Published online Apr 18, 2016. doi: 10.4254/wjh.v8.i11.513
Peer-review started: September 11, 2015
First decision: October 27, 2015
Revised: February 18, 2016
Accepted: March 24, 2016
Article in press: March 25, 2016
Published online: April 18, 2016
Processing time: 213 Days and 1.3 Hours
Hepatocellular carcinoma (HCC) is the main common primary tumour of the liver and it is usually associated with cirrhosis. The barcelona clinic liver cancer (BCLC) classification has been approved as guidance for HCC treatment algorithms by the European Association for the Study of Liver and the American Association for the Study of Liver Disease. According to this algorithm, hepatic resection should be performed only in patients with small single tumours of 2-3 cm without signs of portal hypertension (PHT) or hyperbilirubinemia. BCLC classification has been criticised and many studies have shown that multiple tumors and large tumors, as wide as those with macrovascular infiltration and PHT, could benefit from liver resection. Consequently, treatment guidelines should be revised and patients with intermediate/advanced stage HCC, when technically resectable, should receive the opportunity to be treated with radical surgical treatment. Nevertheless, the surgical treatment of HCC on cirrhosis is complex: The goal to be oncologically radical has always to be balanced with the necessity to minimize organ damage. The aim of this review was to analyze when and how liver resection could be indicated beyond BCLC indication. In particular, the role of multidisciplinary approach to assure a proper indication, of the intraoperative ultrasound for intra-operative restaging and resection guidance and of laparoscopy to minimize surgical trauma have been enhanced.
Core tip: According to the barcelona clinic liver cancer (BCLC) classification liver resection should be performed only in patients with small single hepatocellular carcinoma of 2-3 cm without signs of portal hypertension (PHT). Nevertheless, many studies have shown that patients with multiple and large hepatocellular carcinoma, as like as those with macrovascular infiltration and PHT, could benefit from liver resection. Consequently BCLC algorithm should be updated and revised. The aim of this review was to analyze when and how liver resection could be indicated beyond BCLC indications. In this perspective, the role of multidisciplinary approach, of intraoperative ultrasound and of laparoscopy have been enhanced.
- Citation: Garancini M, Pinotti E, Nespoli S, Romano F, Gianotti L, Giardini V. Hepatic resection beyond barcelona clinic liver cancer indication: When and how. World J Hepatol 2016; 8(11): 513-519
- URL: https://www.wjgnet.com/1948-5182/full/v8/i11/513.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i11.513
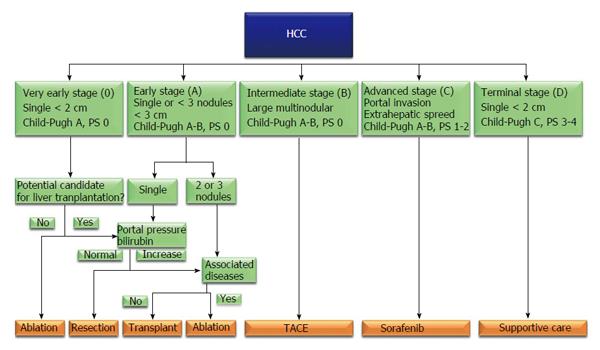
Hepatocellular carcinoma (HCC) is the main common primary tumour of the liver, representing approximately 85%-90% of primary hepatic malignancies; it is ranked as the fifth and seventh most common cancer respectively in males and females, and represents the third leading cause of neoplasm-related deaths worldwide[1,2]. HCC is usually associated with cirrhosis, whose major causes could be identified in viral and alcoholic liver disease, although recent epidemiological data highlighted the increasing etiological role of obesity, diabetes and metabolic syndrome in liver oncogenesis[3]. The treatment of HCC set on cirrhosis is complex: The aim to be oncologically radical has always to be balanced with the necessity to minimize organ damage. In this sense liver transplantation is considered the gold standard treatment, because offers the possibility to treat simultaneously the liver cancer and the damaged organ; on the other hand organ shortage led to the development of restricted indication to liver transplantation, addressing many patients to receive local therapies[4]. In literature, several HCC staging systems based on tumour’s features and liver function have been developed and proposed to guide the therapeutic decisions in such patients; among all the barcelona clinic liver cancer (BCLC) classification (Figure 1) has been approved as guidance for HCC treatment algorithms by the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Disease (AASLD), combining independent prognostic predictors like the background liver status, patient’s performance status and tumor morphological features, and showing a reliable capacity to categorize patients with different prognosis in order to provide recommendations regarding therapeutic options. The BCLC flow chart recommends curative treatments for HCC in very early- or early-stage (stage 0-A), transarterial chemoembolization for intermediate-stage disease (stage B), sorafenib administration for advanced stage HCC (stage C), and supportive care for end stage HCC (stage D)[5,6]. BCLC indication to liver resection seems to be markedly limiting. On contrary, other authors have shown that surgical resection can offer good short- and long-term outcomes even in presence of portal hypertension (PHT), multinodular disease, large nodules or even HCC with macrovascular invasion[7-9]; thus the BCLC classification has been criticised because some patients who may benefit from surgical treatment are excluded from curative resection and these findings have encouraged many experts to disregard the EASL/AASLD therapeutic recommendations)[6-8].
The aim of this review was to analyze when and how liver resection could be indicated beyond BCLC indications.
Historically hepatic resection has been performed with caution to HCC patients because of concerns about morbidity and mortality rates. However, recent improvements in surgical technique and perioperative care have improved hepatic resections outcomes with consequent extension of indications to surgical procedures. In this sense some high volume surgical liver unit recently reported hospital mortality less than 2%[10]. According to BCLC algorithm, liver resection would be indicated only in patients with single tumours of 2-3 cm in diameter without PHT or increased bilirubinemia[6] (Figure 1). BCLC classification has been criticised because it excludes many patients who could benefit from curative resection. PHT, large tumor size, multifocal presentation and vascular invasion are well recognized risk factors for post-operative morbidity and mortality and for poor long-term prognosis, but should not be considered contraindication to surgical treatments.
BCLC algorithm suggests hepatic resection only in presence of small HCC (< 5 cm). On contrary, several authors have recently reported that liver resection can offer good short- and long-term outcomes even in patients with HCC > 5 cm. Main concerns related to restricted surgical indication in patients with large HCC take into account the increased rates of presence of satellite nodules, increased rate of distant metastases and of vascular invasion those are related to increasing tumour size and represent important prognostic factors for poor survival. Furthermore patients with large HCC (> 5 cm) may necessitate a major hepatectomy, which is considered a high-risk procedure especially in HCC set on cirrhosis[11,12]. Anyway radical liver resection can be considered a valuable option in patients with large HCC[13]. Large surgical series recently published reported a significant rate (up to 36%) of large HCC surgically treated with good results[14]. In this sense, it’s remarkable that in literature there are many studies reporting cases of liver resection for HCC > 8-10 cm with good results even considering the poor prognostic results of the main alternative for such BCLC stage B HCC represented by transarterial chemo-embolization (TACE). Zhong et al[15] comparing liver resection to TACE in a wide cohort of patients with large HCC in BCLC B stage (mean size 8.8 cm) showed that tumor resection offers better 5-year overall survival than TACE (41% and 18%, respectively). Proper identification and multidisciplinary discussion of risk factors for surgical morbidity and mortality in such patients (including presence of vascular invasion, cirrhosis, high level of alpha-fetoprotein and the presence of multiple lesions[16]) is critical for patients’ selection and to obtain good outcomes.
Treatment guidelines do not recommend hepatic resection for multifocal HCC. Liver resection in presence of multiple HCC is still controversial; anyway it has been recognized a survival benefit for patients with a number of HCC ≤ 3 and lesions less than 3 cm in diameter (according to Milan criteria)[17]. Multifocal presentation is well recognized independent prognostic factor for early recurrence and poor prognosis[18]. Anyway recent prospective studies showed that hepatic resection in patients with BCLC stage B HCC is well tolerated and related to a low mortality rate, acceptable morbidity and significant survival benefits[4]. Surgical resection yielded better results than TACE in patients with multiple HCCs of the same stage; Zhong et al[19] analyzed outcomes of patients with more and less of 3 HCC tumors who underwent liver resection or TACE. Survival was significantly higher in the surgery subgroup at 1 year (90% vs 59%), 3 years (52% vs 11%), and 5 years (33% vs 6%).
PHT is considered a contraindication for liver resection according to the EASL and AASLD published guidelines for HCC management. PHT may increase the risk of peri-operative haemorrhage, impair liver regeneration, and increase the risk of liver failure. Recent advances in surgical techniques and peri-operative care for patients with cirrhosis have reduced the number of cirrhosis-related complications and deaths. Several authors demonstrated that patients with and without PHT had similar morbidity (28%-39% vs 21%-32.2%) and 90-d mortality (2%-2.1% vs 3.1%-6%). The overall survival at 1, 3 and 5 years is similar or slightly longer in patients without portal vein hypertension (respectively 85%-96%, 67%-80% and 50%-65%) compared to patients with PHT (respectively 83%-90%, 59%-67% and 45%-48%), these results appear significant and encouraging, considering that liver resection, with exclusion of liver transplantation, represent the best choice of radical cure[20-22]. Patients with PHT should be carefully selected for surgery, but PHT should not be considered a contraindication to liver resection.
According to the EASL and AASLD guidelines for management of HCC, patients with macrovascular infiltration are considered in advanced stage (stage C) and should be treated only with chemotheraphy (Sorafenib). Presence of macrovascular invasion is related to an increased risk of metastases and is a well known predictor of poor survival[23]. The median survival for untreated patients with macrovascular portal or major hepatic vein infiltration is 3-5 mo and median survival for such patients treated with sorafenib is 6 mo[18,24]. Selected patients with macrovascular infiltration who underwent liver resection for HCC can achieve longer overall survival, 46%-49% and 11.2%-38% respectively at 3 and 5 years with acceptable morbidity and mortality rate (under 3%-5%)[13,14,25]. Consequently the surgical resection should be considered when planning the treatment’s strategy for such patients and formally included together with other treatment modalities for the cure of BCLC stage C patients.
Patients suffering from cirrhosis are at increased risk of developing significant postoperative complications including ascites, lung infection or pleural effusion, transient encephalopathy, kidney failure, portal vein thrombosis and bleeding due to primary haemostasis dysfunction[26,27]. In order to reduce mortality and morbidity after liver surgery in patients with cirrhosis, surgeons have developed meticulous selection criteria to guide surgical indication in such patients. For all these reasons the decision to submit a cirrhotic patient to a liver resection is complex. It is of paramount importance that pre-operative evaluation of cirrhotic patients with hepatocellular carcinoma would be performed by a multidisciplinary team, in order to match different point of view and possible therapeutic approach. Furthermore, some technical aspects of liver resection should be ehnaced discussing the approach to patients with an advanced stage HCC.
HCC has different presentations those are compounded by the status of liver disease, and the multiple treatment options available make choosing the first line of treatment for a given patient a difficult task. Management of HCC patients should be undertaken by a multidisciplinary team including all the specialties those have a role in the treatment of such patients; if this kind of approach should represent the standard for the treatment of every patient with HCC, the importance of the sharing of indication in disagreement with BCLC algorithm is even increased. Studies those have shown a decrease in morbidity and mortality after liver resection for HCC also showed the importance of a multidisciplinary approach[28-31]. Patients in intermediate/advanced stages should be carefully selected for the best treatment according to the stage of the disease, to the presence of cirrhosis, to the age of patient, to general condition and commorbidity. A team of surgeon, oncologist, hepatologist, radiologist and interventional radiologist, anesthesiologist and pathologist should evaluate the best treatment for each of these patients, in order to perform a tailored treatment that could include more than one approach. BCLC indication to liver resection should be less restrictive; on the other hand the importance of patients’ selection must be considered and great efforts are needed to establish selection criteria to be included in the treatment algorithm.
Intra-operative ultrasound (IOUS) is still considered the most accurate diagnostic technique for detecting focal liver lesions in hepatocellular carcinoma. The main advantages related to an extensive use of ultrasounds in liver surgery for HCC on cirrhosis concerns the intra-operative re-staging and the possibility to perform echo-guided surgical procedures. IOUS may detect additional nodules compared with pre-operative imaging in 33%-41% of patients undergoing liver resection for HCC[32,33]: The removal of new nodules after this early diagnosis may increase the BCLC stage of patients but also contribute to perform a more complete treatment and improve choice of cure. Moreover intra-operative echo guidance, allowing to perform a parenchima-sparing anatomical hepatic surgery in respect of principles of oncologic radicality, is an invaluable tool to engage surgical procedure in intermediate/advanced-stage patients[34]. The use of ultrasound guidance is mandatory for planning the surgical strategy, decide the exact resection plane during the parenchymal transection in order to respect the surrounding vessels and biliary structures. Main concerns related to the restricted indication to liver surgery following BCLC indication regards the possibility of increased peri-operative mortality and morbidity and the poor chance of radical cure and prolonged survival in patients with intermediate/advanced HCC. IOUS, minimizing the extension of the parenchima removed in respect of oncological radicality and offering a re-staging and a consequent more radical treatment, represents an invaluable tool in the perspective of expansion of surgical indication beyond BCLC reccomandations. The extensive use of ultrasound in liver surgery together with technological improvements in recent years allowed per se an expansion of surgical indication in advanced HCC: The possibility to detect intra-operatively connecting veins between adjacent hepatic veins allows to perform radical limited liver resection even in patients with major hepatic vein invasion, in order to reduce the rate of major resection and its consequent increased morbidity and mortality[35,36]. Anatomic liver resection is usually performed because of HCC spreading along the nourishing portal venous branch and consequent growth of satellite nodules within the same anatomical segment. Thus, anatomic resection allows removal of the known tumor, as well as of potential undetectable satellite metastases; the advantages of anatomic resection can be maximized in particular in large HCC which are frequently surrounded by satellite lesions[37,38]. IOUS is of paramount importance to guide and assure an anatomic liver resection, either with traditional puncture technique of the portal branch feeding the tumor, either by means of recently introduced compression technique or other methods as trans-hepatic balloon catheter or CEIOUS portography combined with indigo carmine dye injection[39-43]. In a recent meta-analysis Chen et al[44] analyzed outcomes of 833 patients underwent anatomic liver resection for HCC and 670 patients underwent non-anatomic resection for the same desease The surgical margin per se does not represent a main aspect, because an anatomic resection (segmentectomy or sub-segmentectomy) can be considered adequate even in presence of a narrow margin; the advantages related to limited anatomic resection can be maximized to perform multiple limited resection in multiple HCC, in order to assure local radical tumor removal with a parenchima sparing policy[44]. There are several methods up to now available to perform an anatomic (segmental or subsegmental) US-guided liver resection: Puncture technique proposed by Makuuchi et al[40], insertion of a balloon catheter transhepatically to occlude the feeding portal branch[41] and ultrasound-guided finger compression technique[43].
Laparoscopic surgery for liver tumors requires skilled surgeons and specific technological instruments. Moreover its indications have not been still clearly defined; for such reasons it has not been widely performed even if its employment is progressively expanding. The main concern about the use of laparoscopic technique for malignancies is the risk of inadequate tumor resection; positive margin is a well known prognostic factor for poor survival in surgery for HCC, in this perspective intra-operative ultrasound should be considered an indispensable tool to achieve a safe and effective liver resection. Anyway according to several meta-analyses comparing open vs laparoscopic liver resections for HCC, laparoscopic liver resection is considered a safe procedure with comparable overall and recurrence-free survival rates[45-47]. Laparoscopic approach might improve the postoperative course of cirrhotic patients, because limited mobilization of the liver reduces parenchymal trauma, nonexposure of intestinal viscera restricts fluid requirements and decreased the formation of ascites[26,48]. In a recent study Kanazawa et al[26] compared outcomes of cirrhotic patients underwent laparoscopic and laparotomic liver resection; the two groups was not different by age, sex, stage of cirrhosis, number and size of lesions. In this study the incidence of intractable ascites was significantly higher in the laparotomy group than in the laparoscopy group (71% vs 11%). Furthermore the use of laparoscopy in cirrhotic patients may allow the preservation of wall portosystemic shunts, and in some cases the integrity of round ligament, which can contain collateral vessels. This can result in a lower increase of post-operative PHT and risk of bleeding[26]. Laparoscopic liver resection is associated with less total and major morbidity, shorter hospital stay and lower rate of post-operative early readmissions or number of outpatient clinic appointments compared with open couterpart[49,50]. The above mentioned advantages of laparoscopic approach can be crucial in the perspective of expansion of surgical indication to patients with HCC beyond BCLC indications. In particular patients with PHT or multinodular disease can benefit of a minimally invasive treatment, which can include also combined laparoscopic resection and ablation of multiple HCC in the perspective of a tailored treatment[51,52]. Surgical resection has shown better results in terms of disease free and overall long term surival compared to laparoscopic ablation. Nevertheless, in the treatment of HCC not suitable for liver transplantation or not eligible for resection because of severe PHT and not manageable by percutaneous approach for tumor size or location, laparoscopic ablation should be considered as a valuable choice since it proved to be a safe and effective technique, as it permits to treat lesions with low-morbidity-rate[51]. Laparoscopic approach is also useful to avoid unnecessary laparotomy in patients who show unresectable not previously diagnosed lesions (36% of patients with HCC with surgical indication)[53]. Several authors showed that laparoscopic liver resections can be technically performed regardless tumor size and location, but important reviews have recognized that it can be considered more safe and feasible for lesions located on left lateral (segments II and III) and anterior right (III, VI) segments[45-47]. For this reason the position of lesions is an essential element to establishing the indication to surgical resection for advanced HCC. In order to minimize postoperative complications in fragile patients, the possibility of laparoscopic liver resections is a decisive element in the decision-making process of the best treatment of patients with HCC.
According to BCLC classification hepatic resection should be performed only in patients with small single tumours of 2-3 cm without signs of PHT or hyperbilirubinemia. By contrast many studies have shown that surgical resection can lead to good short and long-term survival in patients with PHT and with multinodular, large or macrovascular invasive HCC. The treatment of these patients is complex, surgery should only be performed in selected patients and a multidisciplinary team is necessary to choose the best treatment for each patient. Intra-operative ultrasound and laparoscopy are necessary tools in a modern liver unit, especially for cirrhotic patients.
BCLC indication should be expanded and redefined: BCLC algorithm should take into account the survival benefit of surgical resection in selected patients with HCC in B-C stages and should discuss the invaluable role of IOUS and the potential role of laparoscopy with the aim to standardize the surgical management. After the recognition that ablative treatment in HCC ≤ 2 cm offer the same survival benefit than surgical removal and consequently can be considered the treatment of choice for such patients[54], it should be recognized that surgical treatment offers the best choice of prolonged survival even to selected patients with intermediate/advanced stage HCC.
P- Reviewer: Chuang WL, Penkova-Radicheva MP, Wang GY S- Editor: Gong XM L- Editor: A E- Editor: Liu SQ
| 1. | Lafaro KJ, Demirjian AN, Pawlik TM. Epidemiology of hepatocellular carcinoma. Surg Oncol Clin N Am. 2015;24:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 2. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J; EASL Panel of Experts on HCC. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3252] [Cited by in RCA: 3255] [Article Influence: 130.2] [Reference Citation Analysis (1)] |
| 3. | Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 412] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | Torzilli G, Donadon M, Marconi M, Palmisano A, Del Fabbro D, Spinelli A, Botea F, Montorsi M. Hepatectomy for stage B and stage C hepatocellular carcinoma in the Barcelona Clinic Liver Cancer classification: results of a prospective analysis. Arch Surg. 2008;143:1082-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 5. | Choi C, Choi GH, Kim TH, Tanaka M, Meng MB, Seong J. Multimodality Management for Barcelona Clinic Liver Cancer Stage C Hepatocellular Carcinoma. Liver Cancer. 2014;3:405-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Schlachterman A, Craft WW, Hilgenfeldt E, Mitra A, Cabrera R. Current and future treatments for hepatocellular carcinoma. World J Gastroenterol. 2015;21:8478-8491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 117] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 7. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Vitali M, Bertuzzo F, De Angelis M, Mantovani G, Iacono C. Hepatocellular carcinoma: surgical perspectives beyond the barcelona clinic liver cancer recommendations. World J Gastroenterol. 2014;20:7525-7533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 8. | Ho MC, Huang GT, Tsang YM, Lee PH, Chen DS, Sheu JC, Chen CH. Liver resection improves the survival of patients with multiple hepatocellular carcinomas. Ann Surg Oncol. 2009;16:848-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 9. | Forner A, Gilabert M, Bruix J, Raoul JL. Treatment of intermediate-stage hepatocellular carcinoma. Nat Rev Clin Oncol. 2014;11:525-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 369] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 10. | Zhou Y, Lei X, Wu L, Wu X, Xu D, Li B. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol. 2014;23:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Extended hepatic resection for hepatocellular carcinoma in patients with cirrhosis: is it justified? Ann Surg. 2002;236:602-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 12. | Schroeder RA, Marroquin CE, Bute BP, Khuri S, Henderson WG, Kuo PC. Predictive indices of morbidity and mortality after liver resection. Ann Surg. 2006;243:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 251] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Zhang ZM, Guo JX, Zhang ZC, Jiang N, Zhang ZY, Pan LJ. Therapeutic options for intermediate-advanced hepatocellular carcinoma. World J Gastroenterol. 2011;17:1685-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Torzilli G, Belghiti J, Kokudo N, Takayama T, Capussotti L, Nuzzo G, Vauthey JN, Choti MA, De Santibanes E, Donadon M. A snapshot of the effective indications and results of surgery for hepatocellular carcinoma in tertiary referral centers: is it adherent to the EASL/AASLD recommendations?: an observational study of the HCC East-West study group. Ann Surg. 2013;257:929-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 427] [Article Influence: 32.8] [Reference Citation Analysis (4)] |
| 15. | Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 16. | Tsoulfas G, Mekras A, Agorastou P, Kiskinis D. Surgical treatment for large hepatocellular carcinoma: does size matter? ANZ J Surg. 2012;82:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Ruzzenente A, Guglielmi A, Sandri M, Campagnaro T, Valdegamberi A, Conci S, Bagante F, Turcato G, D’Onofrio M, Iacono C. Surgical resection versus local ablation for HCC on cirrhosis: results from a propensity case-matched study. J Gastrointest Surg. 2012;16:301-311; discussion 311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 18. | Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Brú C, Rodés J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 919] [Article Influence: 34.0] [Reference Citation Analysis (2)] |
| 19. | Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 387] [Article Influence: 32.3] [Reference Citation Analysis (1)] |
| 20. | Zhong JH, Li H, Xiao N, Ye XP, Ke Y, Wang YY, Ma L, Chen J, You XM, Zhang ZY. Hepatic resection is safe and effective for patients with hepatocellular carcinoma and portal hypertension. PLoS One. 2014;9:e108755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Santambrogio R, Kluger MD, Costa M, Belli A, Barabino M, Laurent A, Opocher E, Azoulay D, Cherqui D. Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford). 2013;15:78-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | He W, Zeng Q, Zheng Y, Chen M, Shen J, Qiu J, Chen M, Zou R, Liao Y, Li Q. The role of clinically significant portal hypertension in hepatic resection for hepatocellular carcinoma patients: a propensity score matching analysis. BMC Cancer. 2015;15:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Hirokawa F, Hayashi M, Miyamoto Y, Asakuma M, Shimizu T, Komeda K, Inoue Y, Uchiyama K. Predictors of poor prognosis by recurrence patterns after curative hepatectomy for hepatocellular carcinoma in Child-Pugh classification A. Hepatogastroenterology. 2015;62:164-168. [PubMed] |
| 24. | Wang Y, Yuan L, Ge RL, Sun Y, Wei G. Survival benefit of surgical treatment for hepatocellular carcinoma with inferior vena cava/right atrium tumor thrombus: results of a retrospective cohort study. Ann Surg Oncol. 2013;20:914-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 25. | Chok KS, Cheung TT, Chan SC, Poon RT, Fan ST, Lo CM. Surgical outcomes in hepatocellular carcinoma patients with portal vein tumor thrombosis. World J Surg. 2014;38:490-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 26. | Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27:2592-2597. [PubMed] |
| 27. | Violi F, Leo R, Vezza E, Basili S, Cordova C, Balsano F. Bleeding time in patients with cirrhosis: relation with degree of liver failure and clotting abnormalities. C.A.L.C. Group. Coagulation Abnormalities in Cirrhosis Study Group. J Hepatol. 1994;20:531-536. [PubMed] |
| 28. | Lencioni R, Chen XP, Dagher L, Venook AP. Treatment of intermediate/advanced hepatocellular carcinoma in the clinic: how can outcomes be improved? Oncologist. 2010;15 Suppl 4:42-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Gish RG, Lencioni R, Di Bisceglie AM, Raoul JL, Mazzaferro V. Role of the multidisciplinary team in the diagnosis and treatment of hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2012;6:173-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 30. | Gomaa AI, Waked I. Recent advances in multidisciplinary management of hepatocellular carcinoma. World J Hepatol. 2015;7:673-687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Gaba RC, Kallwitz ER, Parvinian A, Bui JT, Von Roenn NM, Berkes JL, Cotler SJ. Imaging surveillance and multidisciplinary review improves curative therapy access and survival in HCC patients. Ann Hepatol. 2013;12:766-773. [PubMed] |
| 32. | Torzilli G, Palmisano A, Del Fabbro D, Marconi M, Donadon M, Spinelli A, Bianchi PP, Montorsi M. Contrast-enhanced intraoperative ultrasonography during surgery for hepatocellular carcinoma in liver cirrhosis: is it useful or useless? A prospective cohort study of our experience. Ann Surg Oncol. 2007;14:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Wu H, Lu Q, Luo Y, He XL, Zeng Y. Application of contrast-enhanced intraoperative ultrasonography in the decision-making about hepatocellular carcinoma operation. World J Gastroenterol. 2010;16:508-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Torzilli G, Montorsi M, Donadon M, Palmisano A, Del Fabbro D, Gambetti A, Olivari N, Makuuchi M. “Radical but conservative” is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg. 2005;201:517-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 35. | Torzilli G, Palmisano A, Procopio F, Cimino M, Botea F, Donadon M, Del Fabbro D, Montorsi M. A new systematic small for size resection for liver tumors invading the middle hepatic vein at its caval confluence: mini-mesohepatectomy. Ann Surg. 2010;251:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 36. | Torzilli G, Garancini M, Donadon M, Cimino M, Procopio F, Montorsi M. Intraoperative ultrasonographic detection of communicating veins between adjacent hepatic veins during hepatectomy for tumours at the hepatocaval confluence. Br J Surg. 2010;97:1867-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Slotta JE, Kollmar O, Ellenrieder V, Ghadimi BM, Homayounfar K. Hepatocellular carcinoma: Surgeon’s view on latest findings and future perspectives. World J Hepatol. 2015;7:1168-1183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Zhou Y, Xu D, Wu L, Li B. Meta-analysis of anatomic resection versus nonanatomic resection for hepatocellular carcinoma. Langenbecks Arch Surg. 2011;396:1109-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 39. | Makuuchi M, Hasegawa H, Yamazaki S. Intraoperative ultrasonic examination for hepatectomy. Ultrasound Med Biol. 1983;Suppl 2:493-497. [PubMed] |
| 40. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] |
| 41. | Shimamura Y, Gunvén P, Takenaka Y, Shimizu H, Akimoto H, Shima Y, Arima K, Takahashi A, Kitaya T, Matsuyama T. Selective portal branch occlusion by balloon catheter during liver resection. Surgery. 1986;100:938-941. [PubMed] |
| 42. | Park YS, Lee CH, Park PJ, Kim KA, Park CM. Intraoperative contrast-enhanced sonographic portography combined with indigo carmine dye injection for anatomic liver resection in hepatocellular carcinoma: a new technique. J Ultrasound Med. 2014;33:1287-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Torzilli G, Procopio F, Cimino M, Del Fabbro D, Palmisano A, Donadon M, Montorsi M. Anatomical segmental and subsegmental resection of the liver for hepatocellular carcinoma: a new approach by means of ultrasound-guided vessel compression. Ann Surg. 2010;251:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Chen J, Huang K, Wu J, Zhu H, Shi Y, Wang Y, Zhao G. Survival after anatomic resection versus nonanatomic resection for hepatocellular carcinoma: a meta-analysis. Dig Dis Sci. 2011;56:1626-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Mirnezami R, Mirnezami AH, Chandrakumaran K, Abu Hilal M, Pearce NW, Primrose JN, Sutcliffe RP. Short- and long-term outcomes after laparoscopic and open hepatic resection: systematic review and meta-analysis. HPB (Oxford). 2011;13:295-308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 46. | Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 892] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 47. | Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [PubMed] |
| 48. | Santambrogio R, Aldrighetti L, Barabino M, Pulitanò C, Costa M, Montorsi M, Ferla G, Opocher E. Laparoscopic liver resections for hepatocellular carcinoma. Is it a feasible option for patients with liver cirrhosis? Langenbecks Arch Surg. 2009;394:255-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Xiong JJ, Altaf K, Javed MA, Huang W, Mukherjee R, Mai G, Sutton R, Liu XB, Hu WM. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World J Gastroenterol. 2012;18:6657-6668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 112] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 50. | Slim A, Garancini M, Di Sandro S, Mangoni I, Lauterio A, Giacomoni A, De Carlis L. Laparoscopic versus open liver surgery: a single center analysis of post-operative in-hospital and post-discharge results. Langenbecks Arch Surg. 2012;397:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 51. | Santambrogio R, Barabino M, Bruno S, Costa M, Ceretti AP, Angiolini MR, Zuin M, Meloni F, Opocher E. Long-term outcome of laparoscopic ablation therapies for unresectable hepatocellular carcinoma: a single European center experience of 426 patients. Surg Endosc. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Santambrogio R, Opocher E, Zuin M, Selmi C, Bertolini E, Costa M, Conti M, Montorsi M. Surgical resection versus laparoscopic radiofrequency ablation in patients with hepatocellular carcinoma and Child-Pugh class a liver cirrhosis. Ann Surg Oncol. 2009;16:3289-3298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Lai EC, Tang CN, Ha JP, Tsui DK, Li MK. The evolving influence of laparoscopy and laparoscopic ultrasonography on patients with hepatocellular carcinoma. Am J Surg. 2008;196:736-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 54. | Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 834] [Article Influence: 46.3] [Reference Citation Analysis (0)] |