, Novella MT, Balanzo J, Vilardell F, Mourelle M, Moncada S. Increased serum nitrite and nitrate levels in patients with cirrhosis: relationship to endotoxemia. Hepatology. 1993;18:1139-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 290] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 18. | Campillo B, Bories PN, Benvenuti C, Dupeyron C. Serum and urinary nitrate levels in liver cirrhosis: endotoxemia, renal function and hyperdynamic circulation. J Hepatol. 1996;25:707-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Lin RS, Lee FY, Lee SD, Tsai YT, Lin HC, Lu RH, Hsu WC, Huang CC, Wang SS, Lo KJ. Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J Hepatol. 1995;22:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 271] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 20. | Testro AG, Visvanathan K. Toll-like receptors and their role in gastrointestinal disease. J Gastroenterol Hepatol. 2009;24:943-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 93] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 21. | Bunchorntavakul C, Chamroonkul N, Chavalitdhamrong D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J Hepatol. 2016;8:307-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 157] [Article Influence: 15.7] [Reference Citation Analysis (2)] |
| 22. | Titó L, Rimola A, Ginès P, Llach J, Arroyo V, Rodés J. Recurrence of spontaneous bacterial peritonitis in cirrhosis: frequency and predictive factors. Hepatology. 1988;8:27-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 248] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Runyon BA. Management of Adult Patients with Ascites Due to Cirrhosis: Update 2012. AASLD Practice Guideline: AASLD, 2012. . |
| 24. | Fernández J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, Vila C, Pardo A, Quintero E, Vargas V. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology. 2007;133:818-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 456] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 25. | Saab S, Hernandez JC, Chi AC, Tong MJ. Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short-term survival in cirrhosis: a meta-analysis. Am J Gastroenterol. 2009;104:993-1001; quiz 1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Ngamruengphong S, Nugent K, Rakvit A, Parupudi S. Potential preventability of spontaneous bacterial peritonitis. Dig Dis Sci. 2011;56:2728-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Bruns T, Zimmermann HW, Stallmach A. Risk factors and outcome of bacterial infections in cirrhosis. World J Gastroenterol. 2014;20:2542-2554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 87] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |