INTRODUCTION
Hepatitis delta virus (HDV) is the etiological agent of one of the most severe forms of viral hepatitis in humans. Discovered and isolated in Italy in the mid-70s, the virus was demonstrated to be endemic worldwide, with prevalence rates varying greatly in different geographical areas, regardless of the prevalence of hepatitis B virus (HBV), whose presence is mandatory for HDV propagation[1-3]. Clinical expression is wide and although sometimes it follows a benign course, more often the disease is clinically important with rapid progression to cirrhosis, liver decompensation and death, as compared to HBV monoinfection[4,5]. Several studies published since HDV discovery have provided further knowledge into biology, pathogenesis, epidemiology, and natural history of HDV infection, however the mechanism responsible for such a severe disease is still under debate. In this review we will focus on some of the most peculiar aspects of HDV infection, giving emphasis to the improvements made since virus discovery on the subjects of epidemiology, clinical course and natural history of the disease but also underlying what still needs to be done.
EPIDEMIOLOGY
HDV is a small defective RNA virus that requires the mandatory presence of HBV for its propagation to hepatocytes but not for viral replication[2]. The virus was discovered and isolated by Rizzetto et al[1] in the mid-70s, while investigating a group of chronic HBV infected patients with a severe disease. The existence of a new antigen-antibody system was shown and it was observed in patients with a severe course of HBV infection, only. Later on, the virus was demonstrated to be an RNA molecule of low molecular weight, encapsidated by the three HBV envelope proteins known as large, medium and small hepatitis B surface antigen (HBsAg)[6]. It was named the delta agent[7].
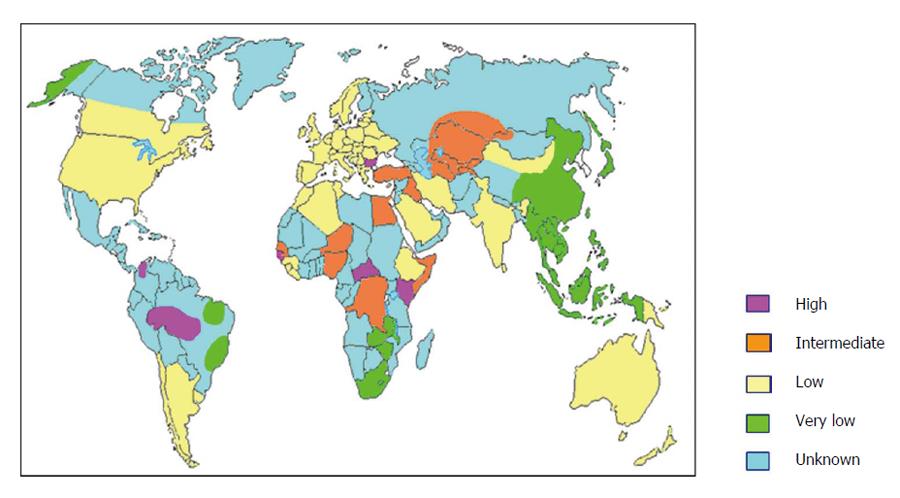
Epidemiological studies carried out in the 1980s demonstrated that the virus was present worldwide with prevalence varying widely in different areas of the world, regardless of the prevalence of HBV infection[3]. Today we know that about 350 million people in the world are considered to be chronic carriers of HBV infection and about 5% of the HBV carriers are believed to have HDV infection, leading to an estimated global burden of about 15-20 million cases of chronic HDV infection in the world. However, these data could be largely underestimated for several reasons. Data on HDV prevalence are still missing from large areas of the world, not exclusively clustered in countries with low socio-economic development (Figure 1). Moreover, a systematic screening for anti-HDV in HBV carriers is still not performed. For sure, main areas of prevalence are the Mediterranean basin, the Middle East, Central and Northern Asia, West and Central Africa, the Amazonian basin, Northern South America and the Asia-Pacific region (Figure 1). Mongolia is a country with particularly high prevalence of HDV, with about one third of chronic hepatitis being attributable to HDV[8].
Figure 1 Estimated world epidemiology of hepatitis delta virus infection.
The prevalence of HDV infection has significantly changed over the last three decades in different areas of the world. Indeed, mainly in Southern Europe[9-11], Taiwan[12] and Turkey[13] the prevalence has significantly declined mainly due to mass vaccination campaign against HBV, systematic screening of blood and blood products, amelioration of safety procedures against blood borne infections in healthcare workers, use of disposable needles and medical devices, improved socioeconomic conditions and protection against sexually transmitted diseases. In Italy, the prevalence of anti-HDV in chronic HBsAg carriers was 24.7% in 1978-1981, 28% in 1987 but declined to 14% in 1992 and to 8.3% in 1997[9,11]. In Turkey the prevalence observed in chronic HBV carriers and cirrhosis between 1980 and 2005 were 20% and 32% respectively, with wide differences within the country, being Eastern and Southeastern regions of the country characterised by higher rates[14]. Conversely, countries like Pakistan and Iran have shown increasing prevalence over time and the presence of HDV infection has been discovered in new regions such as Russia, Northern India, Southern Albania, mainland China and some Pacific Islands[15]. However, the decreasing trend of HDV prevalence has come to a stop in several areas of the world, with stable rates of infection demonstrated in London[16], Germany[17,18] and Italy[10]. Surprisingly, the prevalence rates of HDV infection seem to be increasing in France[19]. The main reason for this recrudescence is the increasing immigration from Eastern Europe, Africa, Turkey and former Soviet Union. In the United States, few studies in the 70s’-80s’ have reported prevalence of 3.8% in blood donors, 15% in HIV coinfection, 30% in developmentally disabled with chronic HBV, and up to 67% in injection drug users[20-23]. More recent data reported that 50% of injection drug users with chronic HBV infection were also HDV infected[24]. Finally, among 499 chronic HBV infected patients from Northern California, 8% tested HDV positive[25].
Hepatitis D is also prevalent in the Amazonian region of western Brazil, in Venezuela and Western Pacific populations[26,27].
CLINICAL COURSE
The virus shares the same routes of transmission of HBV, therefore it is transmitted by parenteral route through exposure to infected blood or body fluids. Since a very small inoculum has been demonstrated to be sufficient for virus transmission[28], intravenous drug users are particularly at risk of infection[29]. Sexual transmission is possible particularly in people with high-risk sexual activity[30]. Finally, inapparent parenteral transmission has been reported as common in areas at high endemicity, where intrafamiliar spread of the infection is common.
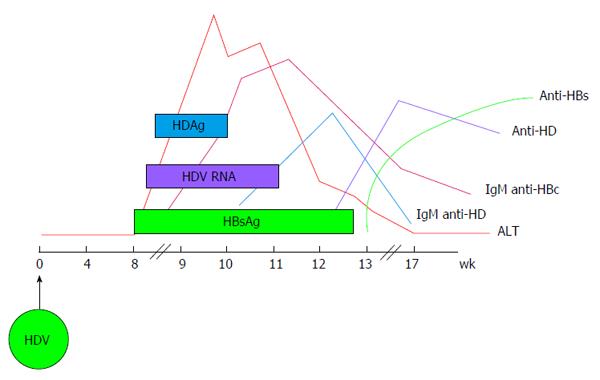
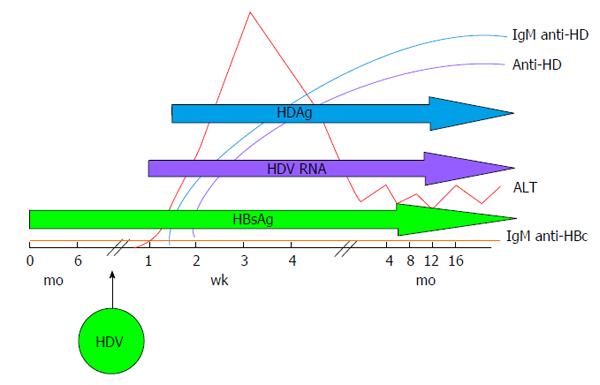
HDV infection can be transmitted either as co-infection, i.e., the simultaneous transmission of both HBV and HDV or as super-infection, i.e., the acquisition of HDV by an already chronic carrier of HBV and the course of the infection varies greatly, accordingly. Indeed, during co-infection the host response to HBV is responsible for HDV persistence and viral clearance as observed in more than 95% of adult cases[31]. Acute HDV co-infection can be characterised by a more severe course than acute HBV mono-infection, often resulting in acute liver failure. HDV super-infection of an individual already chronic carrier of HBV, results in chronic HDV infection in the vast majority of cases[32]. It can present as an acute hepatitis in a previously unknown HBV chronic carrier and it is often misdiagnosed as an acute HBV or as a worsening of chronic HBV infection. The typical course of HDV coinfection is characterised by the appearance of serum HBsAg and HDAg during the increase of alanine aminotransferases (ALT), followed by HDV viremia (Figure 2). The ALT peak during coinfection is typically characterised by a double phase: The first is due to HBV replication while the second is related to HDV replication. IgM anti-HDV appear quickly, followed by seroconversion to IgG anti-HD. HBV infection in this phase is revealed by the presence of IgM anti-HBc and HBV viremia. The self-limiting coinfection is transient and persistence of serum IgG anti-HD is a marker of past infection. The typical course of HDV superinfection is characterised by a very rapid appearance and very high levels of HDV viremia and serum HDAg (Figure 3). The more rapid course of superinfection compared to coinfection is due to the chronic carriage of HBsAg. This situation entails that HDV infects for the first time an organism in which the mechanism of HBsAg production is already set and perfectly functioning, giving rise to the assembly of new HDV virions much rapidly than when the two viruses infect together for the first time. Severe acute hepatitis characterised by ALT elevation follows the peak of viremia and is coincidental with IgM anti-HD rise. Markers of HBV infection are usually inhibited with IgM anti-HBc and HBV DNA typically testing negative. Superinfection’s progress is chronicity in more than 70% of cases.
Figure 2 Typical serologic pattern of coinfection by hepatitis B virus and hepatitis delta virus.
The ALT peak is characterised by a double phase being the first due to HBV replication and the second to HDV replication. IgM anti-HDV appear quickly, followed by seroconversion to IgG anti-HD. HBV infection in this phase is revealed by the presence of IgM anti-HBc and HBV viremia. The self-limiting coinfection is transient and persistence of serum IgG anti-HD is the only marker of past HDV infection. HDV: Hepatitis delta virus; HBV: Hepatitis B virus; HBsAg: Hepatitis B surface antigen; ALT: Alanine aminotransferases.
Figure 3 Typical serologic pattern of hepatitis delta virus superinfection: Severe acute hepatitis is characterised by alanine aminotransferases elevation that follows the peak of hepatitis delta virus viremia and is coincidental with IgM anti-HD rise.
Markers of HBV infection are usually inhibited. IgM anti-HBc and HBV DNA typically test negative. HDV: Hepatitis delta virus; HBsAg: Hepatitis B surface antigen; ALT: Alanine aminotransferases.
NATURAL HISTORY
The state of chronic carrier of HBV, represents the ideal environment for the maintenance of HDV replication. As a consequence, the chronic form of hepatitis Delta is, in the vast majority of cases, the sequela of an acute superinfection. Despite this knowledge, the clinical course of chronic HDV infection may be characterised by highly variable patterns ranging from mild, slowly progressive disease to aggressive disease characterised by a severe course that may lead to the fearsome form of acute on chronic liver failure and death in a few weeks, resolvable with liver transplantation, only. Epidemiological studies on genotypes distribution have demonstrated that different clinical courses may rely upon the presence of genotypes more virulent than others. Indeed, progressive forms of chronic HDV infection have been reported from Far East and South America, where genotypes 2 and 3, respectively, have been described and associated to rapidly progressive diseases. The distribution of genotype 1 is worldwide and the disease caused by this virus may have a variable course. Genotype 4, typically isolated in the Far East is usually characterised by a mild form of hepatitis. Finally, genotypes 5-8 have been described in West and Central Africa but there are not enough epidemiological data coming from these areas allowing conclusions on the clinical course characterised by these last viral types[33]. The different clinical course determined by genotypes may depend by different virion assembly as demonstrated by in vitro studies. These studies have demonstrated a higher assembly efficiency for genotype 1 as compared to genotype 2[34]. Supporting these findings are data demonstrating RNA replication fitness as low as 100-fold in a clone of genotype 2 from Taiwan as compared to a clone of genotype 1 from Italy[35]. Finally it has been suggested that HBV genotypes also may affect the clinical course as well as disease severity of chronic HDV infection. Indeed HBV genotype C appears to be associated to a worse clinical outcome while HBV genotype A appears to be able to determine lower HDV viral load[36].
As a general concept, cirrhosis occurs within few years from HDV infection in about two thirds of cases, and there is a three-fold higher risk of progression to cirrhosis as compared to patients with chronic HBV monoinfection only. Our recent publication on the natural history of 299 patients followed-up for a median of 28 years indicated the occurrence of cirrhosis at an annual rate of 4%[4]. However, the occurrence of cirrhosis may be characterised by a stable clinical state as well as progression to liver decompensation and development of hepatocellular carcinoma (HCC). Early reports on the natural history of chronic HDV infection indicated that the occurrence of HCC was quite unusual in this setting. Nevertheless, a more recent report on 200 Child-Pugh A class cirrhotic patients followed for 6.6 years, indicated that HDV infected patients had a 3.2-fold increased risk of developing HCC compared to the risk of HBV monoinfected patients[5]. Furthermore, the analysis of data on the risk of developing liver decompensation indicated again a 2.2-fold increased risk for chronic HDV infection with regard to chronic HBV monoinfected[5]. We demonstrated that in our experience clinical decompensation was the dominant complication of HDV cirrhotic patients with an annual incidence rate of 2.7% and that persistent HDV replication was associated with the risk of developing liver decompensation[4]. Finally, our data showed that persistent HDV replication was the only predictor of liver-related mortality[4].
The pathogenetic mechanisms of HDV-related liver disease is still a matter of controversy. Indeed, although conclusive evidence supporting a direct correlation between levels of HDV replication and progression to higher levels of liver disease are still weak, our group has recently demonstrated that HDV RNA levels above 600.000 copies/mL are discriminating for cirrhosis development[37]. On the other hand, in spite of data available indicating that hepatitis D is mostly an immune-mediated disease, the observation of unusual histologic findings in fulminant hepatitis induced by HDV genotype 3 leaves room to the hypothesis of a direct cytopathic mechanism[38].
Whatever the mechanism, the optimal therapy against HDV should take into account the different pathogenetic hypothesis combining an immuno-stimulating role able to enhance the immunity against HDV and an anti-viral role, able to reduce and possibly eradicate the virus, leading to a long lasting control of the infection.
TREATMENT
Treatment of chronic HDV infection is difficult. The ideal drug would be able of inducing HDV clearance but also HBV clearance. Several studies have been published over time regarding treatment of chronic HDV infection with recombinant interferon (IFN)[39]. The indications emerging from these studies were that IFN is efficient in reducing transaminases but not in HDV RNA clearance. Better results were obtained with higher doses (9MU thrice weekly) and for longer time (12 mo)[40]. Subsequent studies have investigated the use of nucleos(t)ide analogues. Lamivudine alone did not emerge as effective at reducing HDV RNA concentrations and did not increase sustained virological response when combined with IFN[41]. Famciclovir and Ribavirin alone or in combination with IFN did not improve the virological response[42,43]. Entecavir was demonstrated to reduce ALT and HBV DNA in a small subset of patients but not HDV viremia[44]. More recently, several reports have investigated the use of pegylated interferon for chronic HDV infection, showing higher rates of virological response as compared to standard IFN, but still quite disappointing (17%-43%)[45-47]. Finally, the addition of adefovir or tenofovir to pegylated IFN did not improve the virological response rates[48].
Attempts on looking for new antiviral compounds are currently ongoing. Since prenilation of large-HDAg is essential for viral assembly and secretion and in mouse models prenylation inhibitors have demonstrated interesting results in terms of inhibition of assembly and release of HDV with consequently a rapid clearance of HDV RNA in serum, these compounds are under evaluation in humans as well[49]. Moreover, myristoylated synthetic peptides specific for the N-terminal region of the pre-S1 domain of HBsAg are able to inhibit viral attachment and HDV infectivity. These peptides could represent another therapeutic opportunity.
CONCLUSION
Ever since virus discovery more than three decades ago, several important studies have shed light on viral structure, mechanisms of viral replication, viral heterogeneity, clinical course and natural history of HDV infection. The declining prevalence observed mainly in the areas of initial endemicity like Europe, has significantly diverted the interest and research efforts from HDV in the belief that it was a vanishing disease. The awareness of massive immigration from areas of very high HDV prevalence has recently brought new attention on one of the most severe forms of viral hepatitis in humans. Physicians in their daily practice should keep in mind that any chronic carrier of HBV is potentially at risk of HDV superinfection and an efficient treatment against HDV is still unavailable.
P- Reviewer: Borzio M, Elalfy H S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/