Published online Apr 18, 2016. doi: 10.4254/wjh.v8.i11.520
Peer-review started: December 23, 2015
First decision: January 15, 2016
Revised: January 21, 2016
Accepted: March 14, 2016
Article in press: March 16, 2016
Published online: April 18, 2016
Processing time: 111 Days and 9.1 Hours
AIM: To investigate if echocardiographic and hemodynamic determinations obtained at the time of transjugular intrahepatic portosystemic shunt (TIPS) can provide prognostic information that will enhance risk stratification of patients.
METHODS: We reviewed medical records of 467 patients who underwent TIPS between July 2003 and December 2011 at our institution. We recorded information regarding patient demographics, underlying liver disease, indication for TIPS, baseline laboratory values, hemodynamic determinations at the time of TIPS, and echocardiographic measurements both before and after TIPS. We recorded patient comorbidities that may affect hemodynamic and echocardiographic determinations. We also calculated Model for End-stage Liver Disease (MELD) score and Child Turcotte Pugh (CTP) class. The following pre- and post-TIPS echocardiographic determinations were recorded: Left ventricular ejection fraction, right ventricular (RV) systolic pressure, subjective RV dilation, and subjective RV function. We recorded the following hemodynamic measurements: Right atrial (RA) pressure before and after TIPS, inferior vena cava pressure before and after TIPS, free hepatic vein pressure, portal vein pressure before and after TIPS, and hepatic venous pressure gradient (HVPG).
RESULTS: We reviewed 418 patients with portal hypertension undergoing TIPS. RA pressure increased by a mean ± SD of 4.8 ± 3.9 mmHg (P < 0.001), HVPG decreased by 6.8 ± 3.5 mmHg (P < 0.001). In multivariate linear regression analysis, a higher MELD score, lower platelet count, splenectomy and a higher portal vein pressure were independent predictors of higher RA pressure (R = 0.55). Three variables predicted 3-mo mortality after TIPS in a multivariate analysis: Age, MELD score, and CTP grade C. Change in the RA pressure after TIPS predicted long-term mortality (per 1 mmHg change, HR = 1.03, 95%CI: 1.01-1.06, P < 0.012).
CONCLUSION: RA pressure increased immediately after TIPS particularly in patients with worse liver function, portal hypertension, emergent TIPS placement and history of splenectomy. The increase in RA pressure after TIPS was associated with increased mortality. Age, splenectomy, MELD score and CTP grade were independent predictors of long-term mortality after TIPS.
Core tip: Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure accompanied by morbidity and mortality. We hypothesize that echocardiographic and hemodynamic determinations obtained at the time of TIPS can provide prognostic information that will enhance risk stratification of patients. We measured echocardiographic and hemodynamic variables before and immediately after the TIPS procedure in a large cohort of patients at our institution. Our findings corroborate previous literature stating that right atrial pressure increased after TIPS. Our study demonstrates several predictors of long-term mortality after TIPS, such as age, splenectomy, and Model for End-stage Liver Disease score; this data can help assess the risk for patients undergoing TIPS.
- Citation: Ascha M, Abuqayyas S, Hanouneh I, Alkukhun L, Sands M, Dweik RA, Tonelli AR. Predictors of mortality after transjugular portosystemic shunt. World J Hepatol 2016; 8(11): 520-529
- URL: https://www.wjgnet.com/1948-5182/full/v8/i11/520.htm
- DOI: https://dx.doi.org/10.4254/wjh.v8.i11.520
Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure performed to treat complications of portal hypertension such as bleeding esophageal varices, refractory ascites and hepatic hydrothorax[1-3]. The placement of a covered stent creates an anastomosis between the hypertensive portal vein and the inferior vena cava via the hepatic vein; this non-surgically decompresses the portal pressure. Although TIPS is minimally invasive, patients with advanced liver disease-particularly those with comorbidities-can have complications related to the procedure. The Model of End-stage Liver Disease (MELD) score was originally conceived to determine survival outcomes in patients receiving TIPS. In their original study, Malinchoc et al[4] created a model utilizing serum bilirubin, serum creatinine, international normalized ratio (INR), and cause of underlying liver disease, all of which were used to predict three-month mortality in patients undergoing TIPS. In today’s practice, the MELD score is primarily used to determine the extent of liver failure and subsequent placement on organ transplant waiting lists in addition to predicting risk and mortality of TIPS placement. However, there remains a limited amount of data available that can ascertain which variables convey a higher risk of complications from TIPS.
TIPS is a procedure that should be employed meticulously, as it can be accompanied by morbidity and mortality. Existing literature has elucidated variables that are traditionally associated with a poor outcome after TIPS, which include increasing age, male gender, high Child-Turcotte-Pugh (CTP) score, high MELD score, urgent placement of TIPS for uncontrolled variceal hemorrhage, renal dysfunction, ascites, and pre-existing hepatic encephalopathy[5-10]. However, there is a dearth of studies assessing the prognostic value of echocardiographic and hemodynamic determinations at the time of TIPS.
Liver cirrhosis is characterized by a hyperdynamic circulation, with an increased cardiac preload and a decreased cardiac afterload; this pre-existing hemodynamic stress in cirrhotic patients may be worsened after TIPS placement. After TIPS placement, there is a rapid increase in blood flow from the splanchnic circulation to both the right heart and pulmonary circulation[11-13]. This increase in volume can precipitate right ventricular (RV) failure and pulmonary hypertension[13,14]. The pulmonary pressures may increase, particularly if the vasculature cannot vasodilate to accommodate the increase in cardiac output. In addition, TIPS permits more direct delivery of vasoactive and neurohumoral mediators, which are normally cleared by the liver, to the pulmonary circulation[5,14]. This higher load of vasoactive mediators may increase the RV afterload[14]. Due to these hemodynamic changes, it has been recommended that the TIPS procedure be considered with caution in patients with limited cardiac reserve[11,14]. While there are no clinical studies that identify a single RA pressure measurement that constitutes an absolute threshold above which TIPS should not be performed, intervention should be reconsidered or performed cautiously when right atrial (RA) pressure is greater than 20 mmHg; furthermore, a pulmonary arterial pressure greater than 45 mmHg may contraindicate TIPS placement.
Evidently, TIPS is not suitable for every patient that presents with portal hypertension, and contraindications must be ruled out prior to stent placement. Further research is indispensable to optimizing patient selection in order to achieve maximum survival benefits. We hypothesize that the echocardiographic and hemodynamic determinations obtained at the time of TIPS can provide prognostic information that will enhance risk stratification of patients for this procedure. We particularly sought to assess whether RA pressure could provide prognostic information, given that a higher RA pressure may reflect a higher intravascular volume and a degree of systolic/diastolic RV dysfunction, conditions that could worsen after TIPS placement. We tested our hypothesis in a large number of patients who underwent TIPS placement at the Cleveland Clinic.
This retrospective study received approval from the Cleveland Clinic Institutional Review Board (study number: 12-579). Written informed consent was waived. We reviewed the medical records of 467 patients who underwent TIPS placement between July 2003 and December 2011. Patients were identified using the billing codes for TIPS. Subjects were excluded from the analyses if they underwent liver transplantation before TIPS, TIPS placement was unsuccessful, or if the initial TIPS procedure was performed at an outside facility. Forty-nine patients met exclusion criteria and were thus excluded from analysis.
We recorded information regarding patient demographics, underlying liver disease, indication for TIPS, baseline laboratory values (albumin, bilirubin, INR, creatinine, and platelets), hemodynamic determinations at the time of TIPS, and echocardiographic measurements both before and after TIPS. We also recorded patient comorbidities that may affect hemodynamic and echocardiographic determinations, including arterial hypertension, cardiac heart failure, heart valvular disease, chronic obstructive pulmonary disease, interstitial lung disease, scleroderma, splenectomy, sarcoidosis, sleep apnea, hypothyroidism, chronic kidney disease on hemodialysis, human immunodeficiency virus status, and cocaine use. In addition, we calculated MELD score and CTP class.
We recorded the following pre-TIPS echocardiographic determinations: Left ventricular ejection fraction (LVEF), RV systolic pressure (RVSP), subjective RV dilation, and subjective RV function. We recorded the following hemodynamic measurements: RA pressure before and after TIPS, inferior vena cava pressure before and after TIPS, free hepatic vein pressure, portal vein pressure before and after TIPS, and hepatic venous pressure gradient (HVPG). RV and pulmonary artery pressures were not routinely measured. It should be noted that all variables collected after TIPS were measured immediately after the procedure was performed. We also collected information regarding the type, diameter and length of the stent placed.
We recorded the following post-TIPS echocardiographic determinations: LVEF, RVSP, subjective RV dilation and function.
The TIPS procedure was performed according to previously described techniques, with modifications as needed[15]. After instillation of local anesthesia, the internal jugular vein was cannulated under direct ultrasound guidance and an introducer was placed. Through the introducer, an angled catheter was advanced into the RA and the pressure was recorded. The catheter was then maneuvered into the right hepatic vein. A long sheath was advanced to the proximal right hepatic vein, followed by a Fogarty balloon. Wedged and free hepatic vein pressures are not routinely collected during the TIPS procedure; these values are usually known from prior transjugular liver biopsy procedures performed on these patients. Carbon dioxide was injected as the contrast agent while digital images were obtained in an attempt to opacify the portal venous system. A parenchymal tract was created from the right hepatic vein to the right portal vein using a sheathed modified Colapinto needle. Alternatively, the TIPS procedure was performed with the modified Rosch-Uchida set. The kit used depends on the preference of the physician performing the procedure. A catheter was advanced into the portal vein and an initial pressure measurement was obtained. Nonionic contrast material was injected in order to display the anatomy and confirm the entry site. The parenchymal tract was then dilated and a stent was placed with the goal of obtaining an HVPG < 12 mmHg for patients with GI bleeding. There is no defined HVPG for patients with ascites; too low of a gradient puts these patients at risk for hepatic encephalopathy. At our institution, we aim to obtain an HVPG around 7-8 mmHg, but this is not absolute and is adjusted to the clinical circumstances such as LFTs and the presence of encephalopathy pre-TIPS. The majority of our TIPS procedures are performed for control of ascites. A final angiogram was used to confirm good flow through the TIPS. In addition, the RA pressure and portal vein pressure were measured again immediately after the TIPS procedure was completed.
Means and SD are provided for continuous variables, while numbers of patients with percentages are given for categorical variables. Hemodynamic variables before and after TIPS were compared using paired t-test. Binary logistic regression was used to identify variables that predict 3-mo mortality and results are reported as odds ratio with 95%CI. We evaluated the association between RA pressure and other variables with univariate linear regression. We tested the relationship between survival and variables of interest with Cox proportional-hazards modeling adjusted for age and gender. The start point for the analysis was the date of the TIPS and the end of follow-up was marked by the patient’s death or the end of study in December 2011. Patients were censored at the time of orthotopic liver transplant (OLT). Factors associated with survival in the univariate analysis (P value < 0.05) were entered into a multivariate model (forward selection). Results are expressed as hazard ratios (HRs) with the corresponding 95%CI. Predictors with HRs > 1 are associated with a higher risk for the outcome tested. We constructed receiver operating characteristic (ROC) curves to determine the sensitivity and specificity of different cutoffs of RA pressure and estimated RVSP for discriminating patients who died during follow-up. All P values reported are two tailed and P-values < 0.05 were considered significant. The statistical analyses were performed using SPSS version 17 (SPSS, Inc, Chicago, IL) and MedCalc, version 14.12.0 (Ostend, Belgium). The statistical methods of this study were reviewed by Dr. Adriano Tonelli from the Cleveland Clinic Foundation.
We included 418 patients with portal hypertension in the study. The mean ± SD age was 55.8 ± 11.6 years and 242 patients (57.9%) were male. The primary causes of portal hypertension were cryptogenic and non-alcoholic steatohepatitis (NASH) induced cirrhosis [n = 132 (31.6%)], alcohol induced liver disease [n =105 (25.1%)] and hepatitis C virus [n = 105 (25.1%)]. Less common etiologies for portal hypertension and comorbidities are listed in Table 1.
| Demographics | n (%) or mean ± SD |
| n | 418 |
| Age (yr) | 55.8 ± 11.6 |
| Male gender | 242 (57.9) |
| Etiologies of portal hypertension | |
| NASH cirrhosis | 132 (31.6) |
| Alcohol induced liver disease | 105 (25.1) |
| HCV | 105 (25.1) |
| Primary sclerosing cholangitis | 16 (3.8) |
| Primary biliary cirrhosis | 11 (2.6) |
| Others1 | 49 (11.7) |
| Patient comorbidities | |
| Systemic hypertension | 155 (37.1) |
| Hypothyroidism | 50 (12.0) |
| COPD/ILD | 34 (8.1) |
| Sleep apnea | 20 (4.8) |
| Cardiac heart failure | 18 (4.3) |
| Chronic kidney disease on hemodialysis | 17 (4.1) |
| Valvular heart disease | 17 (4.1) |
| Sarcoidosis | 5 (1.2) |
| Splenectomy | 5 (1.2) |
| Scleroderma | 4 (1.0) |
| Cocaine use | 4 (1.0) |
| HIV | 1 (0.2) |
| Indications for TIPS | |
| GI bleeding | 182 (43.5) |
| Refractory ascites | 157 (37.6) |
| Hepatic hydrothorax | 51 (12.2) |
| Others2 | 28 (6.7) |
| Basic laboratory parameters | |
| Serum albumin (g/dL) | 2.9 ± 0.7 |
| Serum bilirubin (mg/dL) | 3.0 ± 5.4 |
| INR | 1.3 ± 0.4 |
| Serum creatinine (mg/dL) | 1.3 ± 1.1 |
| Platelets (K/μL) | 115.3 ± 77.6 |
Indications for TIPS included gastrointestinal bleeding [n = 182 (43.5%)], refractory ascites [n = 157 (37.6%)], hepatic hydrothorax [n = 51 (12.2%)] and other causes [n = 28 (6.7%)] (Table 1). A total of 113 (27.8%) TIPS procedures were done emergently. Laboratory evaluations of patients before TIPS (n = 416) are as follows (mean ± SD): Serum albumin (g/dL) 2.9 ± 0.7, serum bilirubin (mg/dL) 3.0 ± 5.4, INR 1.3 ± 0.4, serum creatinine (mg/dL) 1.3 ± 1.1, platelets (K/μL) 115.3 ± 77.6. MELD score pre-TIPS revealed a mean ± SD of 13.3 ± 6.9. Meanwhile, CTP classes A, B and C were present in 46 (11.5%), 224 (55.9%) and 131 patients (31.3%), respectively. The mean ± SD diameter of the stent placed was 9.9 ± 1 mm.
Among the 301 patients who had echocardiography data available, 224 patients (74%) had estimates of the RVSP, which demonstrated a mean ± SD of 31.9 ± 10.9 mmHg. Only 11 patients (3.7%) out of 294 in whom the RV function was evaluated had mild or moderate RV dysfunction prior to TIPS placement. There were several notable hemodynamic changes that occurred immediately after TIPS. The RA pressure increased by a mean ± SD of 4.8 ± 3.9 mmHg (P < 0.001). The HVPG decreased by a mean ± SD of 6.8 ± 3.5 mmHg (P < 0.001). However, this was at the expense of a reduction in the portal vein pressure, which decreased by a mean ± SD of 11.7 ± 5.6 mmHg (P < 0.001). Finally, the RVSP measured by echocardiography (n = 109) increased by 7.4 ± 2.6 mmHg (P < 0.001).
We found several factors to be associated with elevated RA pressure prior to TIPS placement, including MELD score (R = 0.36, P < 0.001), serum bilirubin (R = 0.29, P < 0.001), INR (R = 0.26, P < 0.001), serum creatinine (R = 0.26, P < 0.001), platelets (R = -0.18, P < 0.001) and portal vein pressure (R = 0.46, P < 0.001). Elevated RA Pressure before TIPS is directly related to severity of the patient’s underlying liver disease. Moreover, splenectomy (R = 0.11, P = 0.03) and emergent TIPS placement (R = 0.24, P < 0.001) were associated with higher RA pressure in univariate linear regression analysis. RA pressure was not significantly different among the major etiologies of portal hypertension (ETOH, chronic hepatitis, NASH or others). In patients in whom TIPS were placed for acute variceal bleeding the RA before TIPS was higher (7.8 ± 5.9 mmHg) than TIPS placed for ascites (6.0 ± 3.9 mmHg, P = 0.001). Similarly, RA pressure after TIPS was higher in patients who received TIPS for acute variceal bleeding (12.4 ± 6.5 mmHg vs 10.9 ± 4.0 mmHg, P = 0.01) instead of ascites.
In multivariate linear regression analysis, a higher MELD score, lower platelet count, splenectomy and a higher portal vein pressure were independent predictors of higher RA pressure (R = 0.55, P < 0.001) (Table 2). Adding the etiology of portal hypertension and/or the reason for TIPS as variables did not affect the model. RA pressure was not found to be associated with RVSP or RV function obtained with echocardiography.
A total of 97 (24.7%) patients died within the 3-mo period after TIPS. Twenty-six patients underwent OLT during this time frame and were excluded from this analysis. Table 3 shows variables that predicted mortality at 3-mo in a univariate binary logistic regression. Etiology of portal hypertension and reason for TIPS were not significant predictors of this outcome. Only three variables remained predictors of 3-mo mortality after TIPS in a multivariate binary analysis. These included age (per 1 year, OR = 1.04, 95%CI: 1.02-1.08, P = 0.003), MELD score (per 1 unit, OR = 1.14, 95%CI: 1.08-1.19, P < 0.001) and CTP grade C (compared to A, OR = 4.75, 95%CI: 1.02-22.17, P < 0.001).
| Variables | OR | 95%CI | P |
| Age (per 1 yr) | 1.03 | 1.01-1.06 | 0.003b |
| CKD on HD (yes) | 5.93 | 1.94-18.16 | 0.002b |
| MELD (per unit change) | 1.15 | 1.10-1.19 | < 0.001b |
| CTP B (compared to A) | 4.56 | 1.06-19.67 | 0.04a |
| CTP C (compare to A) | 13.90 | 3.21-60.20 | < 0.001b |
| RVSP (per mmHg) | 1.03 | 1.00-1.06 | 0.02a |
| Emergent placement (yes) | 2.56 | 1.57-4.19 | < 0.001b |
| RA pressure before TIPS (per 1 mmHg) | 1.1 | 1.05-1.15 | < 0.001b |
| Portal vein pressure before TIPS (per 1 mmHg) | 1.04 | 1.01-1.08 | 0.02a |
| RA pressure after TIPS (per 1 mmHg) | 1.07 | 1.03-1.12 | 0.002b |
| Portal vein pressure after TIPS (per 1 mmHg) | 1.06 | 1.02-1.10 | 0.006b |
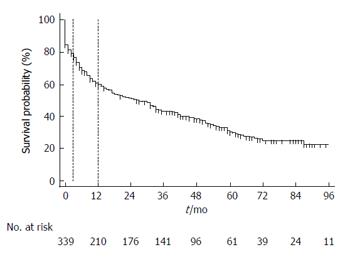
Patients were followed for a median (interquartile range) of 26.7 (2-45) mon. A total of 68 (16.3%) patients underwent OLT after TIPS. Of the remaining patients, 261 (74.6%) patients died before transplantation during follow-up. Median survival after TIPS was 26 mo (95%CI: 17-33) (Figure 1). Table 4 shows several variables that predicted long-term mortality post TIPS in a univariate Cox survival analysis. Etiology of portal hypertension or reason for TIPS was non-significant predictors of long-term mortality in our cohort. Table 5 shows significant predictors of long-term mortality after TIPS (age, splenectomy, MELD score, CTP groups B and C) according to a multivariate analysis that excluded echocardiographic parameters. When echocardiographic parameters were factored into the model, MELD score (per 1 unit, HR = 1.05, 95%CI: 1.02-1.08, P < 0.001) and RV function (per increase in 1 degree of severity, HR = 2.24, 95%CI: 1.34-3.74, P < 0.002) were significant predictors of long-term mortality. Of the hemodynamic determinations studied, only the change in the RA pressure after TIPS predicted long-term mortality (per 1 mmHg change, HR = 1.03, 95%CI: 1.01-1.06, P < 0.012).
| Variables | HR | 95%CI | P |
| Age (per 1 yr) | 1.02 | 1.01-1.03 | 0.001b |
| HCV (ETOH reference) | 1.45 | 1.03-2.04 | 0.03a |
| Splenectomy (yes) | 3.32 | 1.36-8.10 | 0.008b |
| CHF (yes) | 1.32 | 1.01-1.74 | 0.04a |
| Hypothyroidism (yes) | 1.20 | 1.00-1.42 | 0.04a |
| CKD on HD (yes) | 1.56 | 1.17-2.06 | 0.002b |
| Portal vein pressure before TIPS (per 1 mmHg) | 1.03 | 1.01-1.04 | 0.008b |
| RA pressure before TIPS (per 1 mmHg) | 1.03 | 1.01-1.06 | 0.01a |
| RA pressure after TIPS (per 1 mmHg) | 1.03 | 1.00-1.05 | 0.02a |
| MELD score (per 1 unit) | 1.07 | 1.05-1.09 | < 0.001b |
| Albumin (per 1 mg/dL) | 0.70 | 0.58-0.84 | < 0.001b |
| Billirubin (per 1 mg/dL) | 1.05 | 1.03-1.07 | < 0.001b |
| INR (per 1 unit change) | 1.30 | 1.07-1.60 | 0.01a |
| Creatinine (per 1 mg/dL) | 1.26 | 1.14-1.39 | < 0.001b |
| CTP category B (reference A) | 1.98 | 1.24-3.18 | 0.004b |
| CTP category C (reference A) | 3.02 | 1.85-4.94 | < 0.001b |
| EF echocardiogram pre TIPS (per 1% increase) | 0.98 | 0.95-1.00 | 0.04a |
| RVSP pre TIPS (per 1 mmHg increase) | 1.02 | 1.01-1.04 | 0.005b |
| Moderate RV dysfunction (normal RV reference) | 2.84 | 1.18-6.85 | 0.02a |
| EF post TIPS (per 1% increase) | 0.98 | 0.96-1.00 | 0.03a |
| RVSP post TIPS (per 1 mmHg increment) | 1.02 | 1.01-1.04 | 0.01a |
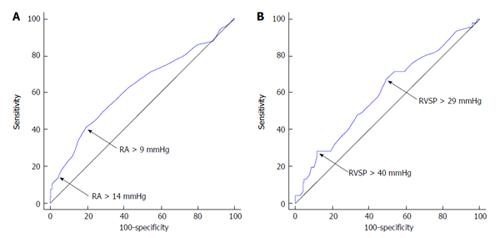
We also constructed ROC curves using the classification variables mortality at 3-mo (excluding liver transplant) and mortality or liver transplant at one year. The first variable tested was RA pressure measure by right heart catheterization immediately before TIPS. The area under the curve (AUC) for 3-mo mortality was 0.63 (95%CI: 0.58-0.68, P < 0.001) (Figure 2A) with an optimal cut-off by Youden index of > 9 mmHg (sensitivity of 41.3%, specificity of 80.5%). In addition, RA pressure > 14 mmHg had a sensitivity of 14.1% and specificity of 95.7%. The AUC for mortality or liver transplant at one year was 0.58 (95%CI: 0.53-0.64, P = 0.009) and a RA pressure of > 9 mmHg showed a sensitivity of 18.1% and specificity of 66.4% in predicting this outcome.
An ROC curve testing a second variable, RVSP estimated by echocardiography, was also constructed. The AUC for 3-mo mortality was 0.60 (95%CI: 0.53-0.67, P = 0.04) (Figure 2B) with a Youden index of > 29 mmHg (sensitivity of 67.4 % and specificity of 50.6%). At a cut-off > 40 mmHg, the sensitivity was 28.3% and specificity was 88.0%. Moreover, at a cut-off > 50 mmHg, the sensitivity was 10.9% and specificity was 95.6% for predicting 3-mo mortality. The AUC predicting mortality or liver transplant at one year for RVSP was 0.58 (0.51-0.65, P = 0.047) with a Youden index of > 29 mmHg (sensitivity of 41.8% and specificity of 42.0%). At a cut-off > 40 mmHg, the sensitivity was 10.0% and specificity was 79.0% and at a cut-off > 50 mmHg, the sensitivity was 3.60% and specificity was 91.4%.
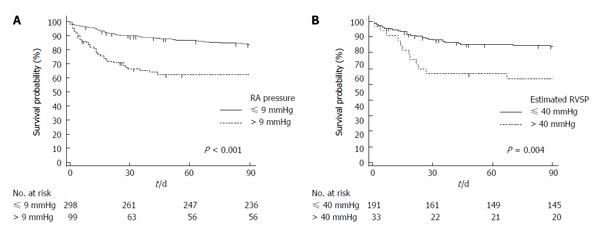
A Kaplan-Meier analysis was performed, and three-month survival after TIPS based on RA pressure > 9 mmHg vs≤ 9 mmHg and estimated RVSP pressure > 40 mmHg vs≤ 40 mmHg is presented in Figure 3.
We analyzed a large cohort of patients with portal hypertension who underwent TIPS for a variety of reasons, chiefly to assess hemodynamic variables before and after TIPS as potential predictors for mortality. We found an increase in RA pressure and a decrease in portal vein pressure after TIPS. The increase in RA pressure immediately after TIPS was associated with worsening liver function, portal hypertension, emergent TIPS placement and history of splenectomy. Of all the hemodynamic variables measured at the time TIPS, the increase in RA pressure after TIPS was associated with increased long-term mortality and a RA pressure > 9 mmHg before TIPS predicted 3- and 12-mo mortality with specificity of 81% and 66%, respectively. It is important to note that in our models, the etiology of portal hypertension and the reasons for placing TIPS had no impact on short- or long-term survival.
Other studies examined the hemodynamic changes before and after TIPS. Kovács et al[16] assessed the short-term hemodynamic and cardiac magnetic resonance imaging changes after TIPS in 11 patients with liver cirrhosis and intractable esophageal varices or refractory ascites. They concluded that the amount of shunted blood after TIPS was more than the preload reserve of the right and left ventricle, and this was manifested by the significant increase of the pulmonary capillary wedge pressure and persistent enlargement of the left and right atria. Van der Linden et al[14] studied the short and mid-term hemodynamic changes after TIPS in 16 sedated biopsy proven cirrhotic patients. They noted an increase in the mean pulmonary artery pressure (PAP), cardiac index, and RA pressure after TIPS. After a transient balloon occlusion of the shunt, they measured these hemodynamics variables once again. Interestingly, all hemodynamic determinations returned to baseline except for the mean PAP, which remained significantly elevated. This hemodynamic change persisted after one month, suggesting that the increase in pulmonary pressure after TIPS is not only due to volume overload but also due to neurohumoral changes. This is consistent with findings from previous studies that evaluated the hemodynamic changes after TIPS placement[11,17].
Azoulay et al[11] investigated 12 cirrhotic patients who underwent the TIPS procedure due to refractory ascites or refractory esophageal variceal bleeding. Hemodynamics were measured before TIPS, at 30 min, and one month after TIPS. Significant changes recorded included the decrease in the HVPG from 15 ± 3 to 7 ± 3 mmHg at 30 min after TIPS, and the subsequent decrease to 8 ± 3 mmHg at one month. The cardiac index increased from 4.5 ± 1.3 to 5.7 ± 1.5 at 30 min after TIPS and subsequently to 7.4 ± 1.4 L/(min·m2) at one month. Colombato et al[17] studied in 15 cirrhotic patients the systemic, splanchnic and pulmonary hemodynamics before TIPS, at 15-30 min and at two months after TIPS. Immediately after TIPS, the cardiac index increased by 32% and at two months the increase was attenuated but remained significantly elevated. In our study, the only hemodynamic variable measured that significantly predicted long-term mortality after TIPS was RA pressure. It is worth noting that our cohort was much larger: 418 patients in our study vs 15 patients in Colombato et al[17]’s study.
Mortality after TIPS continues to be elevated despite better selection of patients and improvements in the technical aspects of the procedure. In fact, the overall 30-d mortality ranges between 3% and 44%; meanwhile the one year mortality varies from 11% to 58%. The mortality is greater in high risk patients, in whom it can be as high as 90% within a few weeks after the procedure[5,6,18,19]. In our study, we observed a 3-mo and 12-mo mortality after TIPS (censored by liver transplant) of 23.6% and 40.3%, respectively. Given this high mortality rate, it is advantageous to identify predictors of short- and long-term mortality in patients considered for TIPS. Variables previously reported to adversely impact outcomes after TIPS include age[19,20], gender, need for emergent TIPS, encephalopathy[6], ascites, variceal hemorrhage[6], CTP class C[10,20], MELD score, bilirubin > 3[6,21], INR[21], creatinine, alanine aminotransferase > 100 IU/L[6], sodium level[10], albumin[21] and portosystemic gradient.
Our study yielded results that corroborate the aforementioned literature. In our study, both echocardiographic (RVSP) and hemodynamic (RA and portal vein pressures both before and after TIPS) variables were predictors of 3-mo survival after TIPS. However, the effect of these determinations became non-significant when adjusting for age, MELD score and CTP grade. Interestingly, a large number of variables impacted long-term survival. Those with independent value included age, MELD score, CTP grade, splenectomy and RV function. We also noted that the higher MELD score and CTP grade impacts adversely the short and long-term prognosis after TIPS. These findings have been described in the literature as predictors of short and long term mortality after TIPS creation in cirrhotic patients[5,10,20]. Parvinian et al[19] evaluated the specificity of RA pressure in predicting mortality after TIPS at 30- and 90-d in a series of 125 patients. They demonstrated 30-d mortality of 18% and 90-d mortality of 28%. According to univariate analysis, baseline RA pressure and final RA pressure were significantly associated with survival at 30- and 90-d, in addition to Child-Pugh score and MELD score. As in our study, multivariate analysis did not include RA pressure as an independent predictor of mortality at 90-d, supporting these results in a large patient cohort.
In this study, we particularly focused on the prognostic importance of hemodynamic and echocardiographic determinations. Patients with advanced cirrhosis and portal hypertension can develop cardiomyopathy with left ventricular diastolic dysfunction[21], hyperdynamic state, volume overload, and less commonly portopulmonary hypertension; these conditions can be aggravated with the insertion of TIPS[14,17,22-24]. The placement of TIPS rapidly increases the RV preload and afterload, which can lead to overt heart failure, pulmonary hypertension and death[11,14,16,22-37]. RA pressure obtained before TIPS could be of value in clinical practice; physicians may elect to abort a TIPS procedure based on this hemodynamic parameter.
Our study has limitations that include the: (1) retrospective collection of data; (2) the lack of data on cardiac output, pulmonary artery and pulmonary capillary wedge pressures which are determinations not routinely obtained at the time of TIPS; and (3) echocardiographic determinations were not done at the time of TIPS. The nature of our patient cohort also poses some limitations. Despite these limitations this study presents data on a large number of TIPS procedures performed during the course of eight years. It describes factors that affect short (3-mo) and long-term prognosis. Most importantly, we found that an important factor with predictive value is RA pressure, which increases after TIPS most prominently in patients with more severe liver disease.
RA pressure increased immediately after TIPS particularly in patients with worse liver function, portal hypertension, emergent TIPS placement and history of splenectomy. The increase in RA pressure after TIPS was associated with increased mortality. Age, splenectomy, MELD score and CTP grade were independent predictors of long-term mortality after TIPS.
We would like to thank the interventional radiology laboratory personnel for their outstanding work. We are indebted to Jennie Newman licensed practical nurse for her invaluable assistance in this project.
Transjugular intrahepatic portosystemic shunt (TIPS) is a procedure that can be accompanied by morbidity and mortality. There is a lack of studies assessing the prognostic value of echocardiographic and hemodynamic determinations at the time of TIPS. The hypothesize that the echocardiographic and hemodynamic determinations obtained at the time of TIPS can provide prognostic information that will enhance risk stratification of patients for this procedure.
Risk stratification of patients with liver disease who are undergoing TIPS is imperfect. It is evident that a number of hemodynamic changes occur after the procedure; their effect on patient outcomes still warrants investigation. The authors examine echocardiographic and hemodynamic variables in this cohort of patients in order to glean information regarding survival and outcomes in patients undergoing TIPS. Furthermore, through a multivariate analysis they also investigate other variables that may significantly influence patient outcomes. This will help to optimize patient benefit from the TIPS procedure.
In their study, they found that right atrial pressure increased immediately after TIPS particularly in patients with worse liver function, portal hypertension, emergent TIPS placement and history of splenectomy. The increase in right atrial pressure after TIPS was associated with increased mortality. Age, splenectomy, Model of End-stage Liver Disease score and Child Turcotte Pugh grade were independent predictors of long-term mortality after TIPS.
These findings could be used to enhance patient selection for TIPS.
This retrospective study is important clinical value to select the patients for TIPS and evaluate the prognosis for patients who underwent TIPS placement.
P- Reviewer: Minicis SD, Qin JM, Wong GLH S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Rössle M, Haag K, Ochs A, Sellinger M, Nöldge G, Perarnau JM, Berger E, Blum U, Gabelmann A, Hauenstein K. The transjugular intrahepatic portosystemic stent-shunt procedure for variceal bleeding. N Engl J Med. 1994;330:165-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 522] [Cited by in RCA: 481] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 2. | Ochs A, Rössle M, Haag K, Hauenstein KH, Deibert P, Siegerstetter V, Huonker M, Langer M, Blum HE. The transjugular intrahepatic portosystemic stent-shunt procedure for refractory ascites. N Engl J Med. 1995;332:1192-1197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 275] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Gordon FD, Anastopoulos HT, Crenshaw W, Gilchrist B, McEniff N, Falchuk KR, LoCicero J, Lewis WD, Jenkins RL, Trey C. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 145] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 4. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2103] [Article Influence: 80.9] [Reference Citation Analysis (0)] |
| 5. | Garcia-Pagán JC, Heydtmann M, Raffa S, Plessier A, Murad S, Fabris F, Vizzini G, Gonzales Abraldes J, Olliff S, Nicolini A. TIPS for Budd-Chiari syndrome: long-term results and prognostics factors in 124 patients. Gastroenterology. 2008;135:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 261] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Chalasani N, Clark WS, Martin LG, Kamean J, Khan MA, Patel NH, Boyer TD. Determinants of mortality in patients with advanced cirrhosis after transjugular intrahepatic portosystemic shunting. Gastroenterology. 2000;118:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 152] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 7. | Russo MW, Jacques PF, Mauro M, Odell P, Brown RS. Predictors of mortality and stenosis after transjugular intrahepatic portosystemic shunt. Liver Transpl. 2002;8:271-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Rajan DK, Haskal ZJ, Clark TW. Serum bilirubin and early mortality after transjugular intrahepatic portosystemic shunts: results of a multivariate analysis. J Vasc Interv Radiol. 2002;13:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Patch D, Nikolopoulou V, McCormick A, Dick R, Armonis A, Wannamethee G, Burroughs A. Factors related to early mortality after transjugular intrahepatic portosystemic shunt for failed endoscopic therapy in acute variceal bleeding. J Hepatol. 1998;28:454-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 85] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Jalan R, Elton RA, Redhead DN, Finlayson ND, Hayes PC. Analysis of prognostic variables in the prediction of mortality, shunt failure, variceal rebleeding and encephalopathy following the transjugular intrahepatic portosystemic stent-shunt for variceal haemorrhage. J Hepatol. 1995;23:123-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 129] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 11. | Azoulay D, Castaing D, Dennison A, Martino W, Eyraud D, Bismuth H. Transjugular intrahepatic portosystemic shunt worsens the hyperdynamic circulatory state of the cirrhotic patient: preliminary report of a prospective study. Hepatology. 1994;19:129-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Blendis L, Wong F. The hyperdynamic circulation in cirrhosis: an overview. Pharmacol Ther. 2001;89:221-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 13. | Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rössle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44:743-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 121] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Van der Linden P, Le Moine O, Ghysels M, Ortinez M, Devière J. Pulmonary hypertension after transjugular intrahepatic portosystemic shunt: effects on right ventricular function. Hepatology. 1996;23:982-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Perarnau JM, Le Gouge A, Nicolas C, d’Alteroche L, Borentain P, Saliba F, Minello A, Anty R, Chagneau-Derrode C, Bernard PH. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 16. | Kovács A, Schepke M, Heller J, Schild HH, Flacke S. Short-term effects of transjugular intrahepatic shunt on cardiac function assessed by cardiac MRI: preliminary results. Cardiovasc Intervent Radiol. 2010;33:290-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Colombato LA, Spahr L, Martinet JP, Dufresne MP, Lafortune M, Fenyves D, Pomier-Layrargues G. Haemodynamic adaptation two months after transjugular intrahepatic portosystemic shunt (TIPS) in cirrhotic patients. Gut. 1996;39:600-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 72] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Harrod-Kim P, Saad WE, Waldman D. Predictors of early mortality after transjugular intrahepatic portosystemic shunt creation for the treatment of refractory ascites. J Vasc Interv Radiol. 2006;17:1605-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Parvinian A, Shah KD, Couture PM, Minocha J, Knuttinen MG, Bui JT, Gaba RC. Older patient age may predict early mortality after transjugular intrahepatic portosystemic shunt creation in individuals at intermediate risk. J Vasc Interv Radiol. 2013;24:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Williams D, Waugh R, Gallagher N, Perkins K, Dilworth P, Duggan A, Selby W. Mortality and rebleeding following Transjugular Intrahepatic Portosystemic Stent Shunt for variceal haemorrhage. J Gastroenterol Hepatol. 1998;13:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Tyburski JG, Noorily MJ, Wilson RF. Prognostic factors with the use of the transjugular intrahepatic portosystemic shunt for bleeding varices. Arch Surg. 1997;132:626-630; discussion 630-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Postgrad Med J. 2009;85:44-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 286] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 23. | Pozzi M, Carugo S, Boari G, Pecci V, de Ceglia S, Maggiolini S, Bolla GB, Roffi L, Failla M, Grassi G. Evidence of functional and structural cardiac abnormalities in cirrhotic patients with and without ascites. Hepatology. 1997;26:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Finucci G, Desideri A, Sacerdoti D, Bolognesi M, Merkel C, Angeli P, Gatta A. Left ventricular diastolic function in liver cirrhosis. Scand J Gastroenterol. 1996;31:279-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 89] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Salerno F, Cazzaniga M, Pagnozzi G, Cirello I, Nicolini A, Meregaglia D, Burdick L. Humoral and cardiac effects of TIPS in cirrhotic patients with different “effective” blood volume. Hepatology. 2003;38:1370-1377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Rabie R, Cazzaniga M, Salerno F, Wong F. The effect of cirrhotic cardiomyopathy on the post-TIPS outcome of patients treated for complications of portal hypertension. Hepatology. 2006;44:444A. |
| 27. | Rabie RN, Cazzaniga M, Salerno F, Wong F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009;104:2458-2466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 129] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 28. | Lee SS, Liu H. Cardiovascular determinants of survival in cirrhosis. Gut. 2007;56:746-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 29. | Rodríguez-Laiz JM, Bañares R, Echenagusia A, Casado M, Camuñez F, Pérez-Roldán F, de Diego A, Cos E, Clemente G. Effects of transjugular intrahepatic portasystemic shunt (TIPS) on splanchnic and systemic hemodynamics, and hepatic function in patients with portal hypertension. Preliminary results. Dig Dis Sci. 1995;40:2121-2127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Cazzaniga M, Salerno F, Pagnozzi G, Dionigi E, Visentin S, Cirello I, Meregaglia D, Nicolini A. Diastolic dysfunction is associated with poor survival in patients with cirrhosis with transjugular intrahepatic portosystemic shunt. Gut. 2007;56:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 173] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 31. | Willoughby PH, Beers RA, Murphy KD. Pulmonary edema after transjugular intrahepatic portosystemic shunt. Anesth Analg. 1996;82:895-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 32. | Braverman AC, Steiner MA, Picus D, White H. High-output congestive heart failure following transjugular intrahepatic portal-systemic shunting. Chest. 1995;107:1467-1469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 33. | Modock J. Acute pulmonary hypertension after transjugular intrahepatic portosystemic shunt: a potentially deadly but commonly forgotten complication. Gastroenterol Nurs. 2014;37:33-8; quiz 39-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Merli M, Valeriano V, Funaro S, Attili AF, Masini A, Efrati C, De CS, Riggio O. Modifications of cardiac function in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt (TIPS). Am J Gastroenterol. 2002;97:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Salerno F, Merli M, Cazzaniga M, Valeriano V, Rossi P, Lovaria A, Meregaglia D, Nicolini A, Lubatti L, Riggio O. MELD score is better than Child-Pugh score in predicting 3-month survival of patients undergoing transjugular intrahepatic portosystemic shunt. J Hepatol. 2002;36:494-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 216] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 36. | Gaba RC, Khiatani VL, Knuttinen MG, Omene BO, Carrillo TC, Bui JT, Owens CA. Comprehensive review of TIPS technical complications and how to avoid them. AJR Am J Roentgenol. 2011;196:675-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 95] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 37. | Parvinian A, Bui JT, Knuttinen MG, Minocha J, Gaba RC. Right atrial pressure may impact early survival of patients undergoing transjugular intrahepatic portosystemic shunt creation. Ann Hepatol. 2014;13:411-419. [PubMed] |