Published online May 27, 2025. doi: 10.4254/wjh.v17.i5.106124
Revised: March 28, 2025
Accepted: April 24, 2025
Published online: May 27, 2025
Processing time: 99 Days and 20.3 Hours
In this study, we are committed to exploring the characteristics of the gut mic
To delineate the gut microbiota traits in individuals with chronic liver ailments (chronic hepatitis B, cirrhosis, HCC), scrutinizes microbiome alterations during the progression of these diseases, and assesses microbiome disparities among various Child-Pugh categories in cirrhosis sufferers.
A cohort of 60 CLD patients from the Third People’s Hospital of Yunnan Province were recruited from February to August 2023, together with 37 healthy counter
Compared to healthy subjects, patients exhibited a reduced presence of Firmicutes and a corresponding decline in butyrate-producing genera. In contrast, an up
The reduced abundance of short-chain fatty acid-producing bacteria in the intestine, alongside the increased abundance of gram-negative bacteria such as Escherichia_Shigella and Parabacteroides, may promote the progression of CLD.
Core Tip: The study emphasized the microbial characteristics of gut microbiome dysbiosis at different stages of chronic liver disease (CLD). In this study, 16SrDNA sequencing was used to detect the gut microbiome characteristics in the CLD population. The reduced abundance of short-chain fatty acid-producing bacteria in the intestine, alongside the increased abundance of gram-negative bacteria such as Escherichia_Shigella and Parabacteroides, may promote the progression of CLD by compromising the integrity of the intestinal mucosal barrier. The results of this study may provide new insights for developing novel diagnostic and therapeutic strategies for CLD.
- Citation: Ma C, Yang J, Fu XN, Luo JY, Liu P, Zeng XL, Li XY, Zhang SL, Zheng S. Microbial characteristics of gut microbiome dysbiosis in patients with chronic liver disease. World J Hepatol 2025; 17(5): 106124
- URL: https://www.wjgnet.com/1948-5182/full/v17/i5/106124.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i5.106124
The complex intestinal ecosystem is crucial in sustaining health and catalyzing disease progression. Research confirms that the human gut microbiota (GM) includes approximately 1000 bacterial species[1], whose collective genomic content is 150-fold greater than that of the human genome[2,3]. The GM is are indispensable for the synthesis of nutrients, metabolism of materials, development of the immune system, and establishment of a biological barrier. The intestinal barrier, being a dynamic structure, requires constant stability to thwart the intrusion of detrimental substances and pathogens into the body’s internal environment. Recent studies have shown that changes in the abundance of bacterial phyla or the emergence of new bacterial groups can disrupt this balance, negatively affecting the integrity of the intestinal barrier[4]. Furthermore, extensive studies have established a connection between the dysbiosis of GM and the heightened intestinal permeability, which contribute to the development of various chronic liver diseases (CLDs)[5,6]. The com
Despite ongoing efforts by countries to eliminate viral hepatitis, Nonetheless, viral hepatitis is still a global public health concern that causes thousands of deaths due to acute and chronic infection, cirrhosis, and liver cancer[7]. In addition to viral hepatitis, with improvements in living standards, it is currently estimated that NAFLD affects approximately 30% of the adult population worldwide, making it the most prevalent CLD in Asia[8]. Regarding chronic hepatitis B (CHB), GM dysbiosis has been reported in infected individuals. A study by Shen et al[9], which utilized metagenomics and metabolomics, revealed significant alterations in the GM and metabolites of patients with hepatitis B virus (HBV)-related CLD, where disease progression and antiviral treatment were the primary factors driving these changes. Peripheral immunity may serve as an intermediary link between GM and the pathogenesis of CHB. Similarly, Ren et al[10] demonstrated that long-term antiviral treatment combined with fecal microbiota transplantation could reconstruct the GM, leading to HBV e-antigen clearance, a reduction in viral load, and improved liver function in HBV e-antigen-positive patients. Recent studies on NAFLD and GM have also emphasized the role of dysbiosis in triggering and sustaining intestinal inflammation in NAFLD patients. Various metabolites, including short-chain fatty acids (SCFAs), bile acids, lipopolysaccharides, and trimethylamine N-oxide, are believed to contribute to the pathogenesis of NAFLD. Additionally, the potential of microbiota-related ecological therapies for NAFLD treatment has been highlighted[11]. However, despite numerous studies describing the GM in various liver diseases, the characteristic genera at different stages of CLD [such as CHB, cirrhosis, and hepatocellular carcinoma (HCC)] remain to be explored.
In this study, by analyzing the GM of patients with CLD and healthy controls, we compared the microbiota characteristics between the two groups and examined the GM structure of CLD patients across the stages of “CHB-cirrhosis-HCC”. This study delves deeper into the GM of cirrhosis patients across different Child-Pugh classifications, with the objective of identifying specific bacterial genera that emerge during the progression of the disease, thereby enhancing clinical diagnosis and management strategies for chronic liver conditions.
This study comprised 60 subjects diagnosed with CLD, including 16 with CHB, 37 with cirrhosis, and 7 with HCC, who were evaluated at the Third People’s Hospital of Yunnan Province between February and August 2023, alongside 37 healthy volunteers. Within the cohort of individuals with cirrhosis, 22 were classified under Child-Pugh A, 9 under Child-Pugh B, and 6 under Child-Pugh C. All experiments were conducted in accordance with the relevant guidelines and regulations, and informed consent has been obtained from all participants or their legal guardians.
Inclusion criteria: (1) Participants with confirmed diagnoses of CLD, adhering to established guidelines: CHB per the “Guidelines for the Prevention and Treatment of CHB (2022 Edition)” by the Hepatology Branch of the Chinese Medical Association; cirrhosis according to the “Guidelines for the Diagnosis and Treatment of Cirrhosis (2019 Edition)”; HCC following the “Guidelines for the Diagnosis and Treatment of Primary Liver Cancer (2020 Edition)”; (2) Healthy volunteers demonstrating normal routine laboratory tests and imaging results during the research period, in overall good health; (3) Age range between 18 to 85 years; (4) Long-term residents (a minimum of ten years) in the study locale; (5) No consumption of medications that alter intestinal flora, such as antibiotics or probiotics, within one month before entering the study; and (6) Willing participation and documented informed consent. Exclusion criteria: (1) Pregnant or breastfeeding individuals; (2) Subjects with coexisting conditions likely to impact intestinal flora, including but not limited to diabetes, hypertension, inflammatory bowel disease, kidney disorders, mental health issues, or other malignancies unrelated to primary liver cancer; (3) Those opting out of the study or declining continuation for any reason; and (4) Those who have received radiotherapy and chemotherapy or have been judged unsuitable for inclusion by the research team.
Collect fresh fecal samples from participants within two hours of defecation to prevent contamination by urine or water. Utilize sterile containers to gather the central portion of the stool, ensuring the collected amount exceeds 200 mg. Securely package and seal the samples, storing them at -80 °C in liquid nitrogen until analysis. Transport all samples using dry ice for centralized testing.
Isolate genomic DNA from collected stool samples and perform PCR amplification targeting the 16S V3-V4 regions with specific primers (forward primer: CCTACGGGNGGCWGCAG, reverse primer: GACTACHVGGGTATCTAATCC). Confirm amplification results using 2% agarose gel electrophoresis and purify the amplicons utilizing the AMPure XT beads cleanup kit. Proceed with quality checks, then sequence on the NovaSeq 6000 system using the NovaSeq 6000 SP Reagent Kit (500 cycles) for 2 × 250 bp paired-end sequencing. Conduct sequence assembly, filtering, and noise reduction with DADA2, then cluster into operational taxonomic units for taxonomic classification against the NT-16S database. Examine both alpha and beta diversity to discern and compare microbial community structures across different groups.
Select statistical tests suitable for the sample attributes: Begin with Shapiro-Wilk tests to evaluate data normality. For normally distributed data, utilize t-tests or analysis of variance to compare between groups. Utilize Mann-Whitney U tests or Kruskal-Wallis tests for datasets that do not follow a normal distribution. P < 0.05 is often interpreted as evidence of statistical significance. Conduct all statistical analyses utilizing SPSS software (version 25.0).
No notable variations were detected in gender, alanine aminotransferase, and serum creatinine levels when comparing the healthy controls to the patients with CLD. Nonetheless, notable differences were observed regarding age, aspartate aminotransferase, total bilirubin, albumin, white blood cell count, platelet count, prothrombin time, and activated partial thromboplastin time (Table 1). In the text, ZC denotes the healthy control group, HZ represents the CHB group, LC refers to the liver cirrhosis (LC) group, and HCC indicates the HCC group.
| Characteristics | Category (number of subjects) | P value | |||
| Number of subjects | HZ (n = 16) | LC (n = 37) | HCC (n = 7) | ZC (n = 37) | - |
| Gender (male/female) | 9/7 | 21/16 | 5/2 | 23/14 | 0.625 |
| Age (years), mean ± SD | 59.5 ± 12.6 | 65.1 ± 12.3 | 55.4 ± 20.5 | 60.8 ± 7.7 | 0.033a |
| ALT | 16.9 (34.8) | 18.7 (16.1) | 25.1 (61.2) | 20.1 (15.7) | 0.211 |
| AST | 28.8 (74.9) | 39.1 (36.9) | 65.4 (52.9) | 18.0 (5.8) | 0.000b |
| TBil | 13.4 (10.3) | 24.1 (50.4) | 36.9 (185.4) | 11.4 (5.7) | 0.000c |
| Albumin | 44.1 (6.8) | 33.3 (7.6) | 32.6 (9.0) | 47.3 (4.9) | 0.000d |
| Scr | 70.0 (16.5) | 74.0 (42.5) | 62.0 (178.0) | 72.0 (25.5) | 0.382 |
| WBC | 4.3 (2.0) | 3.8 (2.2) | 5.7 (2.2) | 6.4 (2.7) | 0.000e |
| PLT | 201.5 (74.0) | 103.0 (81.5) | 78.0 (115.0) | 230.0 (56.0) | 0.000f |
| PT | 11.2 (1.0) | 13.4 (3.6) | 13.0 (5.3) | 10.4 (0.8) | 0.000g |
| APTT | 27.4 (4.7) | 30.2 (4.9) | 31.3 (10.1) | 26.8 (2.3) | 0.000h |
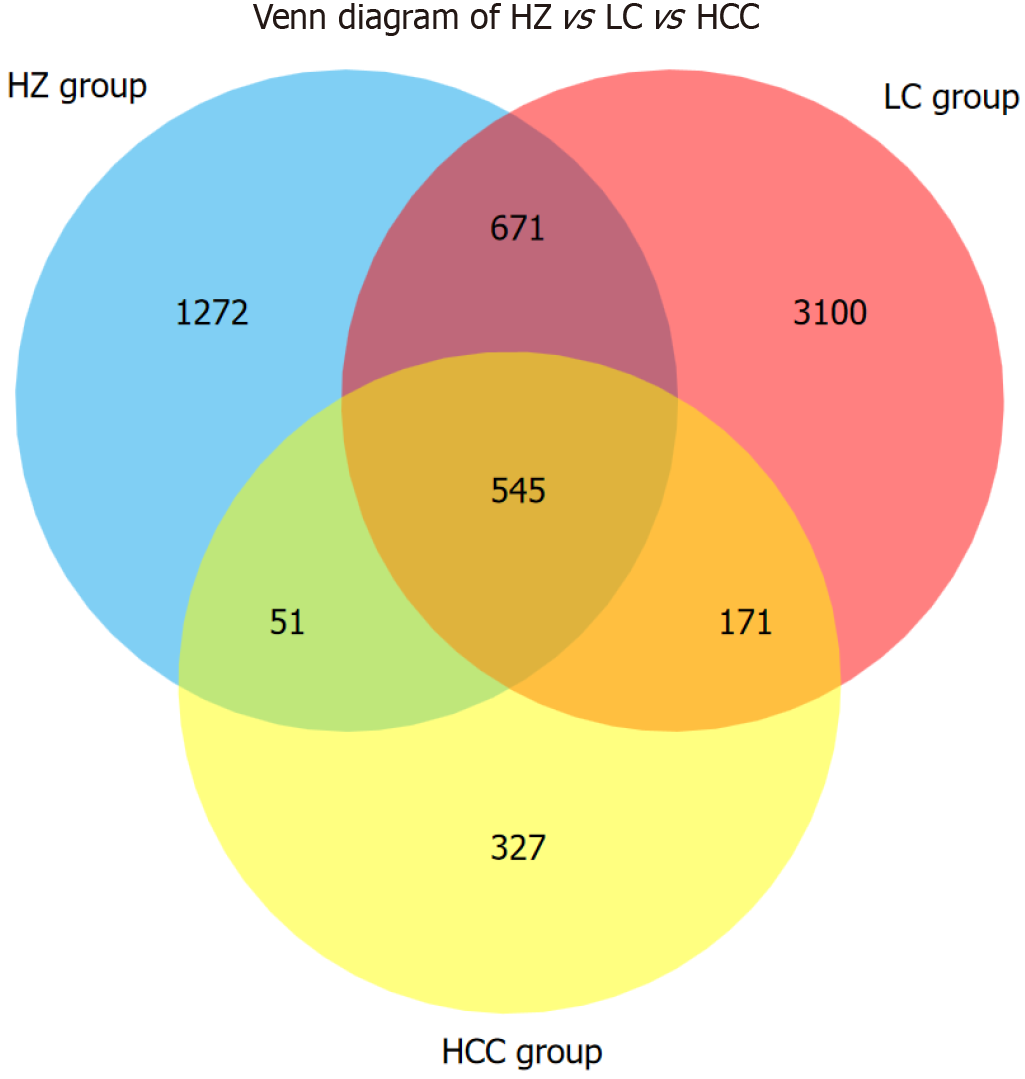
Venn diagram analysis of 6137 amplicon sequence variants (ASVs) found that 545 ASVs were common across the three disease groups; 1216 ASVs were shared between the HZ and LC groups; 596 ASVs were shared between the HZ and HCC groups; and 716 ASVs were shared between the LC and HCC groups. Unique ASVs numbered 1272 for the HZ group, 3100 for the LC group, and 327 for the HCC group (Figure 1).
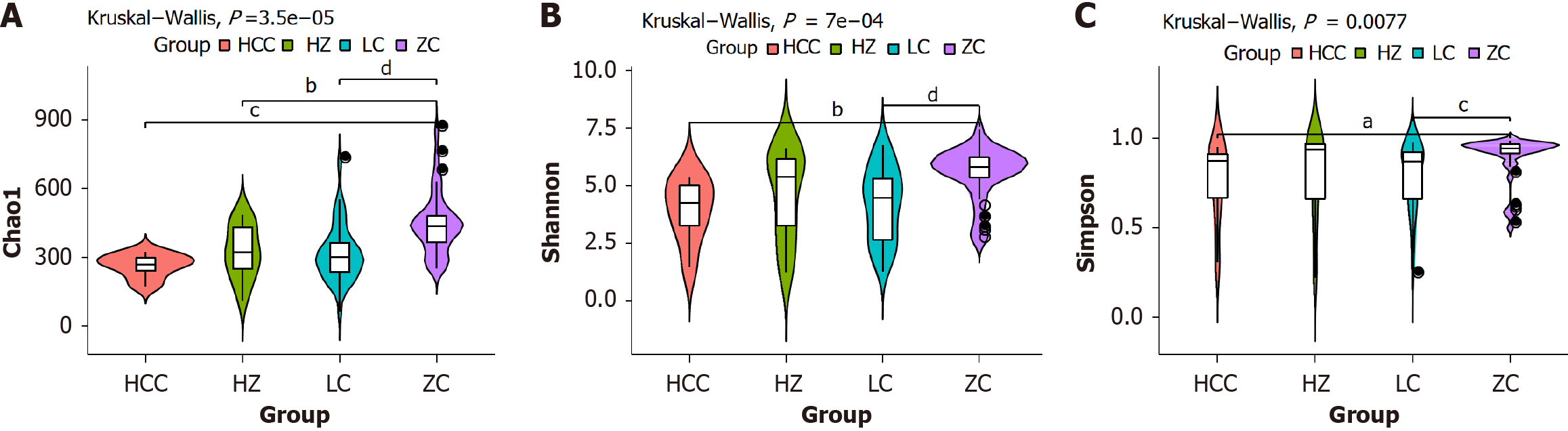
The analysis of the Chao1 index between the CHB cohort and the healthy control cohort demonstrated a statistically significant difference (P < 0.05). In contrast, the Shannon and Simpson indices did not exhibit any significant differences
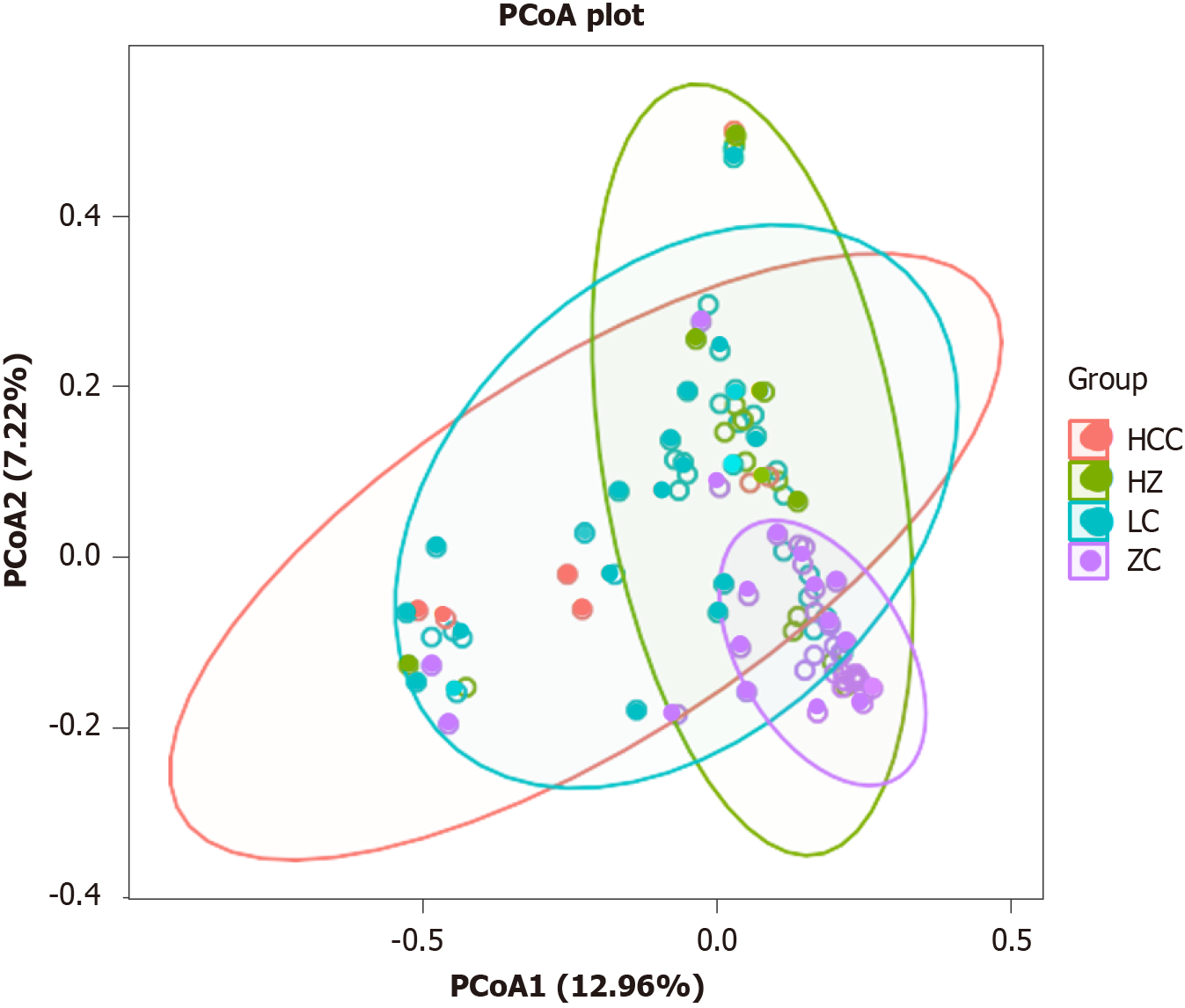
To elucidate the spatial distribution of microbial communities among samples, we employed an analysis based on the Bray-Curtis distance matrix. The principal coordinates analysis (PCoA) showed a symmetric distribution across all samples. The PCoA plot (PCoA1 + PCoA2 = 20.18%) illustrated that the microbial community structures of the CHB group, LC group, and HCC group were similar to each other but markedly deviated from the healthy control group, with this difference reaching statistical significance (t = 0.245618, P = 0.001) (Figure 3).
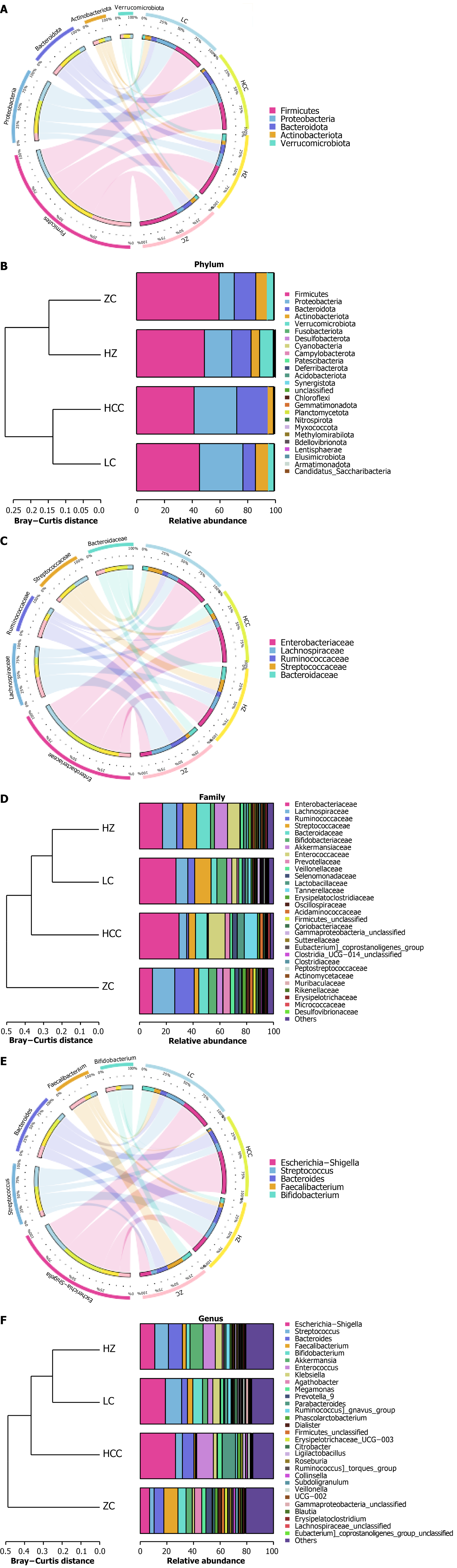
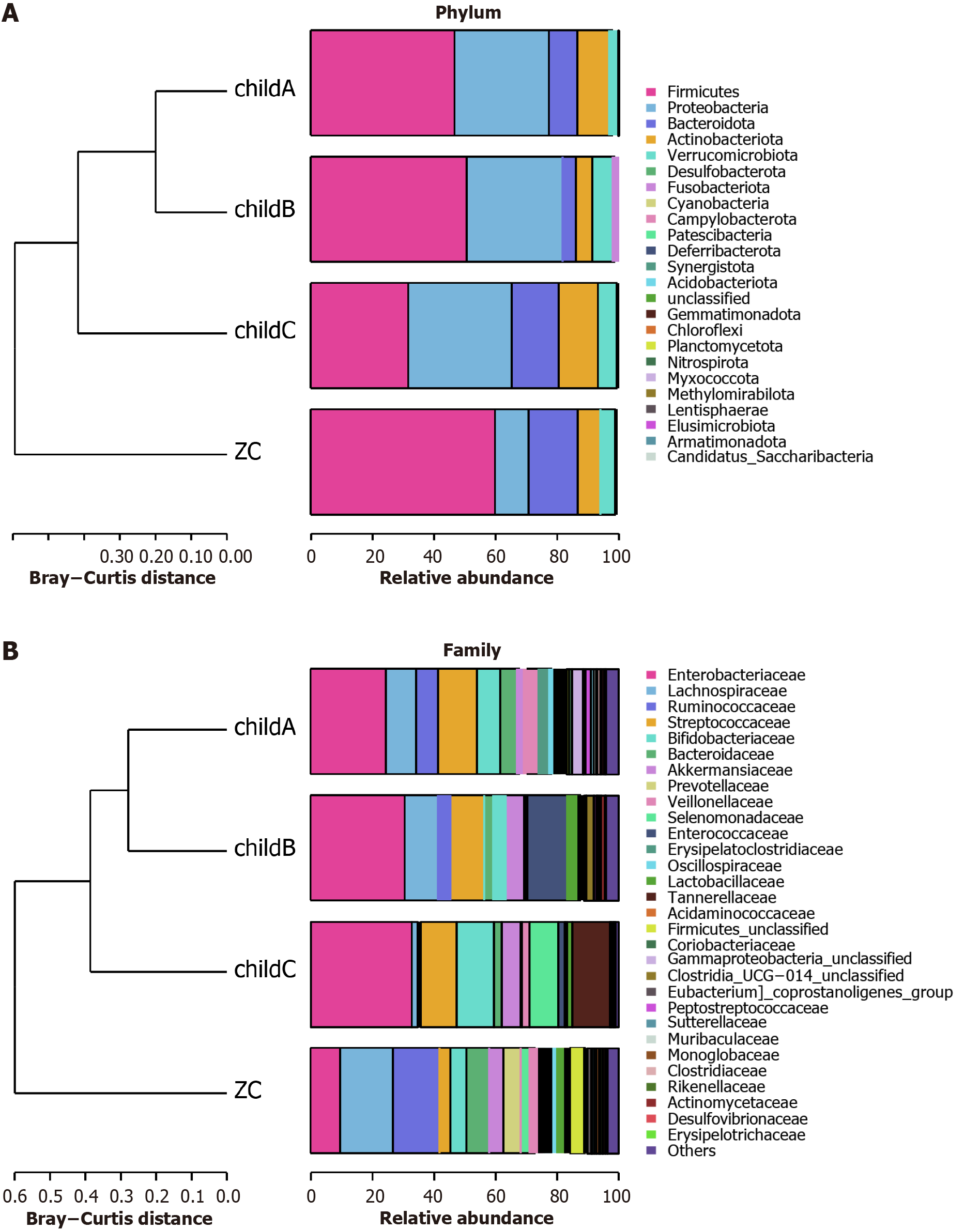
Analysis of species abundance of intestinal microflora at phylum, family and genus level in healthy control group and CLD group: At the phylum level, the major groups identified within the healthy control, CHB, LC, and HCC cohorts comprised Firmicutes, Proteobacteria, Bacteroidota, Actinobacteriota, and Verrucomicrobiota (Figure 4A). There was a notable decrease in the representation of Firmicutes across the CHB, LC, and HCC cohorts (Figure 4B), with respective proportions recorded at 59.48%, 48.94%, 45.36%, and 41.49%. At various stages of the “CHB-cirrhosis-HCC” progression, there was a notable decrease in the population of Firmicutes. In contrast, there was a significant elevation of Proteobacteria in these groups compared to the healthy controls (Figure 4A and B), rising from 11.00% in the healthy cohort to 19.68%, 31.27%, and 30.76% in the various disease states, respectively.
At the family level, the most commonly observed genera included Enterobacteriaceae, Lachnospiraceae, Ruminococcaceae, Streptococcaceae, and Bacteroidaceae (Figure 4C). In comparison to the controls, there was a decline in Lachnospiraceae and Ruminococcaceae across the disease spectra, while Enterobacteriaceae, Streptococcaceae, and Enterococcaceae saw increases (Figure 4D). Notably, the percentage of Enterobacteriaceae rose from 9.65% in the control group to 17.16%, 27.21%, and 29.50% in the respective disease groups. Lachnospiraceae percentages decreased from 16.69% in the control group to 10.57%, 8.88%, and 5.72% respectively. This illustrates a continual increase in Enterobacteriaceae and a decrease in Lachnospiraceae as the diseases progressed (Figure 4C and D).
Among these groups, the five dominant genera also included Escherichia_Shigella, Streptococcus, Bacteroides, Faecalibacterium, and Bifidobacterium (Figure 4E). There was a significant rise in Escherichia_Shigella, Streptococcus, Klebsiella, and Enterococcus within the disease cohorts compared to the healthy controls, while Faecalibacterium and Agathobacter saw notable reductions. Moreover, at various stages of the “CHB-cirrhosis-HCC” progression, the abundances of Escherichia_ Shigella and Parabacteroides continuously increased (Figure 4F). The proportions of Escherichia_Shigella were 7.02%, 10.95%, 18.92%, and 26.52% in the healthy control group, CHB group, cirrhosis group, and HCC group, respectively. The pro
Analysis of species abundance at different Child-Pugh family levels in patients with LC: At the phylum and family levels, Proteobacteria saw a rise in the Child-Pugh A, Child-Pugh B, and Child-Pugh C groups compared to the healthy control. As liver function worsened, a corresponding increase in Proteobacteria was noted (Figure 5A), with figures of 30.65%, 31.15%, and 33.67% respectively. At the family level, the presence of Ruminococcaceae decreased as liver function declined, recording 7.22%, 3.13%, and 1.07% in the Child-Pugh groups, substantially lower than the 15.13% in the control group. A similar downward trend was seen for Bacteroidaceae, with proportions of 7.41% in the healthy control and 5.37%, 3.14%, and 2.53% in the Child-Pugh groups respectively. In contrast, the presence of Enterobacteriaceae progressively increased, registering 24.47%, 30.62%, and 32.76% in the Child-Pugh groups, higher than the 9.56% in the control (Figure 5B).
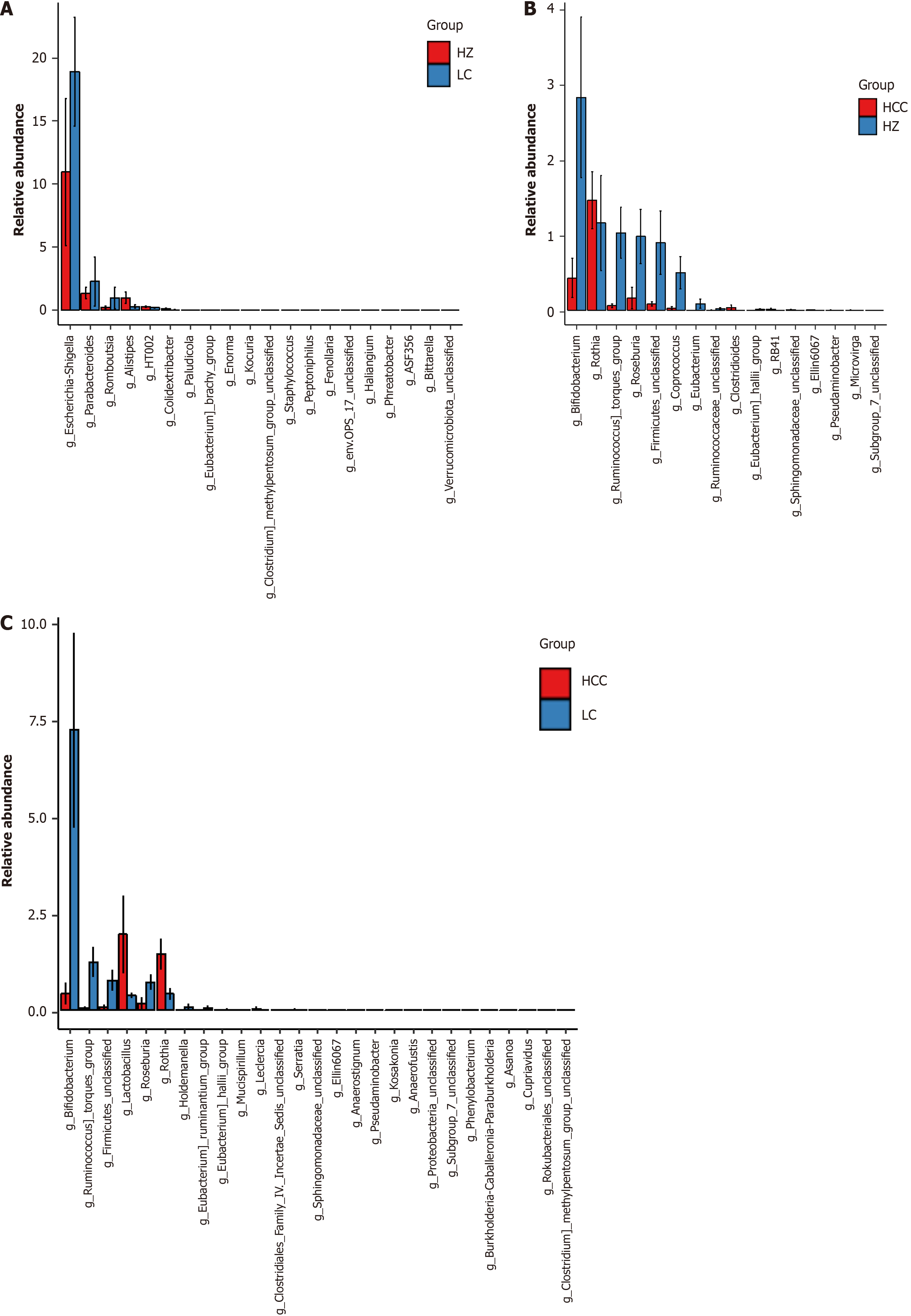
Analysis of different species of intestinal microflora in different groups of CLD: By employing the average relative abundance of species, the Mann-Whitney U test was utilized to examine GM data from individuals diagnosed with CHB, LC, and HCC, pinpointing genera with P values < 0.05 as significantly modified. In contrast to the CHB cohort, the LC cohort exhibited elevated levels of Escherichia_Shigella, Parabacteroides, and Romboutsia, with P values recorded at 0.04, 0.04, and 0.02 respectively. Conversely, Alistipes presented a reduction, indicated by a P value of 0.01 (Figure 6A). Meanwhile, the HCC group exhibited an elevated presence of Rothia, marked by a P value of 0.04, and decreased levels of Bifidobacterium, Ruminococcus_torques_group, Roseburia, and Coprococcus, with P values of 0.03, 0.03, 0.04, and 0.01 respectively (Figure 6B). The HCC group showed reduced levels of Bifidobacterium, Ruminococcus_torques_group, and Roseburia, with P values of 0.04, 0.01, and 0.02 respectively, and increased levels of Lactobacillus and Rothia, at P values of 0.03 and 0.01 respectively (Figure 6C).
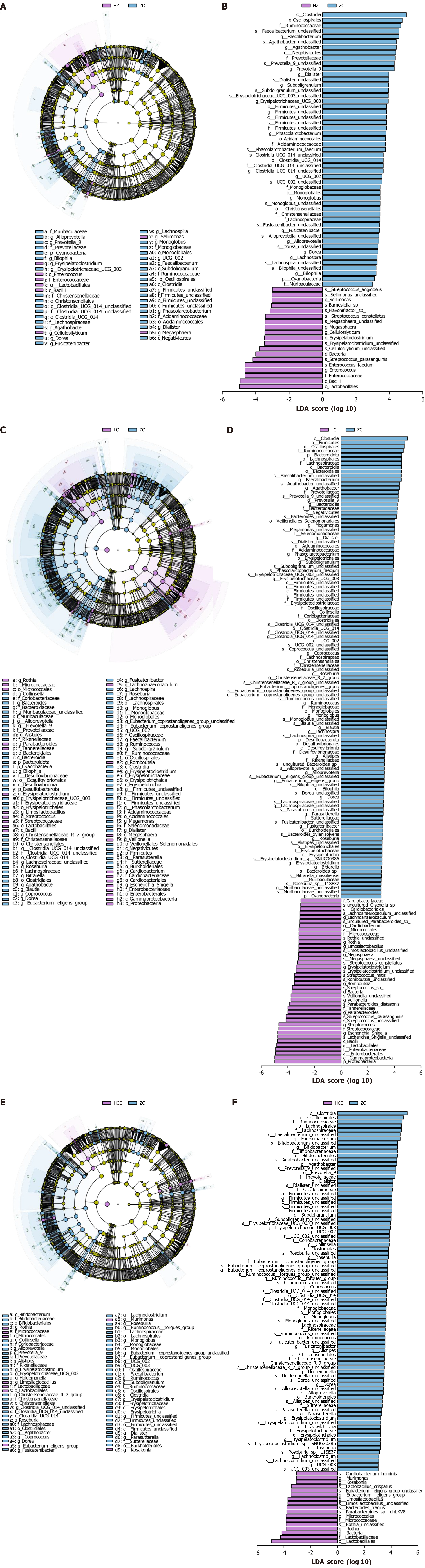
Analysis of dominant flora in CLD group and healthy control group: The linear discriminant analysis effect size method was utilized to detect variations in species composition across all taxonomic ranks. This analysis identified distinct species differences between each CLD group and the healthy control, naming these differential species as dominant genera, which may act as potential biomarkers. Notable variations in GM composition at the genus level were observed among the CLD groups. In the CHB cohort, 19 distinct taxonomic groups were noted, with Enterococcus, Erysipelatoclostridium, Cellulosilyticum, Megasphaera, and Sellimonas being the most predominant (Figure 7A and B). The LC group displayed 40 significant taxonomic differences, with Escherichia_Shigella, Streptococcus, Parabacteroides, Veillonella, and Romboutsia ranking highest (Figure 7C and D). In the HCC cohort, 17 distinct groups were identified, prominently featuring Rothia, Limosilactobacillus, Eubacterium__eligens_group, Kosakonia, and Murimonas (Figure 7E and F).
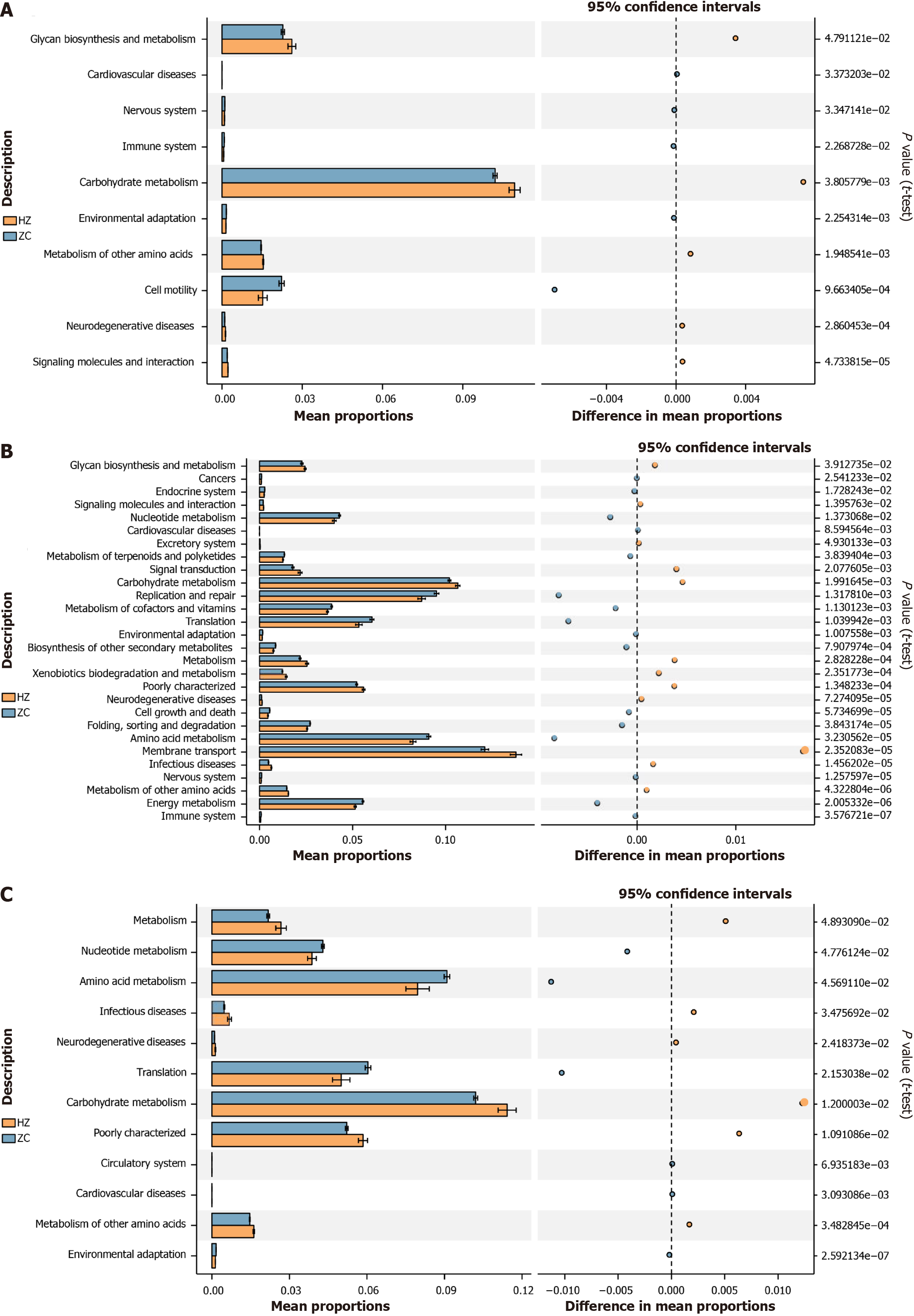
The analysis identified significant disparities between the CHB and healthy control groups in functional genes involved in glycan biosynthesis and metabolism (P = 0.05), carbohydrate metabolism (P < 0.001), metabolism of other amino acids (P < 0.001), and cell motility (P < 0.001). It was also noted that carbohydrate metabolism genes were predominantly abundant in both groups. Specifically, the CHB group exhibited elevated gene content for glycan biosynthesis and metabolism, carbohydrate metabolism, and metabolism of other amino acids (Figure 8A).
Regarding the cirrhosis group relative to the healthy control, significant variances were observed in membrane transport (P < 0.001), carbohydrate metabolism (P < 0.001), replication and repair (P < 0.001), amino acid metabolism (P < 0.001), translation (P < 0.001), energy metabolism (P < 0.001), nucleotide metabolism (P = 0.01), metabolism of cofactors and vitamins (P < 0.001), and glycan biosynthesis and metabolism (P = 0.04). Predictions indicated higher gene content in the cirrhosis group for membrane transport, carbohydrate metabolism, and glycan biosynthesis and metabolism (Figure 8B).
In the HCC group, notable differences were found in metabolism (P = 0.05), nucleotide metabolism (P = 0.05), amino acid metabolism (P = 0.05), translation (P = 0.02), carbohydrate metabolism (P = 0.01), and metabolism of other amino acids (P < 0.001). carbohydrate metabolism displayed the highest gene content in both the HCC and healthy control groups, with more elevated levels in the former (Figure 8C).
Under typical conditions, the intestinal microbiota provides crucial benefits such as supporting the immune system, hindering pathogenic colonization, and enhancing nutrient digestion and absorption[12]. Recent developments in metagenomics and metabolomics have underscored[13,14] how intestinal barrier dysfunction and bacterial translocation critically shape the inflammatory microenvironment that propels liver disease toward cirrhosis and HCC. An imbalance in intestinal microecology detrimentally affects anti-tumor immune surveillance and accelerates liver disease progression toward HCC. In cirrhosis patients, increased bacterial load in liver tissue can initiate the fibrosis-inflammation pathway and facilitate the immunosuppressive cycle of cancer[14,15].
Comparative analyses employing the Shannon and Simpson indices revealed notable decreases in both the richness and diversity of the GM among the groups affected by CHB, cirrhosis, and HCC, all while maintaining consistent sample detection conditions. The species abundance data revealed diminished levels of Firmicutes in these groups relative to the healthy controls, with Lachnospiraceae decreasing as the disease progressed from CHB through cirrhosis to HCC. The species abundance plot results indicated that the abundance of Firmicutes was lower in all three CLD groups compared to the healthy control group. Moreover, the abundance of Lachnospiraceae consistently decreased as the disease progressed from “CHB-cirrhosis-HCC”. In addition, more detailed changes were observed at the genus level. The abundances of Escherichia_Shigella and Parabacteroides increased progressively across the stages of “CHB-cirrhosis-HCC”. In contrast, the abundances of Dialister, Roseburia, Subdoligranulum, and Blautia steadily decreased. Notably, Dialister, Roseburia, and Blautia belong to the Firmicutes and are key producers of SCFAs, particularly butyrate, in the human gut. Numerous studies have demonstrated that SCFAs are beneficial for colonic cell function, reducing inflammation, protecting the intestinal barrier, and promoting a balanced microbiome, all of which are essential for maintaining gastrointestinal health[16,17]. Furthermore, studies have shown that SCFAs can influence liver metabolism by acting as signaling molecules, primarily through the activation of adenosine monophosphate-activated protein kinase[18]. This activation induces the expression of peroxisome proliferator-activated receptor γ- stimulated uncoupling protein 2, promoting hepatic autophagy. In rodent models fed a high-fat diet, butyrate supplementation not only reduced the expression of pro-inflammatory factors in the liver and adipose tissue but also suppressed inflammation and promoted changes in the gut microbiome. These changes included an increase in the abundance of SCFA-producing bacteria and a reduction in the abundance of harmful bacteria that release endotoxins[19]. Furthermore, SCFAs can modulate immune responses by activating G-protein-coupled receptors (GPR) on immune cells[20]. The GPR43 receptor on intestinal epithelial cells activates the family pyrin domain containing 3 inflammasome, increasing interleukin-18 production, which is crucial for maintaining epithelial integrity and intestinal homeostasis[21]. Additionally, recent studies[22] have indicated that GPR43 activation can induce apoptosis in cancer cells and inhibit their proliferation, thereby preventing colorectal cancer.
Therefore, the results of this study suggest that intestinal inflammation in CLD patients may be linked to a reduction in SCFAs. Future research, including larger sample sizes and metabolomic approaches, could further investigate changes in SCFA levels and related metabolic pathways in the intestines of CLD patients at various stages of the disease. Ad
Furthermore, the research noted elevated concentrations of Proteobacteria, Enterobacteriaceae, Escherichia_Shigella, Parabacteroides, Streptococcus, Klebsiella, and Enterococcus within the GMs of individuals with liver disease when contrasted with healthy controls. Notably, Enterobacteriaceae, Escherichia_Shigella and Parabacteroides exhibited a steady increase as the disease progressed from “CHB-cirrhosis-HCC”. In patients with cirrhosis categorized by various Child-Pugh grades, a reduction in liver reserve function correlated with elevated levels of Proteobacteria and Enterobacteriaceae, while levels of Ruminococcaceae and Bacteroidaceae were found to be diminished. Recent studies indicate that extended elevation of Proteobacteria could signify gut dysbiosis, potentially acting as a diagnostic marker for dysbiosis and related disease risks[23]. An upsurge in Proteobacteria could exacerbate liver damage as liver reserve capacity diminishes. Notably, as a significant constituent of Proteobacteria, Enterobacteriaceae exhibited a similar trend in increasing prevalence, both throughout the progression of liver diseases and with deteriorating liver function. The prevalence of Enterococcus was significantly elevated in the cohorts with CLD. Research conducted by Duan et al[24] demonstrated that levels of Enterococcus are elevated in patients suffering from alcoholic hepatitis in contrast to non-alcoholic individuals. This bacterium may produce and secrete a bacterial exotoxin referred to as “cytolysin”, which plays a role in the destruction of hepatocytes and subsequent liver damage.
These results emphasize the critical role of Proteobacteria in the onset and progression of CLDs. When examining temporal changes, the abundances of Escherichia_Shigella and Parabacteroides were found to consistently increase during the progression of CLD. However, both Escherichia_Shigella and Parabacteroides are gram-negative bacteria, and current studies have shown that lipopolysaccharide (LPS) serotypes from gram-negative bacteria in the intestine can promote intestinal epithelial dysfunction by altering toll-like receptor 4 signaling[25]. Additionally, some studies suggest that LPS from the gut microbiome may activate caspase-4 and caspase-11, leading to highly inflammatory programmed cell death[26]. Therefore, the increased abundance of gram-negative bacteria such as Escherichia_Shigella and Parabacteroides in the intestine may elevate levels of LPS or other pro-inflammatory substances, thereby increasing systemic inflammation and exacerbating liver injury. This hypothesis warrants further investigation, including an expanded sample size and metabolomic analyses. Furthermore, a study by Yang et al[27] found a positive correlation between the abundance of Escherichia_Shigella and elevated levels of four metabolites: Aflatoxin M1, bile acids, L-histidine, and phenylacetic acid. Escherichia_Shigella may also contribute to intestinal injury and affect amino acid metabolism. Aflatoxin M1, a metabolite of aflatoxin B1, plays a role in liver cancer development. Recent research by Yuan et al[28] also showed that bile acids can promote the nuclear translocation of yes-associated protein, activating hepatic stellate cells and inducing the expression of connective tissue growth factor in hepatocytes, thus exacerbating liver fibrosis. Therefore, the increased abundance of Escherichia_Shigella may lead to the accumulation of these metabolites, further contributing to the progression of CLD.
In comparisons among the three CLD groups, Rothia exhibited significantly increased abundance in the HCC group compared to the CHB and cirrhosis groups. Additionally, Bifidobacterium, (Ruminococcus)_torques_group, and Roseburia showed considerably reduced levels in the HCC group relative to those in the CHB and cirrhosis groups. As previously discussed, Roseburia plays a pivotal role as a butyrate-producing bacterium; its reduced abundance may escalate intestinal inflammation and potentially worsen liver disease[29]. Bifidobacterium is renowned for its probiotic benefits, with its production of acetate esters bolstering intestinal defense through epithelial cell mediation, thus safeguarding against deadly infections[30]. Declines in its population compromise the integrity of the intestinal mucosal barrier, elevating the risk of intestinal infections[31]. Research by Odenwald et al[32] showed that lactulose treatment boosts Bifidobacterium density in the intestine, decreasing systemic infections and mortality rates. It was also noted that Bifidobacterium converts lactulose into high levels of acetate, acidifying the intestinal lumen in humans and mice, thereby inhibiting the proliferation of antibiotic-resistant bacteria. These findings advocate for the inclusion of probiotics in clinical regimes, not only to rectify intestinal dysbiosis and mitigate inflammation but also to potentially enhance antibiotic treatment efficacy. Moreover, a Mendelian randomization study established a correlation between increased levels of the (Ruminococcus)_ torques_group and a decreased risk of developing alcoholic liver disease, suggesting that a reduction in this group may increase the likelihood of HCC, necessitating further research[33].
The PICRUSt analysis revealed that the functional genes in the intestinal microbiome are primarily associated with energy and functional metabolism, which includes biosynthesis, general metabolism, and amino acid metabolism. The CHB and HCC cohorts exhibited elevated levels of functional genes associated with carbohydrate metabolism, whereas the cirrhosis cohort showed a predominance of genes involved in membrane transport. Furthermore, the levels of carbohydrate metabolism genes were elevated in all three CLD groups compared to the healthy control group. Each disease group exhibited unique profiles of enriched functional genes, suggesting these microbial function alterations could influence host metabolism. However, the precise implications of these changes on the host’s pathological conditions remain undetermined by PICRUSt function analysis capabilities.
Furthermore, the limitations of this study include: Firstly, the relatively small sample size, which could introduce statistical biases. Secondly, as a cross-sectional study, it only reflects the intestinal microbiome status of liver disease patients at a specific time point and does not capture the dynamic evolution of the intestinal microbiome. Lastly, the 16SrDNA high-throughput sequencing technology used only reveals microbial characteristics and does not permit in-depth investigation of microbial metabolites, functions, or pathogenic mechanisms.
The reduced abundance of short-chain fatty acid-producing bacteria in the intestine, alongside the increased abundance of gram-negative bacteria such as Escherichia_Shigella and Parabacteroides, may promote the progression of CLD by compromising the integrity of the intestinal mucosal barrier.
| 1. | Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9292] [Cited by in RCA: 8364] [Article Influence: 597.4] [Reference Citation Analysis (4)] |
| 2. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Li S, Jian M, Zhou Y, Li Y, Zhang X, Li S, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 8109] [Article Influence: 506.8] [Reference Citation Analysis (4)] |
| 3. | Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe. 2015;17:852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 458] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 4. | Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med. 2024;19:275-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 613] [Article Influence: 306.5] [Reference Citation Analysis (0)] |
| 5. | Milosevic I, Vujovic A, Barac A, Djelic M, Korac M, Radovanovic Spurnic A, Gmizic I, Stevanovic O, Djordjevic V, Lekic N, Russo E, Amedei A. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int J Mol Sci. 2019;20:395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 393] [Cited by in RCA: 394] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 6. | Xu M, Luo K, Li J, Li Y, Zhang Y, Yuan Z, Xu Q, Wu X. Role of Intestinal Microbes in Chronic Liver Diseases. Int J Mol Sci. 2022;23:12661. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 7. | Cheung A, Kwo P. Viral Hepatitis Other than A, B, and C: Evaluation and Management. Clin Liver Dis. 2020;24:405-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (3)] |
| 8. | Yip TC, Fan JG, Wong VW. China's Fatty Liver Crisis: A Looming Public Health Emergency. Gastroenterology. 2023;165:825-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 9. | Shen Y, Wu SD, Chen Y, Li XY, Zhu Q, Nakayama K, Zhang WQ, Weng CZ, Zhang J, Wang HK, Wu J, Jiang W. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response. Gut Microbes. 2023;15:2155018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 58] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 10. | Ren YD, Ye ZS, Yang LZ, Jin LX, Wei WJ, Deng YY, Chen XX, Xiao CX, Yu XF, Xu HZ, Xu LZ, Tang YN, Zhou F, Wang XL, Chen MY, Chen LG, Hong MZ, Ren JL, Pan JS. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. 2017;65:1765-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 11. | Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T, Kounatidis D, Dalamaga M. Understanding the Role of the Gut Microbiome and Microbial Metabolites in Non-Alcoholic Fatty Liver Disease: Current Evidence and Perspectives. Biomolecules. 2021;12:56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 204] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 12. | Albhaisi SAM, Bajaj JS, Sanyal AJ. Role of gut microbiota in liver disease. Am J Physiol Gastrointest Liver Physiol. 2020;318:G84-G98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 102] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 13. | Liao L, Schneider KM, Galvez EJC, Frissen M, Marschall HU, Su H, Hatting M, Wahlström A, Haybaeck J, Puchas P, Mohs A, Peng J, Bergheim I, Nier A, Hennings J, Reißing J, Zimmermann HW, Longerich T, Strowig T, Liedtke C, Cubero FJ, Trautwein C. Intestinal dysbiosis augments liver disease progression via NLRP3 in a murine model of primary sclerosing cholangitis. Gut. 2019;68:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 14. | Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, Khiabanian H, Adeyemi A, Bataller R, Lefkowitch JH, Bower M, Friedman R, Sartor RB, Rabadan R, Schwabe RF. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 854] [Cited by in RCA: 1077] [Article Influence: 76.9] [Reference Citation Analysis (0)] |
| 15. | Schneider KM, Mohs A, Gui W, Galvez EJC, Candels LS, Hoenicke L, Muthukumarasamy U, Holland CH, Elfers C, Kilic K, Schneider CV, Schierwagen R, Strnad P, Wirtz TH, Marschall HU, Latz E, Lelouvier B, Saez-Rodriguez J, de Vos W, Strowig T, Trebicka J, Trautwein C. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun. 2022;13:3964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 164] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 16. | Geirnaert A, Calatayud M, Grootaert C, Laukens D, Devriese S, Smagghe G, De Vos M, Boon N, Van de Wiele T. Butyrate-producing bacteria supplemented in vitro to Crohn's disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci Rep. 2017;7:11450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 336] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 17. | Deleu S, Machiels K, Raes J, Verbeke K, Vermeire S. Short chain fatty acids and its producing organisms: An overlooked therapy for IBD? EBioMedicine. 2021;66:103293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 490] [Article Influence: 98.0] [Reference Citation Analysis (0)] |
| 18. | Iannucci LF, Sun J, Singh BK, Zhou J, Kaddai VA, Lanni A, Yen PM, Sinha RA. Short chain fatty acids induce UCP2-mediated autophagy in hepatic cells. Biochem Biophys Res Commun. 2016;480:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Zhai S, Qin S, Li L, Zhu L, Zou Z, Wang L. Dietary butyrate suppresses inflammation through modulating gut microbiota in high-fat diet-fed mice. FEMS Microbiol Lett. 2019;366:fnz153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 20. | Li M, van Esch BCAM, Wagenaar GTM, Garssen J, Folkerts G, Henricks PAJ. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur J Pharmacol. 2018;831:52-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 453] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 21. | Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun. 2015;6:6734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 1059] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 22. | Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. Int J Cancer. 2011;128:847-856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496-503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1476] [Cited by in RCA: 2655] [Article Influence: 241.4] [Reference Citation Analysis (0)] |
| 24. | Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, White RC, Clarke TH, Nguyen K, Torralba M, Shao Y, Liu J, Hernandez-Morales A, Lessor L, Rahman IR, Miyamoto Y, Ly M, Gao B, Sun W, Kiesel R, Hutmacher F, Lee S, Ventura-Cots M, Bosques-Padilla F, Verna EC, Abraldes JG, Brown RS Jr, Vargas V, Altamirano J, Caballería J, Shawcross DL, Ho SB, Louvet A, Lucey MR, Mathurin P, Garcia-Tsao G, Bataller R, Tu XM, Eckmann L, van der Donk WA, Young R, Lawley TD, Stärkel P, Pride D, Fouts DE, Schnabl B. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 714] [Cited by in RCA: 634] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 25. | Płóciennikowska A, Hromada-Judycka A, Borzęcka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. 2015;72:557-581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 603] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 26. | Matikainen S, Nyman TA, Cypryk W. Function and Regulation of Noncanonical Caspase-4/5/11 Inflammasome. J Immunol. 2020;204:3063-3069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 27. | Yang L, Xiang Z, Zou J, Zhang Y, Ni Y, Yang J. Comprehensive Analysis of the Relationships Between the Gut Microbiota and Fecal Metabolome in Individuals With Primary Sjogren's Syndrome by 16S rRNA Sequencing and LC-MS-Based Metabolomics. Front Immunol. 2022;13:874021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 48] [Reference Citation Analysis (0)] |
| 28. | Yuan Z, Wang J, Zhang H, Chai Y, Xu Y, Miao Y, Yuan Z, Zhang L, Jiang Z, Yu Q. Glycocholic acid aggravates liver fibrosis by promoting the up-regulation of connective tissue growth factor in hepatocytes. Cell Signal. 2023;101:110508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | La Rosa SL, Leth ML, Michalak L, Hansen ME, Pudlo NA, Glowacki R, Pereira G, Workman CT, Arntzen MØ, Pope PB, Martens EC, Hachem MA, Westereng B. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat Commun. 2019;10:905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 30. | Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T, Taylor TD, Itoh K, Kikuchi J, Morita H, Hattori M, Ohno H. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469:543-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1754] [Article Influence: 116.9] [Reference Citation Analysis (1)] |
| 31. | Tojo R, Suárez A, Clemente MG, de los Reyes-Gavilán CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163-15176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 309] [Cited by in RCA: 350] [Article Influence: 29.2] [Reference Citation Analysis (5)] |
| 32. | Odenwald MA, Lin H, Lehmann C, Dylla NP, Cole CG, Mostad JD, Pappas TE, Ramaswamy R, Moran A, Hutchison AL, Stutz MR, Dela Cruz M, Adler E, Boissiere J, Khalid M, Cantoral J, Haro F, Oliveira RA, Waligurski E, Cotter TG, Light SH, Beavis KG, Sundararajan A, Sidebottom AM, Reddy KG, Paul S, Pillai A, Te HS, Rinella ME, Charlton MR, Pamer EG, Aronsohn AI. Bifidobacteria metabolize lactulose to optimize gut metabolites and prevent systemic infection in patients with liver disease. Nat Microbiol. 2023;8:2033-2049. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 33. | Zhang L, Zi L, Kuang T, Wang K, Qiu Z, Wu Z, Liu L, Liu R, Wang P, Wang W. Investigating causal associations among gut microbiota, metabolites, and liver diseases: a Mendelian randomization study. Front Endocrinol (Lausanne). 2023;14:1159148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/