Published online Sep 27, 2025. doi: 10.4254/wjh.v17.i9.110264
Revised: June 19, 2025
Accepted: August 22, 2025
Published online: September 27, 2025
Processing time: 114 Days and 20.5 Hours
Autoimmune hepatitis (AIH) is a rare cause of chronic liver disease. The exact pa
Core Tip: Immunosuppressive therapy remains the mainstay for patients with autoimmune hepatitis (AIH). However, managing AIH in special situations, such as acute-on-chronic liver failure, pregnancy, old age, and hepatitis B and C co-infection, can be challenging. Between 10% and 20% of patients may not respond to standard first-line immunosuppressive therapy. This review focuses on the individualized management of patients with difficult-to-treat or refractory AIH and recent advances in second- and third-line immunosuppressive therapy.
- Citation: Malakar S, Shamsul Hoda U, Giri S, Samanta A, Roy A, Gupta R, Kumar SR, Agarwal M, Pawar A, Rungta S, Ghoshal UC. Difficult to treat and refractory autoimmune hepatitis: Recent advances in pharmacological management. World J Hepatol 2025; 17(9): 110264
- URL: https://www.wjgnet.com/1948-5182/full/v17/i9/110264.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i9.110264
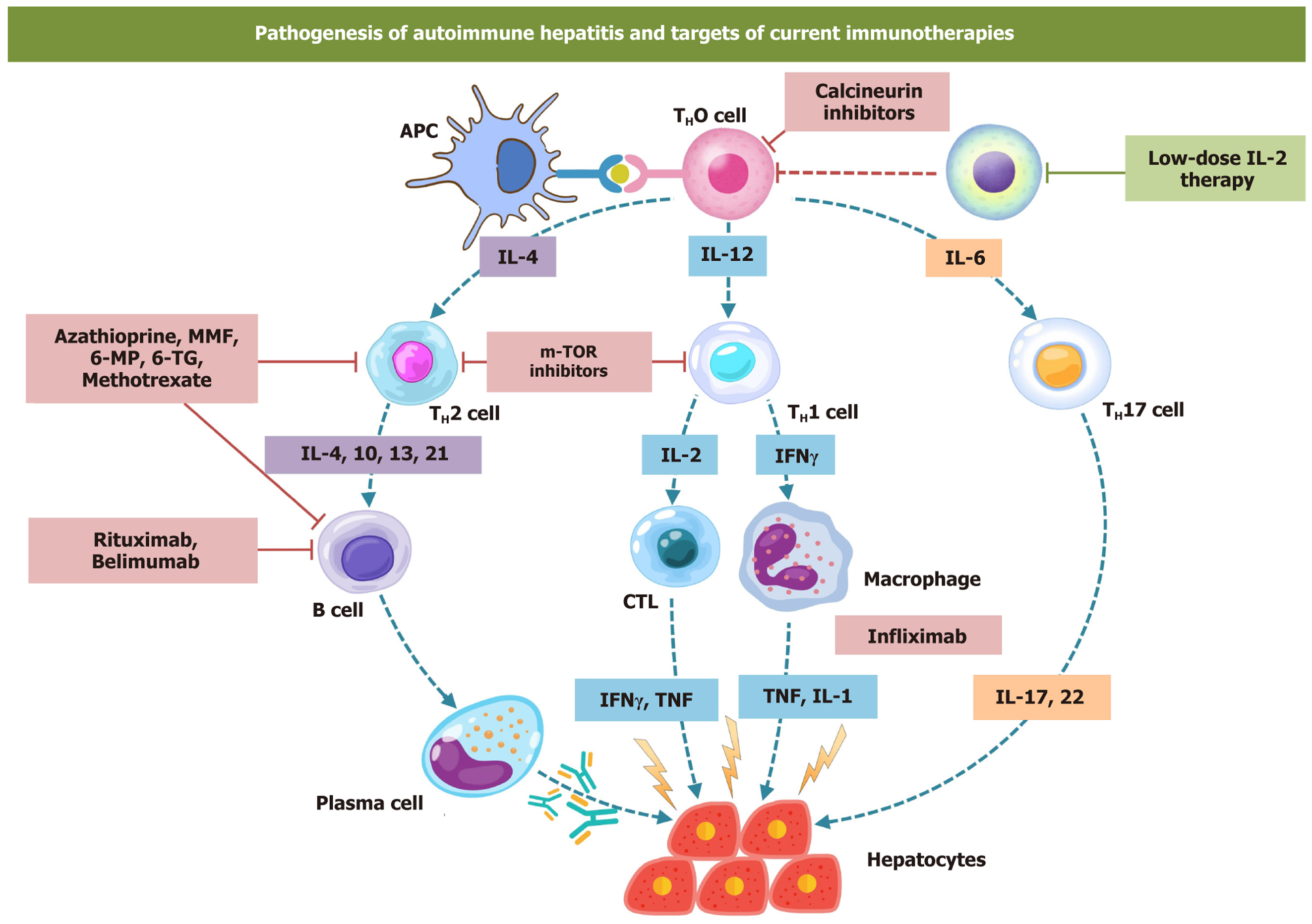
Autoimmune hepatitis (AIH) is a chronic, immune-mediated inflammatory liver disease. It is characterized by the presence of autoantibodies, elevated IgG levels, and specific histological findings such as interface hepatitis (Figure 1)[1]. It is an uncommon cause of chronic liver disease[1,2]. It is more prevalent among women; however, its clinical pre
A nuanced and individualized approach is advocated for the management of AIH[1-4]. Up to 10%–20% of patients may not respond to the primary immunosuppressive treatment[5], and 5%–10% of patients may even discontinue therapy owing to its debilitating adverse events[5,6]. Failure to achieve remission is invariably associated with low transplant-free survival[8]. Moreover, concomitant diseases and conditions, such as diabetes, pregnancy, human immunodeficiency virus (HIV) infection, and other comorbidities, may complicate the course of the disease[9-12]. Such patients are classified as difficult-to-treat AIH. Immunosuppressives should be used cautiously in some critical conditions such as acute severe AIH (AS-AIH), acute-on-chronic-liver failure (ACLF), concomitant HIV infection, metabolic dysfunction-associated steatotic liver disease (MASLD), and cirrhosis[4,9,13]. Concurrent viral hepatitis, overlap syndromes, and MASLD should be ruled out in such patients before switching to other immunosuppressive agents and labelling them as refractory AIH[4,9]. Once the diagnosis of refractory AIH is made, an individualized approach is advocated to choose among different second- and third-line immunosuppressive agents. They include mycophenolate mofetil (MMF), tacrolimus (TAC), cyclosporine (Cys), sirolimus, infliximab (IFX), and belimumab. This review discusses evidence-based management strategies in patients with refractory and difficult-to-treat AIH. It will primarily focus on the second, and third-line pharmacological therapy for the management of patients with AIH.
AS-AIH is defined by the presence of jaundice and coagulopathy [international normalized ratio (INR) ≥ 1.5] in patients with an acute presentation of AIH (≤ 26 weeks between the onset of jaundice and coagulopathy) with no evidence of pre-existing liver disease[13]. A subset of these patients may develop encephalopathy, categorizing them as AS-AIH with acute liver failure (ALF)[4,9]. The diagnosis of AS-AIH is established only in the absence of severe fibrosis or cirrhosis on histology[13,14].
The reported corticosteroid response rate ranges widely from 36% to 100%, with the high variability likely due to differences in the severity of liver disease across studies[13-16]. In AS-AIH, it is crucial to identify patients who will benefit from corticosteroid therapy. The predictors of failure to respond include high INR (> 2.46) and a model for end-stage liver disease (MELD) score > 28.5[17]. The SURFASA scoring system, which combines three parameters, baseline INR, change in INR over 3 days, and change in total bilirubin over 3 days after initiation of steroids, has been validated to predict outcomes[15,18]. A SURFASA score < 0.9 suggests a steroid response of 75%, while a score of > 1.75 indicates a risk of transplant or death of > 85%. AS-AIH has a poor prognosis, and patients should be evaluated for liver transplantation (LT) promptly[18].
AIH-related ACLF is a syndrome characterized by an acute decline in liver function observed in patients with pre-existing chronic liver disease. It is often accompanied by the presence of extrahepatic organ failure[19]. AIH with ACLF (AIH-ACLF) can be distinguished from AIH with ALF by the presence of advanced fibrosis (F3/F4), ductular reactions and large areas of parenchymal collapse with lymphoplasmacytic inflammation[19,20].
The data on steroid use in AIH-ACLF patients are limited. In the largest study, from the Asia Pacific Association for the Study of Liver (APASL) – ACLF Research Consortium (AARC) database, 28 of 82 (34%) patients with AIH-ACLF received steroids. The patients who received steroids had a 90-day survival of 75%, with five patients dying of sepsis. Two had progressive liver failure-related death. Factors associated with a lack of response to corticosteroid therapy included high baseline serum bilirubin, high baseline MELD score (> 27), presence of hepatic encephalopathy (HE) and grade 3/4 fibrosis in liver biopsy[19,20]. Another retrospective study included 29 patients with AIH-ACLF[21]. Following steroid, 90-day and 180-day transplant-free survival were 55.2% [95% confidence interval (95%CI): 39.7%–76.6%] and 30.2% (95%CI: 16.7%–54.6%), respectively[21]. Due to a lack of adequate data on steroids and the poorer outcomes, patients with AIH-ACLF should be evaluated for LT. They can be considered for immunosuppressive therapy only on a case-by-case basis after ruling out and controlling sepsis or overwhelming infection[19-21].
There are a few retrospective studies that have looked at the efficacy of immunosuppressive treatment in patients with decompensated cirrhosis. The largest multicenter study from the International Autoimmune Hepatitis Group included 232 patients with AIH-related decompensated cirrhosis. At diagnosis, 89% had ascites, and 41% had overt HE. A total of 214 (92%) treated patients had higher aminotransferases, bilirubin and modified hepatic activity index. Those with no HE or HE grades 1/2 showed a 60% LT-free survival on treatment. Patients with MELD-Na > 28 had worse LT-free survival rates. Also, a decline in MELD-Na after 4 weeks of treatment had a 100% negative predictive value for death or LT. Around half of the patients achieved recompensation on follow-up[22]. In another small retrospective study of 64 patients treated with corticosteroids, 63% recompensated, while 14% had liver-related death or LT. Changes in total bilirubin and MELD scores at day 7 predicted treatment outcome[23]. Another study compared patients with decompensated cirrhosis and mild or no ascites vs gross ascites vs compensated cirrhosis; 31% of patients with mild ascites vs 4% with gross ascites achieved a biochemical response. Although the initial steroid dose was lower in patients with decom
Twenty-five percent of patients with AIH may be asymptomatic and may just have elevated liver enzymes[4,25-27]. An Italian retrospective study of 305 patients compared the treatment response and outcomes in patients with asymptomatic vs symptomatic AIH. Thirty percent of the patients were asymptomatic at presentation and had lower enzymes; however, treatment response, liver disease progression, and progression to cirrhosis were similar between the two groups[27]. Patients should therefore be started on immunosuppressive therapy when indicated, irrespective of the presence of symptoms[4,25].
Pregnancy: Flares during pregnancy in AIH are not uncommon, with a reported incidence of 21%–33%[28]. Postpartum flares are frequent, occurring in 41% of patients at a median of 11 weeks after delivery[28,29]. AIH may present initially during pregnancy, and standard diagnostic algorithms and treatment regimens should be used. A complete biochemical response during conception and pregnancy is associated with reduced AIH flares and better pregnancy-related outcomes[29,30]. To conceive, patients should be in a biochemical remission for at least 1 year[25]. The commonly used first-line immunosuppressive drugs, steroids and thiopurines can be safely used during pregnancy and lactation[4]. Treatment with MMF or TAC is contraindicated in pregnancy due to teratogenic effects (Table 1), and contraceptives should be used in women of childbearing age on MMF. MMF should be discontinued at least 3 months before conception[4,25].
| Drugs | Mycophenolate mofetil | Tacrolimus | Cyclosporine | Methotrexate | Sirolimus |
| Position in managing refractory AIH | Second line | Second line | Second line | Second line | Second line |
| Mechanism of action | Prodrug converted to mycophenolic acid, which inhibits purine biosynthesis and attenuate proliferation of B and T cells | Calcineurin inhibitors which suppress IL-2 production | Calcineurin inhibitors which suppress IL-2 production | Inhibition of folic acid synthesis | m-TOR inhibitor |
| Dose | 20–40 mg/kg/d | 2–6 mg/kg/d | 2–4 mg/kg/d | 7–20 mg/wk | 1–2 mg/kg/d |
| Pregnancy category1 | D | C | C | X | C |
| Adverse events | Diarrhea, cytopenia and colitis | Nephrotoxicity, gingival hypertrophy, hypertension and dyselectrolytemia | Nephrotoxicity, hyperkalemia | Bone marrow suppression, pulmonary fibrosis, and liver injury | Arthralgia, peripheral edema, skin rashes ang gastrointestinal problems |
| Comments | More effective for azathioprine intolerant than azathioprine refractory AIH. Current RCT shows it can be safe first-line alternative to azathioprine[61-63] | Tacrolimus was effective in 18 of 23 patients with first-line-intolerant or refractory AIH[65] | Five of six patients with steroid refractory AIH responded to cyclosporine[66,120] | Six of 11 patients responded to methotrexate after initial non-response to steroid with or without azathioprine[68,69] | Four of five patients with steroid refractory AIH responded to sirolimus[92] |
Old age: Elderly patients with AIH achieve a similar complete biochemical response as younger patients; however, they have lower rates of relapse[31,32]. In a recent systematic review, treatment-related adverse events were similar in all age groups[31]. Due to the presence of multiple comorbidities and the risk of osteoporosis with corticosteroids in these patients, recent guidelines recommend treating elderly patients with moderate activity on histology and/or with ad
AIH in HIV: AIH in people living with HIV is usually diagnosed after initiation of antiretroviral therapy when the immune status of the patient has been restored[12]. Immune reconstitution inflammatory syndrome after initiation of antiretroviral therapy has been hypothesized to be the trigger for AIH[33,34]. Patients started on corticosteroids and AZA for maintenance showed excellent response to therapy in a case series. It was without HIV viral rebound or infectious complications[33,34].
AIH in hepatitis B and C co-infection: Concurrent infection with hepatitis B virus (HBV) and hepatitis C virus (HCV) is rare; however, it can be found in high-prevalence regions. With the advent of highly efficacious direct-acting antivirals (DAAs), HCV co-infection can be tackled with ease. DAAs have also been shown to resolve autoimmune features by themselves[35,36]. HBV co-infection needs extensive evaluation and therapy in most cases before immunosuppressive therapy. Hepatitis B surface antigen (HBsAg) positive, HBsAg negative with antibody against total core antigen (anti-HBc) positive patients at high risk of reactivation should be initiated on antiviral therapy before immunosuppression[35]. HBsAg-negative and anti-HBc-positive patients should be monitored for HBsAg and HBV deoxyribonucleic acid (DNA) while on immunosuppressive therapy. Patients with chronic hepatitis D virus infection demonstrate a higher frequency of antinuclear antibody and anti-smooth muscle antibody positivity (67% and 43%, respectively) and higher levels of serum IgG, which suggest an autoimmune phenomenon at play[36,37]. However, the relevance of this is unknown.
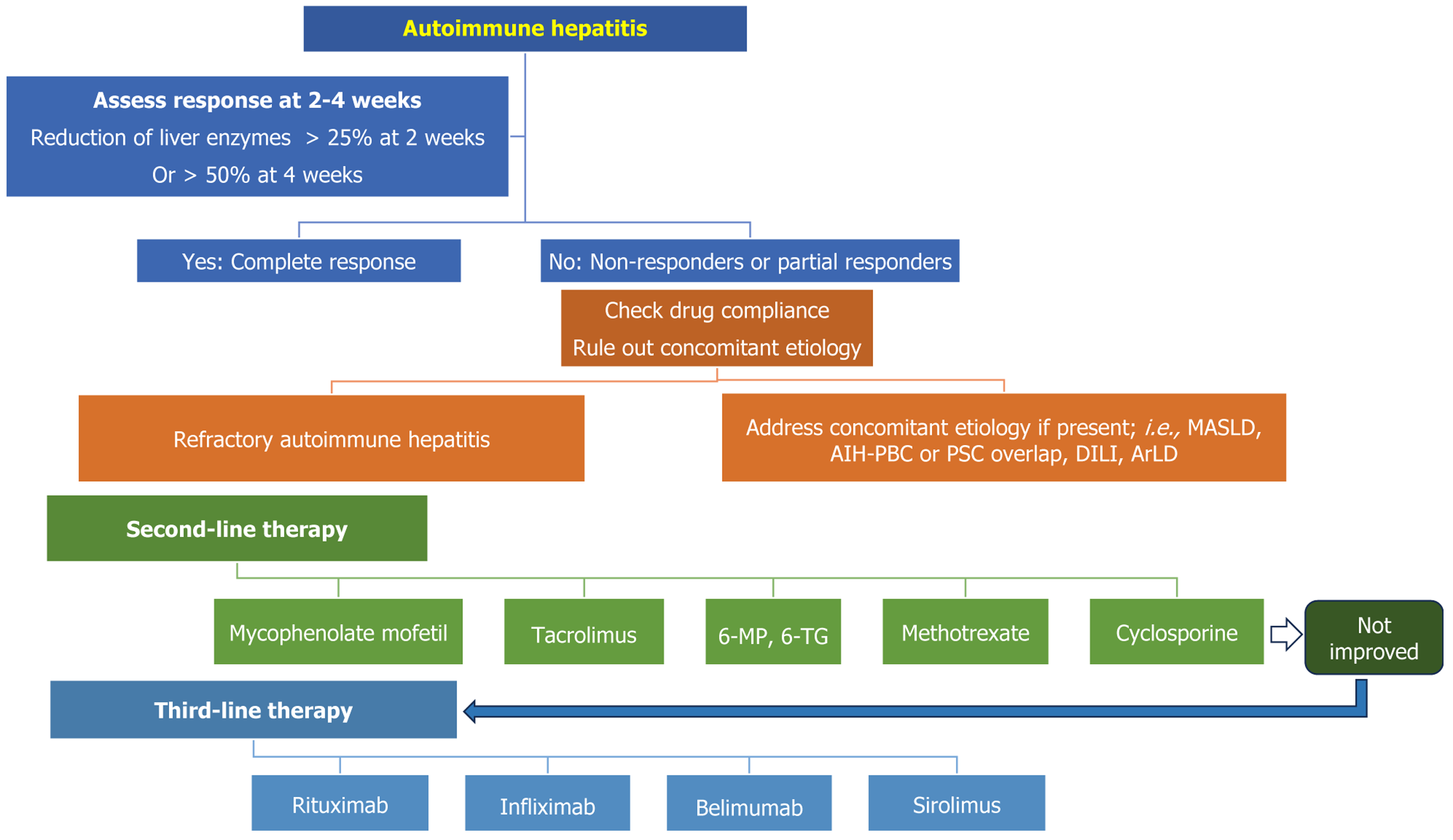
The aim of first-line immunosuppressive therapy is to induce remission and halt fibrosis progression in patients with AIH[4,25,26]. First-line therapy comprises glucocorticoids with or without AZA (Figure 2). The standard dose of prednisone is 20–40 mg/day or 1–2 mg/kg/day. Some societies advocate the use of AZA along with a lower dose of steroids from the beginning of the treatment[4,25]. However, given the side effects profile, it is logical to assess for steroid responsiveness after 2 weeks of the therapy. If the patient responds to the steroid, AZA (50-150 mg/day) is added once bilirubin comes below 5 mg/dL[25]. Thiopurine methyl transferase (TPMT) mutation testing is mandatory before starting AZA[38]. Owing to its higher prevalence, nudix hydrolase (NUDT-15) mutation analysis is a prerequisite for starting AZA in Asian countries[39,40]. Following the initiation of the treatment, liver function tests are done after 1–2 weeks, and a biochemical response is assessed after 4–8 weeks (Figure 2). If patients respond to treatment, the steroid is tapered to 5 mg/day to minimize the risk of steroid-related adverse events[4,26,41]. A dose of ≥ 10 mg/day is associated with higher adverse events[41,42].
Steroid monotherapy is advisable when the anticipated duration of treatment is < 6 months or the patient has contraindications for AZA therapy[4].
Budesonide has been used instead of prednisolone in patients with AIH. A randomized controlled trial (RCT) has demonstrated the superiority of budesonide over prednisone. After 6 months, 60% of patients in the budesonide arm achieved biochemical remission compared to 39% in the prednisone arm, with fewer steroid-related side effects (28% vs 53%). However, the study did not include patients with ALF or cirrhosis. As budesonide undergoes extensive fast-pass metabolism in the liver, it is not recommended in patients with cirrhosis[43]. Budesonide is also associated with the risk of portal vein thrombosis in patients with cirrhosis[44,45].
Nonresponse to steroids is defined as the lack of reduction of liver enzymes by more than 50% after 4 weeks of therapy[46]. However, according to the APASL guidelines, failure of reduction of liver enzymes by 25% within 2 weeks of tr
Delaying second-line therapy in such patients can be associated with worsening of pre-existing liver dysfunction. They should be promptly evaluated for the other concomitant etiology and counseled for second-line therapy (Figure 2)[4,25-27].
Patients who fail to respond to the initial immunosuppressive therapy should be investigated for concurrent etiology for liver injury, such as MASLD, primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), drug-induced liver injury (DILI), complementary and alternative medicine (CAM) or herb-related liver injury, alcohol-related liver injury, and chronic viral hepatitis B and C (Figure 2)[4,8-10].
Twenty to 30% of patients with AIH may have underlying histological evidence of MASLD. A subset of such patients may have steatohepatitis. Concomitant MASLD in AIH is associated with higher liver enzymes, advanced fibrosis, and decompensation[10,47]. Steroids may worsen underlying metabolic dysfunction in such patients[47,48]. Overlap syn
The seroprevalence of hepatitis B and C ranges from 2% to 8%, depending on the population[59]. Routinely, HBsAg and anti-HCV are used to screen for the presence of these infections. However, routine screening with HbsAg may miss a subset of patients infected with the surface-mutant variant of hepatitis B[4,25,26]. Immunosuppression may lead to reactivation of the underlying HBV infection. Thus, screening with HBsAg, total anti-HBc, and anti-HBsAg titer should be the standard of care. Anti-HCV serology is recommended to screen for HCV infection. Patients with chronic kidney disease and HIV infection may not mount a sufficient antibody response. Testing HCV RNA is recommended in such populations[4].
MMF: MMF is the most commonly used second-line therapy in patients with AIH. However, current guidelines recommend the use of MMF as an alternative first-line therapy in patients with AZA-intolerant or nonresponsive AIH (Figure 2)[4,25,60]. MMF is a prodrug, and it is converted to mycophenolic acid, which inhibits purine synthesis. Attenuation of purine synthesis leads to reduced B- and T-cell proliferation[60]. A recent RCT has clearly shown that when combined with steroids, MMF performed better than AZA in treatment-naïve patients with AIH. At 24 weeks, normalization of liver enzymes and IgG was significantly higher in patients receiving MMF (56.4% vs 29%; P = 0.004). No adverse events were reported in the MMF group; however, serious adverse events were noted in 13% of patients in the AZA group[61]. Retrospective data have shown long-term safety and efficacy of MMF as a first-line therapy in patients with AIH[62].
A recent meta-analysis has revealed that MMF works best in patients who initially respond to AZA but have to discontinue it because of side effects[63]. In such patients with AZA intolerance, the pooled response rate with MMF was 82%. However, the response rate was lower (32%) in patients who failed to respond to AZA initially. Pooled discontinuation and adverse events with MMF were 0.08% and 0.14%, respectively[63]. Leukopenia, diarrhea, and teratogenicity are the main concerns with the use of MMF (Table 1)[60].
Calcineurin inhibitors: Cys and TAC have been used in patients with AIH with varied response rates and adverse events. They act by inhibiting interleukin (IL)-2 production and T-cell proliferation[64]. Calcineurin inhibitors (CNIs) have been used as a second-line therapy in pediatric patients with AIH (Table 1). A systematic review and meta-analysis included 76 pediatric patients who failed to respond to steroid or AZA[65]. Overall response rate at 6 months was 36% with MMF and 50% with TAC, and 83% for Cys. The Cys and TAC groups had a significantly higher rate of adverse events (78% and 42%, respectively).
Other real-world data on CNIs as a second- or third-line agent included 20 patients with difficult-to-treat AIH. Ten patients received TAC, and others were managed with Cys. The duration of therapy was longer when the drugs were used as second- or third-line agents. On follow-up, 55% of patients achieved normalization of liver enzymes (Tables 1 and 2)[66].
| Drugs | Infliximab | Rituximab | Belimumab |
| Position in managing refractory AIH | Third line | Third line | Third line |
| Mechanism of action | Monoclonal antibody to TNF-α | Chimeric monoclonal antibody against CD-20 | B-cell-activating factor inhibitor |
| Dose | 5 mg/kg | 2 doses of 1000 mg IV administered 2 wk apart, or 375 mg/m2 rituximab | 10 mg/kg |
| Pregnancy category1 | B | C | C |
| Adverse events | Infectious complications can cause drug induced AIH like injury allergic reaction | Infectious complications | Infection, allergic reactions, progressive multifocal leukoencephalopathy, and mental health issues |
| Comments | Response rate 78% when used as a third line agent[94] | As a third line agent biochemical remission: 67%, 71% flare-free survival after median 6 yr[88-90,121] | Steroid refractory AIH. Two patients had complete biochemical response[99] |
Methotrexate: Methotrexate (MTX) inhibits the synthesis of tetrahydrofolate and interferes with the production of DNA[67]. Compared to other second- or third-line therapies, MTX is associated with a lower response rate in patients with AIH[68]. The conventional side effects of MTX include bone marrow suppression, teratogenicity, and liver fibrosis (Table 1). Despite these limitations, it has been used with a success rate of ~50%[68,69]. Long-term use of MTX is linked to pulmonary and hepatic fibrosis, although recent data suggest an overestimation of liver injury related to MTX[70-72]. Routine (2–3-monthly) liver function test monitoring is recommended in patients receiving MTX[71].
6-mercaptopurine: AZA and 6-mercaptopurine (6-MP) are purine anti-metabolites[73,74]. AZA is converted to 6-MP. 6-MP is methylated by TPMT to form methyl-6-MP, or it may undergo oxidation to produce 6-thiouric acid. The immu
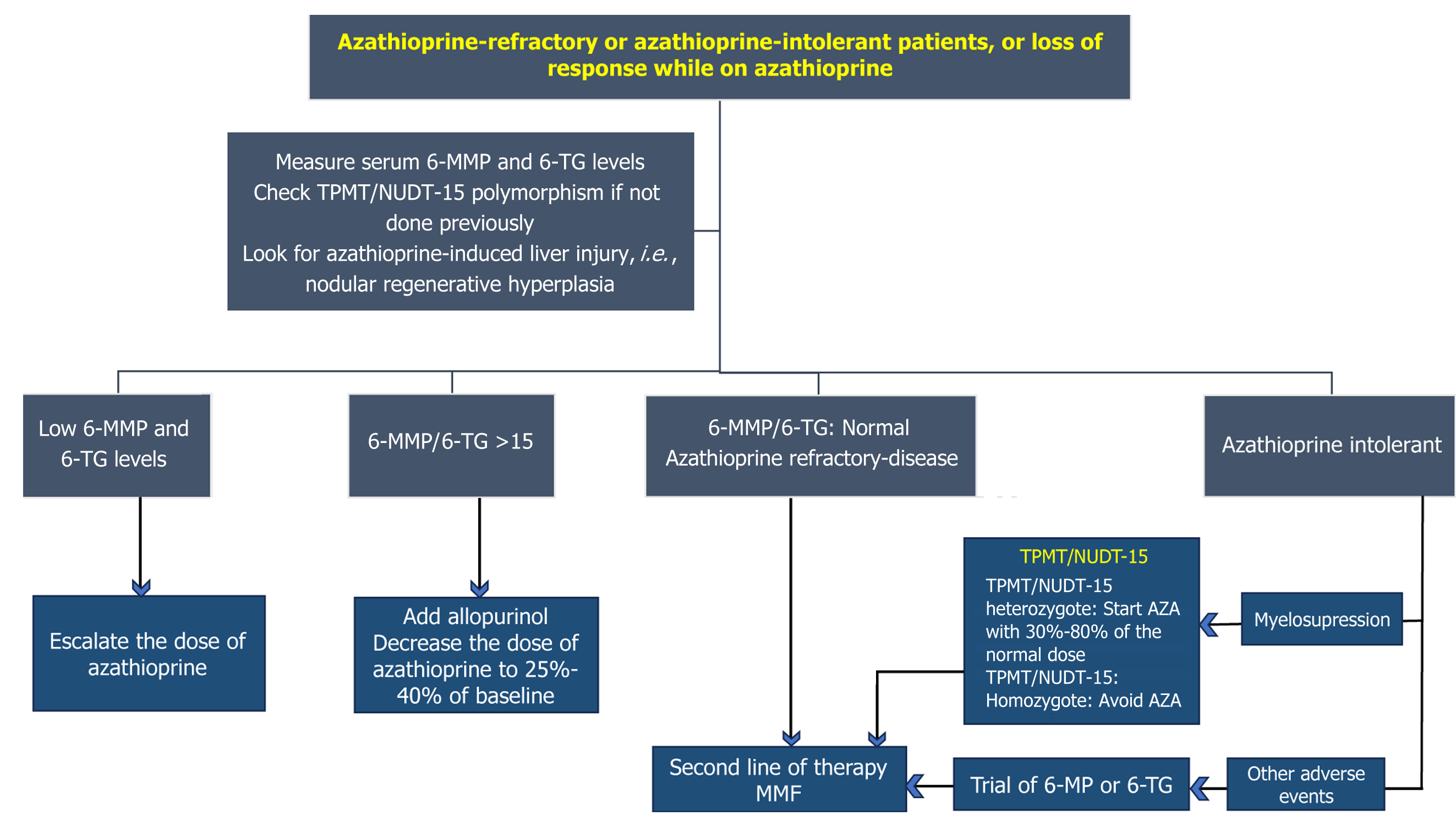
In patients with AZA intolerance, 6-MP is a choice. However, 6-MP is ineffective in patients who fail to respond to AZA. A retrospective study by Hübener et al[77] included 20 patients with AIH who had AZA-intolerance: 15 (75%) res
Long-term use of 6-MP is associated with various side effects, including liver injury[78,79]. Periodic monitoring and strong suspicion can avoid liver toxicity with 6-MP[80].
Thioguanine: Patients with AIH who are intolerant or refractory to AZA or 6-MP can be managed by 6-TG[81]. A study by Legué et al[81] included 17 patients with AZA-intolerant AIH. Following 6-TG therapy, 16 patients had normalization of liver enzymes within 3 months. In another study, 6-TG was safe and effective in patients with AZA or 6-MP-intolerant or refractory cases (Figure 3). Overall response rate was 83%[82].
Loss of remission and clinical flare should be evaluated using the 6-MP/6-TG ratio. Addition of allopurinol should be considered when the ratio is < 4[83,84]. No correlation exists between serum 6-TG level and liver enzyme levels and remission in patients with AIH[85].
Rituximab: B-cell-driven autoimmune injury plays a role in the pathogenesis of AIH (Figure 1). Thus, targeting B cells is a potential opportunity to treat AIH[86,87]. Rituximab targets CD20 on the cell surface. Rituximab is a monoclonal antibody and is effective in patients with AIH[87]. Various case series with long-term data have established its role as second- and third-line therapy in AIH. A case series by Burak et al[88] described six cases of steroid-refractory AIH suc
Mammalian target of rapamycin inhibitors: Sirolimus is a macrolide. It is a mammalian-target of rapamycin (m-TOR) inhibitor, widely used in solid organ transplantation (Figure 1). By inhibiting m-TOR, it attenuates the sensitivity of B cells and T cells to IL-2[91]. It has been used in refractory AIH. A series of five patients with refractory AIH treated with sirolimus showed a success rate of 80% (four patients had a biochemical response, i.e., reduction of alanine aminotransferase > 50%). However, only two patients maintained sustained remission on follow-up (Table 1)[92].
IFX: IFX is a chimeric monoclonal antibody against tumor-necrosis factor (TNF)[93]. It has been used as third-line of therapy in patients with AIH. In an elegant study by Efe et al[94], 20 patients who failed standard therapy, MMF, 6-MP, and TAC received IFX (Figure 2). Another group of 22 patients received IFX because they had concomitant extra-hepatic autoimmune diseases. After a median follow-up period of 17 (3–104) months, a complete biochemical response was maintained in 78% of patients[94]. The major limitations of IFX include infectious complications. Paradoxically, anti-TNF has been associated with the development of drug-induced AIH-like liver injury[95,96]. Despite these limitations and risks of infections like reactivation of latent tuberculosis in endemic countries, IFX is an attractive option for patients with difficult-to-treat AIH (Figure 2)[97].
Belimumab: Belimumab is a B-cell activating factor inhibitor used in managing systemic lupus erythematosus[98]. Arvaniti et al[99] reported two cases of refractory AIH who were managed with belimumab (Table 1). They were used as a third line of therapy[99]. Patients with SLE and concomitant AIH can be salvaged using belimumab.
Experimental therapy: Despite substantial advances in the management of AIH and recent development of newer immunosuppressive therapies, 10%–20% of patients with AIH fail to respond to currently available therapy[100]. This group of patients with decompensated liver diseases and liver failure should be evaluated for LT. However, recent experimental therapies have shown promising results in refractory AIH, which include tofacitinib, fecal microbiota transplantation (FMT), regulatory T (Treg) cell therapy, low-dose IL-2, bacteriophage therapy, ginsenosides, and pre
Tofacitinib: Tofacitinib is a Janus kinase (JAK) inhibitor mostly used in autoimmune disorders and inflammatory bowel disease[101]. Inhibiting JAK, it attenuates downstream signaling pathways and IL production[101]. The ameliorative effects of tofacitinib in immune-mediated liver disease have already been established in animal models[102]. There are reports of successful management of immune-checkpoint-mediated liver injury with tofacitinib[103]. Gökçe et al[104] reported a case of refractory AIH successfully managed with tofacitinib. However, large prospective data are needed to advocate its routine use as a third-line therapy in AIH[105].
FMT: Gut dysbiosis and increased intestinal permeability are some of the major drivers of liver dysfunction in various liver disorders. Increased intestinal permeability, small intestinal bacterial overgrowth and dysbiosis lead to bacterial translocation and lipopolysaccharidemia in patients with cirrhosis[106]. FMT has been tried in various gastrointestinal and liver disorders with promising results. It is currently indicated in patients with recurrent Clostridium difficile infection[106]. By correcting gut dysbiosis, FMT restores the intestinal gut–liver axis and ameliorates the effects of gut-derived toxins in liver dysfunction[106]. In animal models, FMT attenuate the progression of experimental AIH by modulating immune balance[107,108]. Scarcity of long-term data, regulatory and ethical issues are the main concerns regarding the use of FMT in patients with AIH.
Treg therapy and low-dose IL-2: Treg cells are involved in hepatic immune tolerance, and their reserve is important in maintaining immune homeostasis in hepatic parenchyma[109]. Depleted and dysfunctional Treg cells are involved in the pathogenesis of AIH[109]. In a proof-of-concept study by Oo et al[110], successful homing of Treg cell was seen in the hepatic parenchyma following intravenous infusion. Treg cell therapy is an emerging cell-based immunotherapy in patients with refractory AIH[111,112] (Figure 1).
IL-2 stimulates the proliferation of Treg cells. Low-dose IL-2 has been used in patients with refractory AIH[113,114]. Lim et al[115] reported two cases of refractory AIH, which were salvaged using low-dose IL-2.
Bacteriophages: Bacteriophages outnumber the intestinal bacteria and viruses[116]. They have an immunomodulatory role in the intestine and the liver[116]. Phages have been shown to reduce the expression of Toll-like receptor 4 and downregulate nuclear factor B to attenuate the inflammatory pathways and cellular damage[117]. Recent data suggest that phages may ameliorate the immune-mediated tissue damage in various autoimmune disorders and nonalcoholic steatohepatitis[118,119]. Prospective data on the role of phage therapy in patients with refractory AIH are needed to establish its clinical efficacy.
An individualized approach should be followed in managing patients with difficult-to-treat and refractory AIH. Second-line therapy is reserved for patients who fail to respond to initial immunosuppression therapy. Patients who are intolerant to AZA can be managed with MMF or 6-MP. However, AZA-non-responders can be salvaged with MMF or a third-line immunosuppressive drug. Genetic polymorphism involving TPMT and NUDT15 may affect the metabolism of AZA and 6-MP, and screening for these mutations is recommended. Metabolite assays and adding allopurinol can be considered when patients experience secondary loss of response with AZA and 6-MP. Patients with concomitant autoimmune diseases can be managed with IFX, belimumab, or rituximab. Frequent monitoring for adverse events is recommended while using immunosuppressive therapy. Worsening liver enzymes should prompt physicians to look for DILI. Few drugs used to treat AIH have been associated with variable degrees of liver injury; i.e., AIH-like DILI (IFX), nodular regenerative hyperplasia (AZA), and hepatic fibrosis (MTX). Data on the safety and efficacy of sirolimus, 6-MP, belimumab, and MTX come from a small number of patients. Large prospective data are needed for newer second- and third-line drugs for managing refractory AIH. Tofacitinib is a promising experimental drug. Low-dose IL-2, phage the
The authors express their heartfelt gratitude and thank all the individuals for their tireless efforts, insightful comments, suggestions and intellectual assistance to this work.
| 1. | Komori A. Recent updates on the management of autoimmune hepatitis. Clin Mol Hepatol. 2021;27:58-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 2. | Malakar S, Mohindra S, Mishra P, Kothalkar S, Shirol VV, Borah G, Shamsul Hoda U, Shah N, Balankhe K, Pande G, Ghoshal UC. Implications of Gender on the Outcome in Patients With Autoimmune Hepatitis. Cureus. 2024;16:e55477. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Hahn JW, Yang HR, Moon JS, Chang JY, Lee K, Kim GA, Rahmati M, Koyanagi A, Smith L, Kim MS, López Sánchez GF, Elena D, Shin JY, Shin JI, Kwon R, Kim S, Kim HJ, Lee H, Ko JS, Yon DK. Global incidence and prevalence of autoimmune hepatitis, 1970-2022: a systematic review and meta-analysis. EClinicalMedicine. 2023;65:102280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 4. | Wang G, Tanaka A, Zhao H, Jia J, Ma X, Harada K, Wang FS, Wei L, Wang Q, Sun Y, Hong Y, Rao H, Efe C, Lau G, Payawal D, Gani R, Lindor K, Jafri W, Omata M, Sarin SK. The Asian Pacific Association for the Study of the Liver clinical practice guidance: the diagnosis and management of patients with autoimmune hepatitis. Hepatol Int. 2021;15:223-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Taneja S, Mehtani R, De A, Mitra S, Rathi S, Verma N, Premkumar M, Minz R, Duseja A, Das A, Singh V, Dhiman RK, Chawla YK. Spectrum of Autoimmune Liver Disease and Real-World Treatment Experience from a Tertiary Care Hospital. J Clin Exp Hepatol. 2023;13:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 6. | van den Brand FF, van der Veen KS, Lissenberg-Witte BI, de Boer YS, van Hoek B, Drenth JPH, Verdonk RC, Vrolijk JM, van Nieuwkerk CMJ, Bouma G; Dutch Autoimmune Hepatitis Study Group. Adverse events related to low dose corticosteroids in autoimmune hepatitis. Aliment Pharmacol Ther. 2019;50:1120-1126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 7. | Johnson PJ, McFarlane IG, Williams R. Azathioprine for long-term maintenance of remission in autoimmune hepatitis. N Engl J Med. 1995;333:958-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 247] [Article Influence: 8.0] [Reference Citation Analysis (4)] |
| 8. | Whitehead B, Kriegermeier A. Natural history and management of refractory autoimmune hepatitis. Clin Liver Dis (Hoboken). 2022;20:120-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Parker R, Oo YH, Adams DH. Management of patients with difficult autoimmune hepatitis. Therap Adv Gastroenterol. 2012;5:421-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Takahashi A, Arinaga-Hino T, Ohira H, Abe K, Torimura T, Zeniya M, Abe M, Yoshizawa K, Takaki A, Suzuki Y, Kang JH, Nakamoto N, Fujisawa T, Tanaka A, Takikawa H; Japan AIH Study Group (JAIHSG). Non-alcoholic fatty liver disease in patients with autoimmune hepatitis. JGH Open. 2018;2:54-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Rigopoulou EI, Zachou K, Gatselis N, Koukoulis GK, Dalekos GN. Autoimmune hepatitis in patients with chronic HBV and HCV infections: patterns of clinical characteristics, disease progression and outcome. Ann Hepatol. 2013;13:127-135. [PubMed] |
| 12. | Chaiteerakij R, Sanpawat A, Avihingsanon A, Treeprasertsuk S. Autoimmune hepatitis in human immunodeficiency virus-infected patients: A case series and review of the literature. World J Gastroenterol. 2019;25:5388-5402. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Rahim MN, Miquel R, Heneghan MA. Approach to the patient with acute severe autoimmune hepatitis. JHEP Rep. 2020;2:100149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Zheng L, Liu Y, Shang Y, Han Z, Han Y. Clinical characteristics and treatment outcomes of acute severe autoimmune hepatitis. BMC Gastroenterol. 2021;21:93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 15. | De Martin E, Coilly A, Chazouillères O, Roux O, Peron JM, Houssel-Debry P, Artru F, Silvain C, Ollivier-Hourmand I, Duvoux C, Heurgue A, Barge S, Ganne-Carrié N, Pageaux GP, Besch C, Bourlière M, Fontaine H, de Ledinghen V, Dumortier J, Conti F, Radenne S, Debette-Gratien M, Goria O, Durand F, Potier P, Di Martino V, Reboux N, Ichai P, Sebagh M, Mathurin P, Agostini H, Samuel D, Duclos-Vallée JC; FILFOIE consortium – France. Early liver transplantation for corticosteroid non-responders with acute severe autoimmune hepatitis: The SURFASA score. J Hepatol. 2021;74:1325-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 16. | Téllez L, Sánchez Rodríguez E, Rodríguez de Santiago E, Llovet L, Gómez-Outomuro A, Díaz-Fontenla F, Álvarez López P, García-Eliz M, Amaral C, Sánchez-Torrijos Y, Fortea JI, Ferre-Aracil C, Rodríguez-Perálvarez M, Abadía M, Gómez-Camarero J, Olveira A, Calleja JL, Crespo J, Romero M, Hernández-Guerra M, Berenguer M, Riveiro-Barciela M, Salcedo M, Rodríguez M, Londoño MC, Albillos A. Early predictors of corticosteroid response in acute severe autoimmune hepatitis: a nationwide multicenter study. Aliment Pharmacol Ther. 2022;56:131-143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 17. | Moenne-Loccoz R, Severac F, Baumert TF, Habersetzer F. Usefulness of corticosteroids as first-line therapy in patients with acute severe autoimmune hepatitis. J Hepatol. 2016;65:444-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Lin S, Hall A, Kumar R, Quaglia A, Jalan R. Validation of the SURFASA score to define steroid responsiveness in patients with acute autoimmune hepatitis. J Hepatol. 2022;76:485-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Anand L, Choudhury A, Bihari C, Sharma BC, Kumar M, Maiwall R, Siam Tan S, Shah SR, Hamid S, Butt AS, Jafri W, Chawla YK, Taneja S, Duseja A, Dhiman RK, Mahtab MA, Ghazinyan H, Duan Z, Chen Y, Shukla A, Hu J, Abbas Z, Treeprasertsuk S, Lesmana LA, Lesmana CR, Sollano JD, Carpio G, Sahu MK, Kumar G, Sarin SK; APASL ACLF (APASL ACLF Research Consortium) Working Party. Flare of Autoimmune Hepatitis Causing Acute on Chronic Liver Failure: Diagnosis and Response to Corticosteroid Therapy. Hepatology. 2019;70:587-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 20. | Choudhury A, Kulkarni AV, Arora V, Soin AS, Dokmeci AK, Chowdhury A, Koshy A, Duseja A, Kumar A, Mishra AK, Patwa AK, Sood A, Roy A, Shukla A, Chan A, Krag A, Mukund A, Mandot A, Goel A, Butt AS, Sahney A, Shrestha A, Cárdenas A, Di Giorgio A, Arora A, Anand AC, Dhawan A, Jindal A, Saraya A, Srivastava A, Kumar A, Kaewdech A, Pande A, Rastogi A, Valsan A, Goel A, Kumar A, Singal AK, Tanaka A, Coilly A, Singh A, Meena BL, Jagadisan B, Sharma BC, Lal BB, Eapen CE, Yaghi C, Kedarisetty CK, Kim CW, Panackel C, Yu C, Kalal CR, Bihari C, Huang CH, Vasishtha C, Jansen C, Strassburg C, Lin CY, Karvellas CJ, Lesmana CRA, Philips CA, Shawcross D, Kapoor D, Agrawal D, Payawal DA, Praharaj DL, Jothimani D, Song DS, Kim DJ, Kim DS, Zhongping D, Karim F, Durand F, Shiha GE, D'Amico G, Lau GK, Pati GK, Narro GEC, Lee GH, Adali G, Dhakal GP, Szabo G, Lin HC, Li H, Nair HK, Devarbhavi H, Tevethia H, Ghazinian H, Ilango H, Yu HL, Hasan I, Fernandez J, George J, Behari J, Fung J, Bajaj J, Benjamin J, Lai JC, Jia J, Hu JH, Chen JJ, Hou JL, Yang JM, Chang J, Trebicka J, Kalf JC, Sollano JD, Varghese J, Arab JP, Li J, Reddy KR, Raja K, Panda K, Kajal K, Kumar K, Madan K, Kalista KF, Thanapirom K, Win KM, Suk KT, Devadas K, Lesmana LA, Kamani L, Premkumar M, Niriella MA, Al Mahtab M, Yuen MF, Sayed MH, Alla M, Wadhawan M, Sharma MK, Sahu M, Prasad M, Muthiah MD, Schulz M, Bajpai M, Reddy MS, Praktiknjo M, Yu ML, Prasad M, Sharma M, Elbasiony M, Eslam M, Azam MG, Rela M, Desai MS, Vij M, Mahmud N, Choudhary NS, Marannan NK, Ormeci N, Saraf N, Verma N, Nakayama N, Kawada N, Oidov Baatarkhuu, Goyal O, Yokosuka O, Rao PN, Angeli P, Parikh P, Kamath PS, Thuluvath PJ, Lingohr P, Ranjan P, Bhangui P, Rathi P, Sakhuja P, Puri P, Ning Q, Dhiman RK, Kumar R, Vijayaraghavan R, Khanna R, Maiwall R, Mohanka R, Moreau R, Gani RA, Loomba R, Mehtani R, Rajaram RB, Hamid SS, Palnitkar S, Lal S, Biswas S, Chirapongsathorn S, Agarwal S, Sachdeva S, Saigal S, Kumar SE, Violeta S, Singh SP, Mochida S, Mukewar S, Alam S, Lim SG, Alam S, Shalimar, Venishetty S, Sundaram SS, Shetty S, Bhatia S, Singh SA, Kottilil S, Strasser S, Shasthry SM, Maung ST, Tan SS, Treeprasertsuk S, Asthana S, Manekeller S, Gupta S, Acharya SK, K C S, Maharshi S, Asrani S, Dadhich S, Taneja S, Giri S, Singh S, Chen T, Gupta T, Kanda T, Tanwandee T, Piratvishuth T, Spengler U, Prasad VGM, Midha V, Rakhmetova V, Arroyo V, Sood V, Br VK, Wong VW, Pamecha V, Singh V, Dayal VM, Saraswat VA, Kim W, Jafri W, Gu W, Jun WY, Qi X, Chawla YK, Kim YJ, Shi Y, Abbas Z, Kumar G, Shiina S, Wei L, Omata M, Sarin SK; APASL-ACLF Research Consortium (AARC) for APASL-ACLF working party. Acute-on-chronic liver failure (ACLF): the 'Kyoto Consensus'-steps from Asia. Hepatol Int. 2025;19:1-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 27] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 21. | Sharma S, Agarwal S, Gopi S, Anand A, Mohta S, Gunjan D, Yadav R, Saraya A. Determinants of Outcomes in Autoimmune Hepatitis Presenting as Acute on Chronic Liver Failure Without Extrahepatic Organ Dysfunction upon Treatment With Steroids. J Clin Exp Hepatol. 2021;11:171-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 22. | Arvaniti P, Rodríguez-Tajes S, Padilla M, Olivas I, Mauro E, El Maimouni C, Lytvyak E, Verhelst X, Engel B, Taubert R, Lorente-Pérez S, Conde I, Riveiro-Barciela M, Ruiz-Cobo JC, Álvarez-Navascués C, Salcedo M, Gómez J, Janik MK, Mateos B, Efe C, Granito A, Dajti E, Azzaroli F, Horta D, Vila C, Castello I, Pérez-Medrano I, Arencibia A, Gerussi A, Bruns T, Colaprieto F, Lleo A, Van den Ende N, Verbeek J, Díaz-González Á, Morillas RM, Torner-Simó M, Bernal V, Fernández EM, Gevers TJG, Terziroli Beretta-Piccoli B, Gómez E, Cuenca P, de Boer YS, Kerkar N, Assis DN, Liberal R, Drenth JPH, Tana MM, Sebode M, Schregel I, Schramm C, Lohse AW, Montano-Loza AJ, Zachou K, Villamil A, Dalekos GN, Londoño MC; International Autoimmune Hepatitis Group (IAIHG); European Reference Network on Hepatological Diseases (ENR RARE-LIVER); Spanish Registry for Autoimmune and Cholestatic Diseases (ColHai) Registry. Hepatic Encephalopathy and MELD-Na Predict Treatment Benefit in Autoimmune Hepatitis-related Decompensated Cirrhosis. Clin Gastroenterol Hepatol. 2025;S1542-3565(25)00249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 23. | Wang Z, Sheng L, Yang Y, Yang F, Xiao X, Hua J, Guo C, Wei Y, Tang R, Miao Q, Zhang J, Li Y, Fang J, Qiu D, Krawitt EL, Bowlus CL, Gershwin ME, Wang Q, Ma X. The Management of Autoimmune Hepatitis Patients with Decompensated Cirrhosis: Real-World Experience and a Comprehensive Review. Clin Rev Allergy Immunol. 2017;52:424-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Sharma S, Agarwal S, Kaushal K, Anand A, Gunjan D, Yadav R, Saraya A. Presence and type of decompensation affects outcomes in autoimmune hepatitis upon treatment with corticosteroids. JGH Open. 2021;5:81-90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Chung YY, Heneghan MA. Autoimmune hepatitis in pregnancy: Pearls and pitfalls. Hepatology. 2022;76:502-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 26. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1027] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 27. | Muratori P, Lalanne C, Barbato E, Fabbri A, Cassani F, Lenzi M, Muratori L. Features and Progression of Asymptomatic Autoimmune Hepatitis in Italy. Clin Gastroenterol Hepatol. 2016;14:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Llovet LP, Horta D, Eliz MG, Berenguer M, Fábrega E, Sáez-Royuela F, García-Retortillo M, Torrijos YS, Romero-Gómez M, Fernández C, Domínguez EG, Parés A, Londoño MC. Presentation and Outcomes of Pregnancy in Patients With Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2019;17:2819-2821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Westbrook RH, Yeoman AD, Kriese S, Heneghan MA. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun. 2012;38:J239-J244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 30. | Fischer SE, de Vries ES, Tushuizen ME, de Boer YS, van der Meer AJP, de Man RA, Brouwer JT, Kuyvenhoven JP, Klemt-Kropp M, Gevers TJG, Tjwa ETTL, Kuiper EMM, Verhagen MAMT, Friederich PW, van Hoek B. Importance of complete response for outcomes of pregnancy in patients with autoimmune hepatitis. Liver Int. 2023;43:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Durazzo M, Lupi G, Scandella M, Ferro A, Gruden G. Autoimmune hepatitis treatment in the elderly: A systematic review. World J Gastroenterol. 2019;25:2809-2818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Dalekos GN, Azariadis K, Lygoura V, Arvaniti P, Gampeta S, Gatselis NK. Autoimmune hepatitis in patients aged 70 years or older: Disease characteristics, treatment response and outcome. Liver Int. 2021;41:1592-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Murunga E, Andersson M, Rensburg Cv. Autoimmune hepatitis: a manifestation of immune reconstitution inflammatory syndrome in HIV infected patients? Scand J Gastroenterol. 2016;51:814-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | O'Leary JG, Zachary K, Misdraji J, Chung RT. De novo autoimmune hepatitis during immune reconstitution in an HIV-infected patient receiving highly active antiretroviral therapy. Clin Infect Dis. 2008;46:e12-e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Loomba R, Liang TJ. Hepatitis B Reactivation Associated With Immune Suppressive and Biological Modifier Therapies: Current Concepts, Management Strategies, and Future Directions. Gastroenterology. 2017;152:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 475] [Article Influence: 52.8] [Reference Citation Analysis (0)] |
| 36. | Matsumoto K, Kikuchi K, Namura Y, Watanabe A, Tsunashima H, Doi S. Histological improvement in chronic hepatitis C-autoimmune hepatitis overlap syndrome by glecaprevir and pibrentasvir. Clin J Gastroenterol. 2023;16:572-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 37. | Hermanussen L, Lampalzer S, Bockmann JH, Ziegler AE, Piecha F, Dandri M, Pischke S, Haag F, Lohse AW, Lütgehetmann M, Weiler-Normann C, Zur Wiesch JS. Non-organ-specific autoantibodies with unspecific patterns are a frequent para-infectious feature of chronic hepatitis D. Front Med (Lausanne). 2023;10:1169096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (2)] |
| 38. | Mitra S, Ghosh A, Chatterjee S, Chatterjee M, Sinhamahapatra P. Association of TPMT and NUDT15 gene polymorphisms with azathioprine-induced leukopenia: A case-control study in Eastern India. Indian J Pharmacol. 2024;56:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 39. | Grover N, Bhatia P, Kumar A, Singh M, Lad D, Mandavdhare HS, Samanta J, Prasad KK, Dutta U, Sharma V. TPMT and NUDT15 polymorphisms in thiopurine induced leucopenia in inflammatory bowel disease: a prospective study from India. BMC Gastroenterol. 2021;21:327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 40. | Banerjee R, Ravikanth VV, Pal P, Bale G, Avanthi US, Goren I, Girish BG, Mitnala S, Reddy DN. NUDT15 C415T variant compared with TPMT genotyping in predicting azathioprine-induced leucopenia: prospective analysis of 1014 inflammatory bowel disease patients in India. Aliment Pharmacol Ther. 2020;52:1683-1694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 33] [Reference Citation Analysis (0)] |
| 41. | Terziroli Beretta-Piccoli B, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: Standard treatment and systematic review of alternative treatments. World J Gastroenterol. 2017;23:6030-6048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (1)] |
| 42. | Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin Ther. 2017;39:2216-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 371] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 43. | Manns MP, Woynarowski M, Kreisel W, Lurie Y, Rust C, Zuckerman E, Bahr MJ, Günther R, Hultcrantz RW, Spengler U, Lohse AW, Szalay F, Färkkilä M, Pröls M, Strassburg CP; European AIH-BUC-Study Group. Budesonide induces remission more effectively than prednisone in a controlled trial of patients with autoimmune hepatitis. Gastroenterology. 2010;139:1198-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 271] [Article Influence: 16.9] [Reference Citation Analysis (1)] |
| 44. | Manns MP, Jaeckel E, Taubert R. Budesonide in Autoimmune Hepatitis: The Right Drug at the Right Time for the Right Patient. Clin Gastroenterol Hepatol. 2018;16:186-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Wiegand J, Schüler A, Kanzler S, Lohse A, Beuers U, Kreisel W, Spengler U, Koletzko S, Jansen PL, Hochhaus G, Möllmann HW, Pröls M, Manns MP. Budesonide in previously untreated autoimmune hepatitis. Liver Int. 2005;25:927-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 46. | Korean Association for the Study of the Liver (KASL). KASL clinical practice guidelines for management of autoimmune hepatitis 2022. Clin Mol Hepatol. 2023;29:542-592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 47. | European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J Hepatol. 2024;81:492-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 889] [Article Influence: 444.5] [Reference Citation Analysis (1)] |
| 48. | Zachou K, Azariadis K, Lytvyak E, Snijders RJALM, Takahashi A, Gatselis NK, Robles M, Andrade RJ, Schramm C, Lohse AW, Tanaka A, Drenth JPH, Montano-Loza AJ, Dalekos GN; International Autoimmune Hepatitis Group (IAIHG). Treatment responses and outcomes in patients with autoimmune hepatitis and concomitant features of non-alcoholic fatty liver disease. JHEP Rep. 2023;5:100778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 49. | Malakar S, Pande G, Mishra P, Kumar SR, Kumar P, Mohindra S, Rai P, Ghoshal UC. Frequency and spectrum of primary biliary cholangitis and its overlap with autoimmune hepatitis among patients with chronic liver disease. Indian J Gastroenterol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 50. | Rust C, Beuers U. Overlap syndromes among autoimmune liver diseases. World J Gastroenterol. 2008;14:3368-3373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 89] [Cited by in RCA: 116] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 51. | Malakar S, Mishra P, Paturu R, Verma R, Ghoshal UC. Primary sclerosing cholangitis with high immunoglobulin-G4. J Hepatol. 2024;80:e168-e170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 52. | Laskin CA, Vidins E, Blendis LM, Soloninka CA. Autoantibodies in alcoholic liver disease. Am J Med. 1990;89:129-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Lian M, Hua J, Sheng L, Qiu DK. Prevalence and significance of autoantibodies in patients with alcoholic liver disease. J Dig Dis. 2013;14:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 54. | Rigopoulou EI, Gatselis N, Arvaniti P, Koukoulis GK, Dalekos GN. Alcoholic liver disease and autoimmune hepatitis: Sometimes a closer look under the surface is needed. Eur J Intern Med. 2021;85:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Devarbhavi H, Aithal G, Treeprasertsuk S, Takikawa H, Mao Y, Shasthry SM, Hamid S, Tan SS, Philips CA, George J, Jafri W, Sarin SK; Asia Pacific Association of Study of Liver. Drug-induced liver injury: Asia Pacific Association of Study of Liver consensus guidelines. Hepatol Int. 2021;15:258-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 105] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 56. | Fontana RJ, Liou I, Reuben A, Suzuki A, Fiel MI, Lee W, Navarro V. AASLD practice guidance on drug, herbal, and dietary supplement-induced liver injury. Hepatology. 2023;77:1036-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 57. | Ercin CN. New classification of drug induced liver injury (DILI) in AASLD guidance: What is next? Hepatol Forum. 2024;5:61-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 58. | Andrade RJ, Aithal GP, de Boer YS, Liberal R, Gerbes A, Regev A, Terziroli Beretta-Piccoli B, Schramm C, Kleiner DE, De Martin E, Kullak-Ublick GA, Stirnimann G, Devarbhavi H, Vierling JM, Manns MP, Sebode M, Londoño MC, Avigan M, Robles-Diaz M, García-Cortes M, Atallah E, Heneghan M, Chalasani N, Trivedi PJ, Hayashi PH, Taubert R, Fontana RJ, Weber S, Oo YH, Zen Y, Licata A, Lucena MI, Mieli-Vergani G, Vergani D, Björnsson ES; IAIHG and EASL DHILI Consortium. Nomenclature, diagnosis and management of drug-induced autoimmune-like hepatitis (DI-ALH): An expert opinion meeting report. J Hepatol. 2023;79:853-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 99] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 59. | GBD 2019 Hepatitis B Collaborators. Global, regional, and national burden of hepatitis B, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol Hepatol. 2022;7:796-829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 416] [Cited by in RCA: 507] [Article Influence: 126.8] [Reference Citation Analysis (1)] |
| 60. | Sharzehi K, Huang MA, Schreibman IR, Brown KA. Mycophenolate mofetil for the treatment of autoimmune hepatitis in patients refractory or intolerant to conventional therapy. Can J Gastroenterol. 2010;24:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 61. | Snijders RJALM, Stoelinga AEC, Gevers TJG, Pape S, Biewenga M, Tushuizen ME, Verdonk RC, de Jonge HJM, Vrolijk JM, Bakker SF, Vanwolleghem T, de Boer YS, Baven Pronk MAMC, Beuers U, van der Meer AJ, Gerven NMFV, Sijtsma MGM, van Eijck BC, van IJzendoorn MC, van Herwaarden M, van den Brand FF, Korkmaz KS, van den Berg AP, Guichelaar MMJ, Levens AD, van Hoek B, Drenth JPH; Dutch Autoimmune Hepatitis Working Group. An open-label randomised-controlled trial of azathioprine vs. mycophenolate mofetil for the induction of remission in treatment-naive autoimmune hepatitis. J Hepatol. 2024;80:576-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 62. | Dalekos GN, Arvaniti P, Gatselis NK, Gabeta S, Samakidou A, Giannoulis G, Rigopoulou E, Koukoulis GK, Zachou K. Long-term results of mycophenolate mofetil vs. azathioprine use in individuals with autoimmune hepatitis. JHEP Rep. 2022;4:100601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 63. | Santiago P, Schwartz I, Tamariz L, Levy C. Systematic review with meta-analysis: mycophenolate mofetil as a second-line therapy for autoimmune hepatitis. Aliment Pharmacol Ther. 2019;49:830-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 64. | Safarini OA, Keshavamurthy C, Patel P. Calcineurin Inhibitors. 2023 Nov 12. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. [PubMed] |
| 65. | Zizzo AN, Valentino PL, Shah PS, Kamath BM. Second-line Agents in Pediatric Patients With Autoimmune Hepatitis: A Systematic Review and Meta-analysis. J Pediatr Gastroenterol Nutr. 2017;65:6-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 66. | Pape S, Nevens F, Verslype C, Mertens C, Drenth JPH, Tjwa ETTL. Profiling the patient with autoimmune hepatitis on calcineurin inhibitors: a real-world-experience. Eur J Gastroenterol Hepatol. 2020;32:727-732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Zachariae H. Methotrexate side-effects. Br J Dermatol. 1990;122 Suppl 36:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 68. | Haridy J, Nicoll A, Sood S. Methotrexate Therapy for Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2018;16:288-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 69. | Venkataramani A, Jones MB, Sorrell MF. Methotrexate therapy for refractory chronic active autoimmune hepatitis. Am J Gastroenterol. 2001;96:3432-3434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Solomon DH, Glynn RJ, Karlson EW, Lu F, Corrigan C, Colls J, Xu C, MacFadyen J, Barbhaiya M, Berliner N, Dellaripa PF, Everett BM, Pradhan AD, Hammond SP, Murray M, Rao DA, Ritter SY, Rutherford A, Sparks JA, Stratton J, Suh DH, Tedeschi SK, Vanni KMM, Paynter NP, Ridker PM. Adverse Effects of Low-Dose Methotrexate: A Randomized Trial. Ann Intern Med. 2020;172:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 71. | Atallah E, Grove JI, Crooks C, Burden-Teh E, Abhishek A, Moreea S, Jordan KM, Ala A, Hutchinson D, Aspinall RJ, Murphy R, Aithal GP. Risk of liver fibrosis associated with long-term methotrexate therapy may be overestimated. J Hepatol. 2023;78:989-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 72. | Di Martino V. Methotrexate-induced liver fibrosis: The end of a long-held belief. J Hepatol. 2023;78:896-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 73. | Gearry RB, Barclay ML. Azathioprine and 6-mercaptopurine pharmacogenetics and metabolite monitoring in inflammatory bowel disease. J Gastroenterol Hepatol. 2005;20:1149-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 177] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 74. | Kröplin T, Iven H. Methylation of 6-mercaptopurine and 6-thioguanine by thiopurine S-methyltransferase. A comparison of activity in red blood cell samples of 199 blood donors. Eur J Clin Pharmacol. 2000;56:343-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 75. | Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol. 2011;17:4166-4173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 76. | Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, Seidman EG. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 727] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 77. | Hübener S, Oo YH, Than NN, Hübener P, Weiler-Normann C, Lohse AW, Schramm C. Efficacy of 6-Mercaptopurine as Second-Line Treatment for Patients With Autoimmune Hepatitis and Azathioprine Intolerance. Clin Gastroenterol Hepatol. 2016;14:445-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (2)] |
| 78. | Yarze JC, Herlihy KH, Fritz HP, Poulos AM. Azathioprine and 6-mercaptopurine and autoimmune hepatitis. Gastroenterology. 1996;110:2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 79. | Yip JS, Woodward M, Abreu MT, Sparrow MP. How are Azathioprine and 6-mercaptopurine dosed by gastroenterologists? Results of a survey of clinical practice. Inflamm Bowel Dis. 2008;14:514-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Shaye OA, Yadegari M, Abreu MT, Poordad F, Simon K, Martin P, Papadakis KA, Ippoliti A, Vasiliauskas E, Tran TT. Hepatotoxicity of 6-mercaptopurine (6-MP) and Azathioprine (AZA) in adult IBD patients. Am J Gastroenterol. 2007;102:2488-2494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Legué C, Legros L, Kammerer-Jacquet S, Jézequel C, Houssel-Debry P, Uguen T, Le Lan C, Guillygomarc'h A, Moirand R, Turlin B, Guyader D, Bardou-Jacquet E. Safety and Efficacy of 6-thioguanine as a Second-line Treatment for Autoimmune Hepatitis. Clin Gastroenterol Hepatol. 2018;16:290-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 82. | van den Brand FF, van Nieuwkerk CMJ, Verwer BJ, de Boer YS, de Boer NKH, Mulder CJJ, Bloemena E, Bakker CM, Vrolijk JM, Drenth JPH, Tan ACITL, Ter Borg F, Ter Borg MJ, van den Hazel SJ, Inderson A, Tushuizen ME, Bouma G. Biochemical efficacy of tioguanine in autoimmune hepatitis: a retrospective review of practice in the Netherlands. Aliment Pharmacol Ther. 2018;48:761-767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 83. | Kaps-Kopiec D, Czajkowska A, Górska M, Woźniak M, Jarzębicka D, Cielecka-Kuszyk J, Czubkowski P, Pawłowska J. The relationship between 6-thioguanine levels and remission outcomes in children with autoimmune hepatitis. Single center experience. Clin Exp Hepatol. 2023;9:115-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 84. | Deswal S, Srivastava A. Role of Allopurinol in Optimizing Thiopurine Therapy in Patients with Autoimmune Hepatitis: A Review. J Clin Exp Hepatol. 2017;7:55-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 85. | Bolia R, Rajanayagam J, Hardikar W. Lower 6-MMP/6-TG Ratio May Be a Therapeutic Target in Pediatric Autoimmune Hepatitis. J Pediatr Gastroenterol Nutr. 2018;67:695-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Fan JH, Liu GF, Lv XD, Zeng RZ, Zhan LL, Lv XP. Pathogenesis of autoimmune hepatitis. World J Hepatol. 2021;13:879-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Cerny T, Borisch B, Introna M, Johnson P, Rose AL. Mechanism of action of rituximab. Anticancer Drugs. 2002;13 Suppl 2:S3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 88. | Burak KW, Swain MG, Santodomingo-Garzon T, Lee SS, Urbanski SJ, Aspinall AI, Coffin CS, Myers RP. Rituximab for the treatment of patients with autoimmune hepatitis who are refractory or intolerant to standard therapy. Can J Gastroenterol. 2013;27:273-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 89. | Than NN, Hodson J, Schmidt-Martin D, Taubert R, Wawman RE, Botter M, Gautam N, Bock K, Jones R, Appanna GD, Godkin A, Montano-Loza AJ, Lammert F, Schramm C, Manns MP, Swain M, Burak KW, Adams DH, Hirschfield GM, Oo YH. Efficacy of rituximab in difficult-to-manage autoimmune hepatitis: Results from the International Autoimmune Hepatitis Group. JHEP Rep. 2019;1:437-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 90. | Riveiro-Barciela M, Barreira-Díaz A, Esteban P, Rota R, Álvarez-Navascúes C, Pérez-Medrano I, Mateos B, Gómez E, De-la-Cruz G, Ferre-Aracil C, Horta D, Díaz-González Á, Ampuero J, Díaz-Fontenla F, Salcedo M, Ruiz-Cobo JC, Londoño MC. Rituximab is a safe and effective alternative treatment for patients with autoimmune hepatitis: Results from the ColHai registry. Liver Int. 2024;44:2303-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 91. | Augustine JJ, Bodziak KA, Hricik DE. Use of sirolimus in solid organ transplantation. Drugs. 2007;67:369-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 171] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Chatrath H, Allen L, Boyer TD. Use of sirolimus in the treatment of refractory autoimmune hepatitis. Am J Med. 2014;127:1128-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (1)] |
| 93. | Guo Y, Lu N, Bai A. Clinical use and mechanisms of infliximab treatment on inflammatory bowel disease: a recent update. Biomed Res Int. 2013;2013:581631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Efe C, Lytvyak E, Eşkazan T, Liberal R, Androutsakos T, Turan Gökçe D, Terziroli Beretta-Piccoli B, Janik M, Bernsmeier C, Arvaniti P, Milkiewicz P, Batibay E, Yüksekyayla O, Ergenç I, Arikan Ç, Stättermayer AF, Barutçu S, Cengiz M, Gül Ö, Heurgue A, Heneghan MA, Verma S, Purnak T, Törüner M, Akdogan Kayhan M, Hatemi I, Zachou K, Macedo G, Drenth JPH, Björnsson E, Montano-Loza AJ, Wahlin S, Higuera-de la Tijera F. Efficacy and safety of infliximab in patients with autoimmune hepatitis. Hepatology. 2025;81:1660-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 95. | Jenkins A, Austin A, Hughes K, Sadowski B, Torres D. Infliximab-induced autoimmune hepatitis. BMJ Case Rep. 2021;14:e239944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 96. | Wong F, Al Ibrahim B, Walsh J, Qumosani K. Infliximab-induced autoimmune hepatitis requiring liver transplantation. Clin Case Rep. 2019;7:2135-2139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 97. | Weiler-Normann C, Schramm C, Quaas A, Wiegard C, Glaubke C, Pannicke N, Möller S, Lohse AW. Infliximab as a rescue treatment in difficult-to-treat autoimmune hepatitis. J Hepatol. 2013;58:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 13.1] [Reference Citation Analysis (1)] |
| 98. | Hui-Yuen JS, Li XQ, Askanase AD. Belimumab in systemic lupus erythematosus: a perspective review. Ther Adv Musculoskelet Dis. 2015;7:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Arvaniti P, Giannoulis G, Gabeta S, Zachou K, Koukoulis GK, Dalekos GN. Belimumab is a promising third-line treatment option for refractory autoimmune hepatitis. JHEP Rep. 2020;2:100123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 100. | Halliday N, Dyson JK, Thorburn D, Lohse AW, Heneghan MA. Review article: experimental therapies in autoimmune hepatitis. Aliment Pharmacol Ther. 2020;52:1134-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Liu E, Aslam N, Nigam G, Limdi JK. Tofacitinib and newer JAK inhibitors in inflammatory bowel disease-where we are and where we are going. Drugs Context. 2022;11:2021-11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 102. | Wang H, Feng X, Han P, Lei Y, Xia Y, Tian D, Yan W. The JAK inhibitor tofacitinib ameliorates immune-mediated liver injury in mice. Mol Med Rep. 2019;20:4883-4892. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 103. | Wang M, Reynolds KL, Montazeri K, Schaefer EA, Sullivan RJ, Dougan M. Tofacitinib is Effective in Treating Refractory Immune Checkpoint Inhibitor Hepatitis. Clin Gastroenterol Hepatol. 2024;22:1539-1541.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 104. | Gökçe DT, Arı D, Kayhan MA, Efe C. A Case of Difficult-To-Treat Autoimmune Hepatitis Successfully Managed by Tofacitinib. Liver Int. 2025;45:e16173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 105. | Ruiz-Cobo JC, Ruiz-Ortega L, Robles-Alonso V, Riveiro-Barciela M. JAK Inhibitors for Autoimmune Hepatitis: One Swallow Does Not Make a Summer. Liver Int. 2025;45:e16223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 106. | Aroniadis OC, Brandt LJ. Intestinal microbiota and the efficacy of fecal microbiota transplantation in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2014;10:230-237. [PubMed] |
| 107. | Liang M, Liwen Z, Jianguo S, Juan D, Fei D, Yin Z, Changping W, Jianping C. Fecal Microbiota Transplantation Controls Progression of Experimental Autoimmune Hepatitis in Mice by Modulating the TFR/TFH Immune Imbalance and Intestinal Microbiota Composition. Front Immunol. 2021;12:728723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 108. | Ma L, Song J, Chen X, Dai D, Chen J, Zhang L. Fecal microbiota transplantation regulates TFH/TFR cell imbalance via TLR/MyD88 pathway in experimental autoimmune hepatitis. Heliyon. 2023;9:e20591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 109. | Longhi MS, Mieli-Vergani G, Vergani D. Regulatory T cells in autoimmune hepatitis: an updated overview. J Autoimmun. 2021;119:102619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 110. | Oo YH, Ackrill S, Cole R, Jenkins L, Anderson P, Jeffery HC, Jones N, Jeffery LE, Lutz P, Wawman RE, Athwal AK, Thompson J, Gray J, Guo K, Barton D, Hirschfield GM, Wong T, Guest P, Adams DH. Liver homing of clinical grade Tregs after therapeutic infusion in patients with autoimmune hepatitis. JHEP Rep. 2019;1:286-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 111. | Oo YH, Sakaguchi S. Regulatory T-cell directed therapies in liver diseases. J Hepatol. 2013;59:1127-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 112. | Buitrago-Molina LE, Pietrek J, Noyan F, Schlue J, Manns MP, Wedemeyer H, Hardtke-Wolenski M, Jaeckel E. Treg-specific IL-2 therapy can reestablish intrahepatic immune regulation in autoimmune hepatitis. J Autoimmun. 2021;117:102591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 113. | Graßhoff H, Comdühr S, Monne LR, Müller A, Lamprecht P, Riemekasten G, Humrich JY. Low-Dose IL-2 Therapy in Autoimmune and Rheumatic Diseases. Front Immunol. 2021;12:648408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 114. | Zhang R, Zhao Y, Chen X, Zhuang Z, Li X, Shen E. Low-dose IL-2 therapy in autoimmune diseases: An update review. Int Rev Immunol. 2024;43:113-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 115. | Lim TY, Martinez-Llordella M, Kodela E, Gray E, Heneghan MA, Sanchez-Fueyo A. Low-Dose Interleukin-2 for Refractory Autoimmune Hepatitis. Hepatology. 2018;68:1649-1652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 116. | Hsu CL, Duan Y, Fouts DE, Schnabl B. Intestinal virome and therapeutic potential of bacteriophages in liver disease. J Hepatol. 2021;75:1465-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (1)] |
| 117. | Górski A, Jończyk-Matysiak E, Łusiak-Szelachowska M, Weber-Dąbrowska B, Międzybrodzki R, Borysowski J. Therapeutic potential of phages in autoimmune liver diseases. Clin Exp Immunol. 2018;192:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 118. | Międzybrodzki R, Borysowski J, Kłak M, Jończyk-Matysiak E, Obmińska-Mrukowicz B, Suszko-Pawłowska A, Bubak B, Weber-Dąbrowska B, Górski A. In Vivo Studies on the Influence of Bacteriophage Preparations on the Autoimmune Inflammatory Process. Biomed Res Int. 2017;2017:3612015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 119. | Gan L, Feng Y, Du B, Fu H, Tian Z, Xue G, Yan C, Cui X, Zhang R, Cui J, Zhao H, Feng J, Xu Z, Fan Z, Fu T, Du S, Liu S, Zhang Q, Yu Z, Sun Y, Yuan J. Bacteriophage targeting microbiota alleviates non-alcoholic fatty liver disease induced by high alcohol-producing Klebsiella pneumoniae. Nat Commun. 2023;14:3215. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 95] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 120. | Sherman KE, Narkewicz M, Pinto PC. Cyclosporine in the management of corticosteroid-resistant type I autoimmune chronic active hepatitis. J Hepatol. 1994;21:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 121. | Zouridis S, Oo YH, Syn WK. Role of Sirolimus and Rituximab in the Treatment of Autoimmune Hepatitis. ACG Case Rep J. 2024;11:e01414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |