Published online Mar 27, 2025. doi: 10.4254/wjh.v17.i3.101721
Revised: January 1, 2025
Accepted: February 10, 2025
Published online: March 27, 2025
Processing time: 182 Days and 16.2 Hours
In recent years, the utilization of artificial intelligence (AI) technology has gained prominence in the field of liver disease.
To analyzes AI research in the field of liver disease, summarizes the current research status and identifies hot spots.
We searched the Web of Science Core Collection database for all articles and reviews on hepatopathy and AI. The time spans from January 2007 to August 2023. We included 4051 studies for further collection of information, including authors, countries, institutions, publication years, keywords and references. VOS viewer, CiteSpace, R 4.3.1 and Scimago Graphica were used to visualize the results.
A total of 4051 articles were analyzed. China was the leading contributor, with 1568 publications, while the United States had the most international collaborations. The most productive institutions and journals were the Chinese Academy of Sciences and Frontiers in Oncology. Keywords co-occurrence analysis can be roughly summarized into four clusters: Risk prediction, diagnosis, treatment and prognosis of liver diseases. "Machine learning", "deep learning", "convolutional neural network", "CT", and "microvascular infiltration" have been popular research topics in recent years.
AI is widely applied in the risk assessment, diagnosis, treatment, and prognosis of liver diseases, with a shift from invasive to noninvasive treatment approaches.
Core Tip: This study highlights the increasing annual publications on artificial intelligence in liver disease, with applications spanning risk assessment, diagnosis, treatment, and prognosis. China leads in publication output, whereas the United States remains a dominant force in the field. High-impact journals, authors, and institutions are identified, along with trends in international collaboration. Key research hotspots include "machine learning", "deep learning", "convolutional neural networks", "CT imaging", and "microvascular infiltration".
- Citation: Zhou XQ, Huang S, Shi XM, Liu S, Zhang W, Shi L, Lv MH, Tang XW. Global trends in artificial intelligence applications in liver disease over seventeen years. World J Hepatol 2025; 17(3): 101721
- URL: https://www.wjgnet.com/1948-5182/full/v17/i3/101721.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i3.101721
Liver diseases pose a global health challenge, responsible for nearly 2 million fatalities each year, and contributing to one in every 25 deaths globally[1]. The escalating prevalence of liver diseases with asymptomatic or mildly symptomatic presentations further exacerbates this issue[2], imposing an enormous economic burden on society. The high incidence and unfavorable prognosis associated with liver diseases highlight the imperative need for prompt detection and intervention. Such measures are pivotal not only in enhancing patient outcomes and quality of life but also in mitigating the financial impact on healthcare systems. In this context, the emergence of artificial intelligence (AI) technologies presents promising avenues for the early identification and management of liver diseases, paving the way for tailored precision therapies.
AI, designed to mimic human cognitive functions, is poised to revolutionize patient care quality in healthcare. Within AI, Natural Language Processing (NLP) stands out for its ability to derive meaningful clinical insights from extensive unstructured data sources, such as Electronic Health Records. NLP conducts exhaustive and unbiased analyses of these datasets, greatly aiding patient care. Machine learning (ML), another critical component of AI, has demonstrated remarkable proficiency in areas like medical imaging and disease prognosis. ML is categorized into supervised or unsupervised learning, contingent on the use of labeled training data. Deep learning (DL), an advanced branch of ML, is modeled on human brain functions, utilizing multilayered neural networks to independently interpret and learn from data’s hierarchical structures. This is particularly beneficial for tasks involving classification and feature extraction. However, DL's advanced learning abilities require substantial computational resources and entail significant expenses. Challenges include the "black box" phenomenon, where the opacity of neural network algorithms obscures the inter
In contemporary medical practice, a vast array of patient data has been accumulated, presenting a significant opportunity for enhanced clinical care through AI. AI's ability to process and interpret multimodal patient data promises a more holistic and accurate approach in various medical disciplines. Key areas currently employing AI include radiology, cardiology, ophthalmology, and increasingly, hepatology[3]. In hepatology, AI integration promises to improve the prognostic accuracy and diagnostic precision of hepatic disorders, thus enabling better-informed clinical decisions and positively impacting hepatic disease outcomes. Despite extensive research in hepatic disorders using AI, a comprehensive understanding in this field remains elusive. This article employs bibliometric analysis to systematically examine and visually represent scholarly articles on AI applications in hepatology from 2007 to 2023. This analysis highlights the progressive trends in AI research within the domain of hepatic diseases and anticipates future research directions.
In this study, a comprehensive search was conducted within the Web of Science Core Collection (WoSCC) database to identify all articles and reviews pertaining to hepatopathy and AI. The timeframe for this search extended from January 2007 to August 2023. Renowned for its extensive use and acceptance in scientific and bibliometric research, the WoSCC encompasses predominant scientific journals across a multitude of disciplines globally. It provides a vast repository of academic literature, encompassing works from the early 20th century to present, thereby serving as a pivotal resource for this investigation.
We searched for publications related to AI in the field of liver disease within one day to ensure that there were no data updates. After reviewing the literature and organizing Medical Subject Headings terms, we identified relevant subject terms and supplemented them with free terms. The retrieval strategy is as follows: Topic = ("Liver Diseases" or "Hepatopathy" or "Liver Dysfunction" or "Liver Failure" or "Liver Neoplasm*" or "Hepatis" or "Hepatic" or "Fatty liver" or "Hepato*" or "Cirrhosis" or "Liver Abscess") AND topic = ("Artificial Intelligence" or "Computational Intelligence" or "Machine Intelligence" or "Machine Learning" or "AI" or "Computer Vision System" or "Deep Learning" or "Supervised Machine Learning" or "Unsupervised Machine Learning" or "Sentiment Analysis" or "Robotic*" or "Big Data" or "Intelligent Learning" or "Image* Segmentation" or "Knowledge Graph" or "Expert* System*" or "Semantic Segmentation" or "Feature* Learning" or "Feature* Mining" or "Feature* Extraction" or "Feature* Selection" or "Neural Network*" or "Deep Network*" or "Bayes* Network" or "Data Clustering" or "Graph Mining" or "Neural Nets Model*") AND pub
For the analysis and visualization of the collected data, this study predominantly utilized tools such as VOSviewer, Citespace, and Scimago Graphica. These applications were instrumental in mapping and interpreting complex bibliometric networks. Furthermore, R software, version 4.3.1, was employed to graphically represent the evolution and trends of keyword hotspots, providing a nuanced understanding of thematic progression within the field.
In network and clustering analysis, we use the modularity Q and weighted mean silhouette S coefficients to assess the quality and effectiveness of clustering. Modularity Q measures the quality of network clustering through data par
The data used in our study are available from public databases. Permission from the ethics committee is not needed.
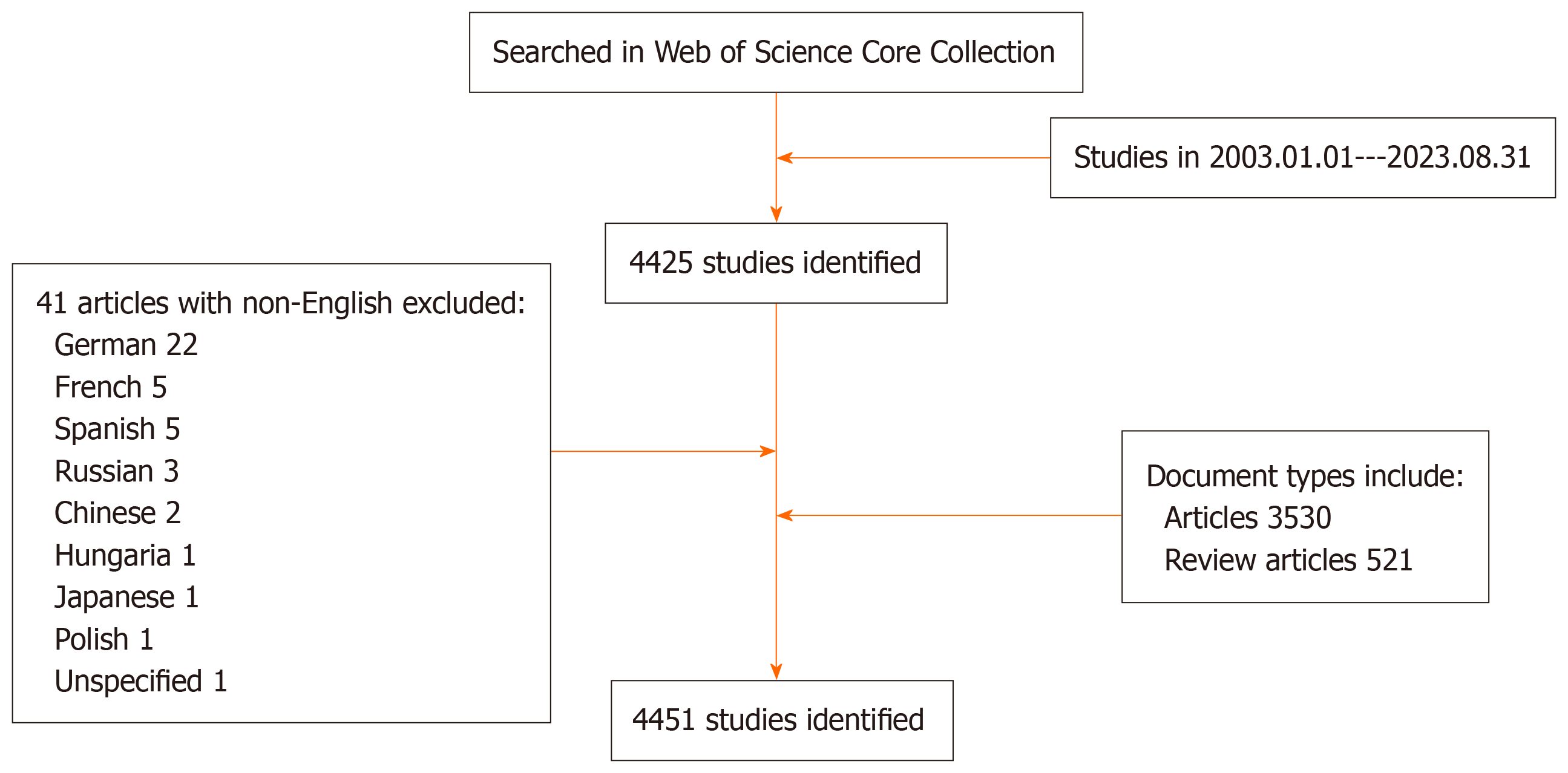
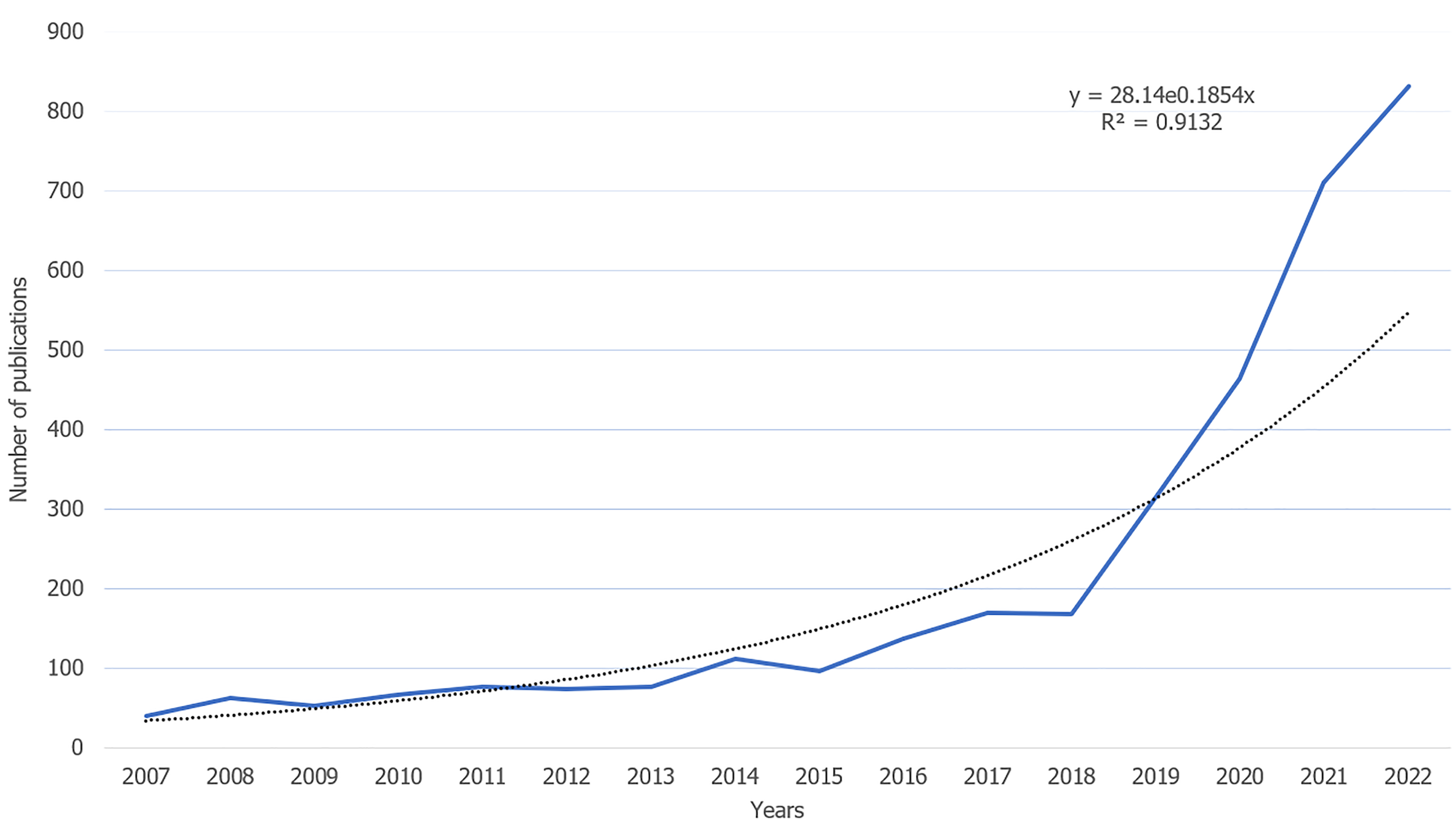
In accordance with the outlined literature search strategy flowchart, a total of 4051 papers were extracted from the WoSCC over the past 17 years, encompassing 3530 original articles and 521 reviews (as depicted in Figure 1). A notable increase in the volume of published papers was observed from 2018-2022, signifying a growing interest in the application of AI technology in the field of liver diseases (illustrated in Figure 2). In alignment with Price's law, which posits an exponential increase in scientific documentation, the number of publications in this domain has been expanding exponentially. This trend is mathematically represented by the exponential curve equation: Y = 28.14e0.1854×. The fitting curve, demonstrating a high degree of correlation with an R² value of 0.9132, suggests a continued upward trajectory in research output, thereby indicating that an increased number of studies in this area can be anticipated in the coming years.
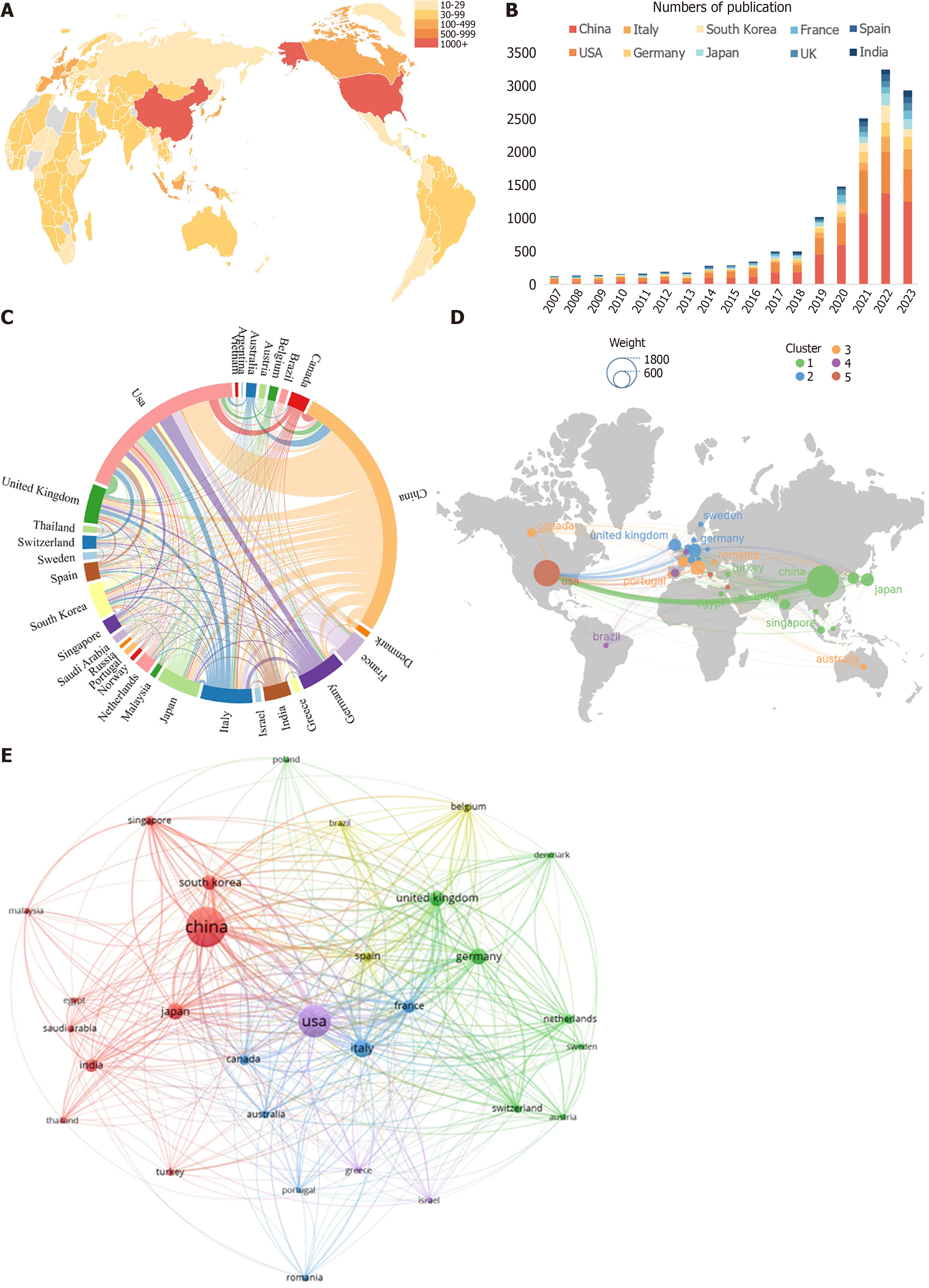
Related papers in this field have been published by authors from a collective total of 88 countries. The top 10 most productive countries contributing to the growth of AI in liver disease research activities globally are outlined in Table 1 and Figure 3A and B. China published the most studies (1568), followed by the United States (1062), Italy (318), Germany (277), Japan (258), the United Kingdom (234) and South Korea (211). China was the leading country (1568), accounting for 38.71% of all publications (4051) worldwide, but the average number of citations per paper was 13.48, ranking behind the United States (23.62). For collaboration analysis between countries, China has cooperated with many countries, most importantly with the United States and the United Kingdom (Figure 3C and D). Additionally, VOSviewer was employed to construct a network map illustrating the collaborative relationships among countries (as shown in Figure 3E). This analysis encompassed 30 countries, each with a minimum publication count exceeding 32. In this map, the lines connecting various nodes signify collaborative interactions between countries, with the line thickness indicating the intensity of these collaborations, quantified as the total link strength (TLS). The co-authorship visualization highlighted that the United States, China, Italy, the United Kingdom, and Germany were the top five countries in terms of TLS. The map of the cooperation network between countries shows that the United States has more connections with other countries, including China, the United Kingdom, Germany, and Italy. This pattern underscores the global scope of research attention in the application of AI in liver disease studies.
| Country | Counts | Ranking on the basis of total documents | Total citations | Ranking on the basis of total citations | Average citation | Ranking on the basis of total link strength | Link strength |
| China | 1568 | 1 | 21137 | 2 | 13.48 | 2 | 678 |
| United States | 1062 | 2 | 25084 | 1 | 23.62 | 1 | 1034 |
| Italy | 318 | 3 | 6629 | 3 | 20.85 | 3 | 605 |
| Germany | 277 | 4 | 6377 | 4 | 23.02 | 5 | 539 |
| Japan | 258 | 5 | 5716 | 5 | 22.16 | 7 | 374 |
| United Kingdom | 234 | 6 | 5312 | 6 | 22.70 | 4 | 559 |
| South Korea | 211 | 7 | 3935 | 8 | 18.65 | 9 | 339 |
| France | 175 | 8 | 4423 | 7 | 25.27 | 6 | 503 |
| India | 165 | 9 | 3245 | 9 | 19.67 | 18 | 167 |
| Canada | 118 | 10 | 3066 | 11 | 25.98 | 11 | 261 |
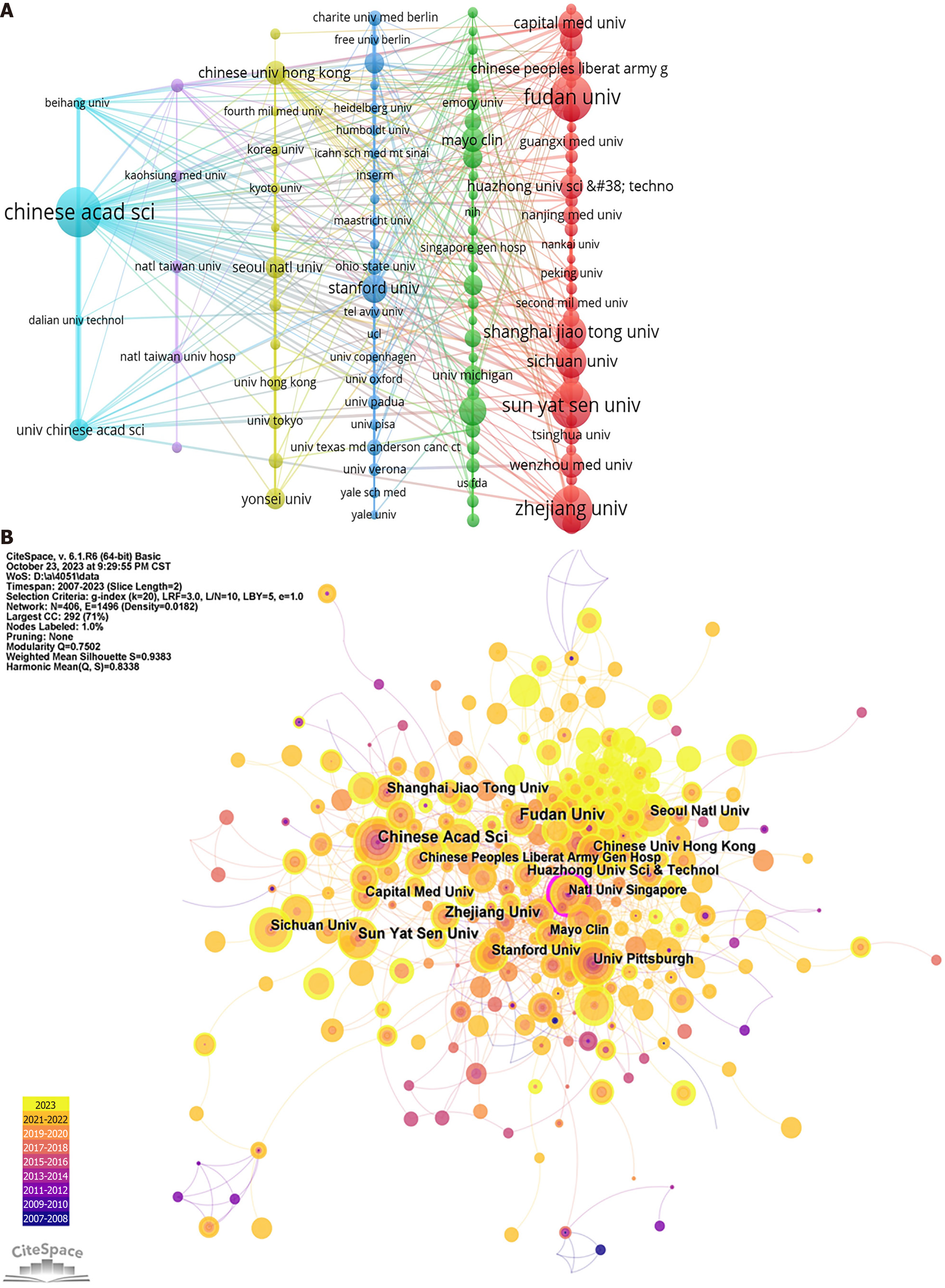
This study identified a total of 4957 institutions contributing to the publication of research on AI in liver disease. As summarized in Table 2, the top 10 institutions with the highest contributions are predominantly based in China, with the exception of the University of Pittsburgh from the United States, which ranks seventh. Notably, the University of Pittsburgh also has the distinction of having the highest average number of citations. The pattern of institutional collaboration is presented in Figure 4A. In terms of publication volume, the Chinese Academy of Sciences led with 104 publications, closely followed by Fudan University (96), Zhejiang University (93), and Sun Yat-sen University (92). Figure 4A shows that among the institutions with over 15 publications, the Chinese Academy of Sciences (TLS = 263), Fudan University (210), and Sun Yat-sen University (179) have the highest TLS. The centrality of organizations in this research area was analyzed via the CiteSpace platform. Figure 4B, generated by CiteSpace, visualizes the cooperation between organizations. Organizations with a purple outer ring in the visualization represent a centrality greater than 0.1, indicating a central role in the network. Notably, the National University of Singapore emerged as a central player (centrality 0.12), highlighting its significant position in the network of institutional collaboration in AI research on liver disease.
| Rank | Country | Counts | Institution | Citation | Mean citation |
| 1 | China | 104 | Chinese Academy of Sciences | 2088 | 20.08 |
| 2 | China | 96 | Fudan University | 1465 | 15.26 |
| 3 | China | 93 | Zhejiang University | 1366 | 14.69 |
| 4 | China | 92 | Sun Yat-Sen University | 1833 | 19.92 |
| 5 | China | 64 | Shanghai Jiao Tong University | 732 | 11.44 |
| 6 | China | 63 | Sichuan University | 606 | 9.62 |
| 7 | United States | 56 | University of Pittsburgh | 1815 | 32.41 |
| 8 | China | 53 | Capital Medical University | 735 | 13.87 |
| 9 | China | 52 | Stanford University | 1308 | 25.15 |
| 10 | China | 47 | Chinese People’s Liberation Army General Hospital | 858 | 18.26 |
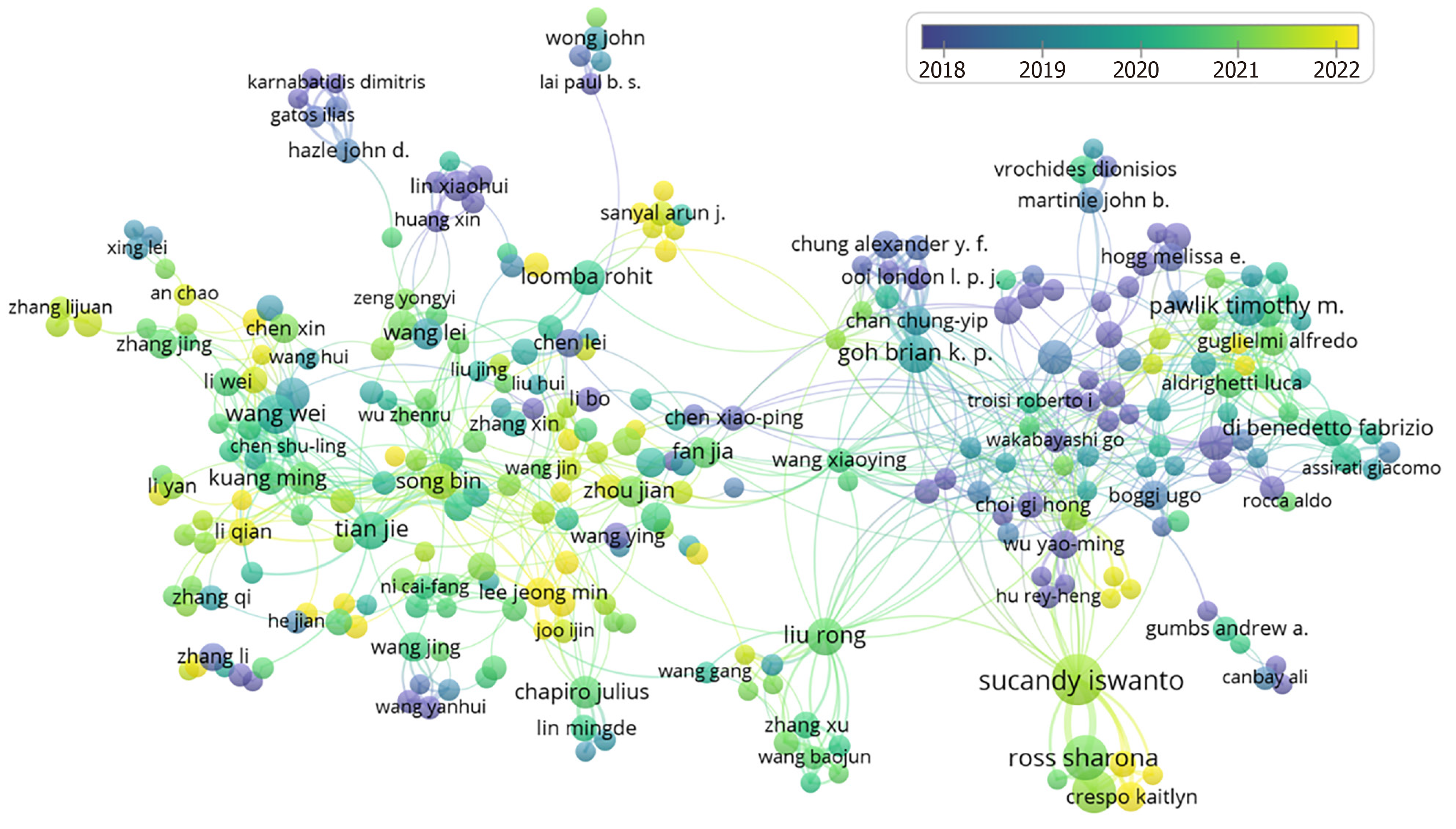
The study included a comprehensive cohort of 129243 authors. For a more focused analysis in VOSviewer, authors who had published more than five articles were specifically included, facilitating the generation of a coauthorship visualization graph, as depicted in Figure 5. Table 3 delineates the ten most prolific authors in terms of publication volume. The leaders of this group are Sucandy Iswanto, Ross Sharona, and Rosemurgy Alexander, who contributed 32, 25, and 23 papers, respectively.
| Rank | Author | Counts | Total citations | Mean citations |
| 1 | Sucandy Iswanto | 32 | 501 | 15.66 |
| 2 | Ross Sharona | 25 | 176 | 7.04 |
| 3 | Rosemurgy Alexander | 23 | 442 | 19.22 |
| 4 | Wang Wei | 19 | 23 | 1.21 |
| 5 | Tian Jie | 17 | 508 | 29.88 |
| 6 | Goh Brian K. P. | 17 | 177 | 10.41 |
| 7 | Liu Rong | 17 | 225 | 13.24 |
| 8 | Pawlik Timothy M. | 17 | 218 | 12.82 |
| 9 | Song Bin | 16 | 131 | 8.19 |
| 10 | Di Benedetto Fabrizio | 15 | 546 | 36.40 |
In this study, a total of 1116 journals were identified as having published articles relevant to the research topic. Among these, 189 journals published no fewer than five articles each. The quality of a journal is often assessed via metrics such as the impact factor (IF, JCR category) and the number of citations received.
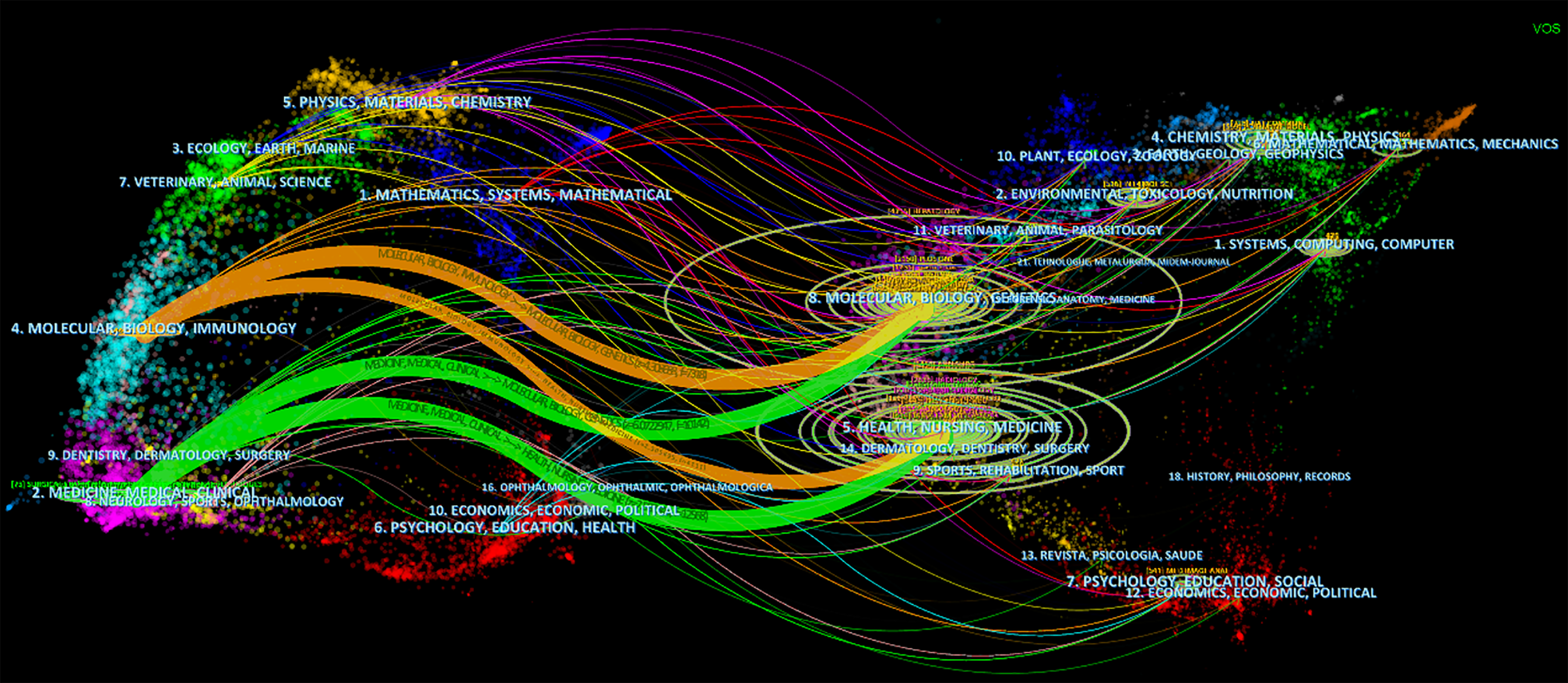
Table 4 lists the top ten journals by publication count, with Frontiers in Oncology (89 articles, IF = 4.7, Q2), Scientific Reports (82 articles, IF = 4.6, Q2), and Asian Journal of Surgery (77 articles, IF = 3.5, Q1) occupying the top three positions. However, in terms of average citations, the leading journals are IEEE Transactions on Medical Imaging (139.60), Annals of Surgery (132.15), Proceedings of the National Academy of Sciences of the United States of America (116.20), and Neurocomputing (105.38). This suggests that the articles published in these journals are of high quality. Figure 6, created via CiteSpace software, presents a dual map of journals, illustrating the relationship between cited and citing journals. This visual representation reveals four main citation pathways. The cited articles predominantly fall into two fields: (1) Medical, clinical, and (2) Molecular, biological, and immunology. However, the citing papers are primarily concentrated in three fields: (1) Molecular, Biology, Genetics; (2) Health, Nursing, Medicine; and (3) Dermatology, Dentistry, Surgery. This delineation provides insight into the interdisciplinary nature and citation dynamics within the scope of the study. The concentration of citing papers in the fields of dermatology and dentistry may be attributed to interdisciplinary collaborations or overlapping methodologies.
| Rank | Journal title | Countries | Count | Impact factor (2023) | JCR (2023) | Total citations |
| 1 | Frontiers in Oncology | Switzerland | 89 | 4.7 | Q2 | 655 |
| 2 | Scientific Reports | England | 82 | 4.6 | Q2 | 1000 |
| 3 | Asian Journal of Surgery | China | 77 | 3.5 | Q1 | 553 |
| 4 | European Radiology | Germany | 73 | 5.9 | Q1 | 1672 |
| 5 | Surgical Endoscopy and Other Interventional Techniques | United States | 73 | 3.1 | Q1 | 1763 |
| 6 | Cancers | Switzerland | 69 | 5.2 | Q2 | 317 |
| 7 | Plos One | United States | 67 | 3.7 | Q2 | 1321 |
| 8 | World Journal of Gastroenterology | China | 55 | 4.3 | Q2 | 950 |
| 9 | Diagnostics | Poland | 51 | - | - | 159 |
| 10 | Medical Physics | United States | 42 | 3.8 | Q2 | 717 |
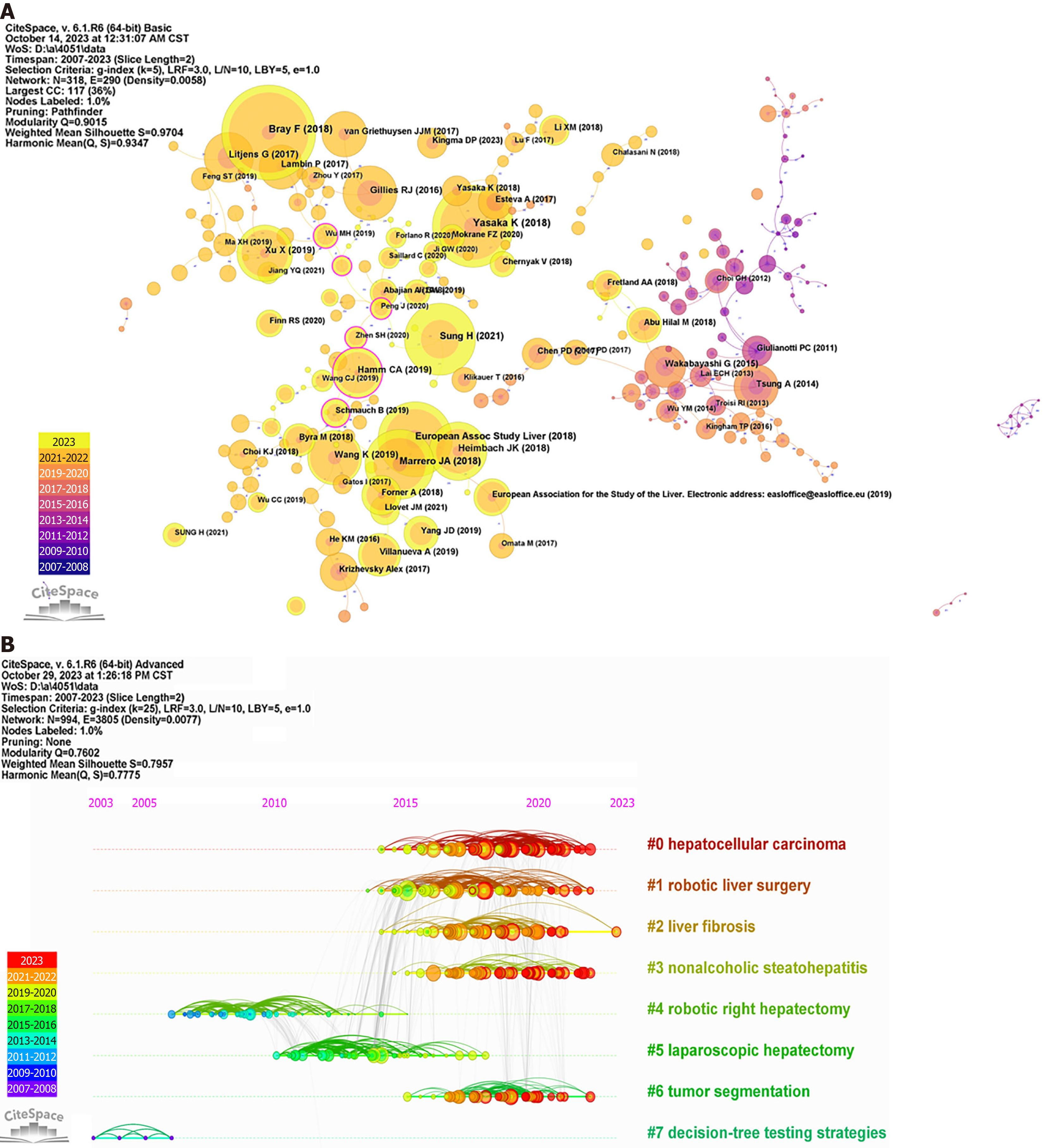
In this research, a total of 4051 papers were analyzed, among which 303 were cited more than 50 times. Table 5 presents the top 10 most cited articles pertaining to the application of AI in liver disease. These publications are indicative of foundational works in the integration of AI within this specific medical domain. The work by Bray et al[5] has the most citations, with 148 citations, followed by Yasaka et al[6] and the European Association for the Study of the Liver, with 128 and 117 citations, respectively. Figure 7A provides a visual representation of the cocitation network of references, demonstrating effective clustering, as evidenced by high modularity Q and weighted mean silhouette S values of 0.9015 and 0.9704, respectively. Additionally, Figure 7B offers a timeline view of cocited articles, highlighting the evolution of research hotspots over time. For clarity, only the top 8 Largest clusters are depicted in this figure. The four largest clusters identified are "hepatocellular carcinoma", "robotic liver surgery", "liver fibrosis", and "nonalcoholic steatohepatitis", highlighting the key areas of focus in AI-related liver disease research.
| Title | Journals | First author | Year | Citations |
| Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries | CA Cancer J Clin | Freddie Bray | 2018 | 148 |
| Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study | Radiology | Koichiro Yasaka | 2018 | 128 |
| EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma | J Hepatol | European Association for the Study of the Liver | 2018 | 117 |
| Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries | CA Cancer J Clin | Hyuna Sung | 2021 | 112 |
| Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases | Hepatology | Jorge A Marrero | 2018 | 109 |
| AASLD guidelines for the treatment of hepatocellular carcinoma | Hepatology | Julie K Heimbach | 2018 | 91 |
| Radiomics: Images Are More than Pictures, They Are Data | Radiology | Robert J Gillies | 2016 | 89 |
| Radiomic analysis of contrast-enhanced CT predicts microvascular invasion and outcome in hepatocellular carcinoma | J Hepatol | Xun Xu | 2019 | 88 |
| Deep learning Radiomics of shear wave elastography significantly improved diagnostic performance for assessing liver fibrosis in chronic hepatitis B: A prospective multicenter study | Gut | Kun Wang | 2019 | 85 |
| A survey on deep learning in medical image analysis | Med Image Anal | Geert Litjens | 2017 | 78 |
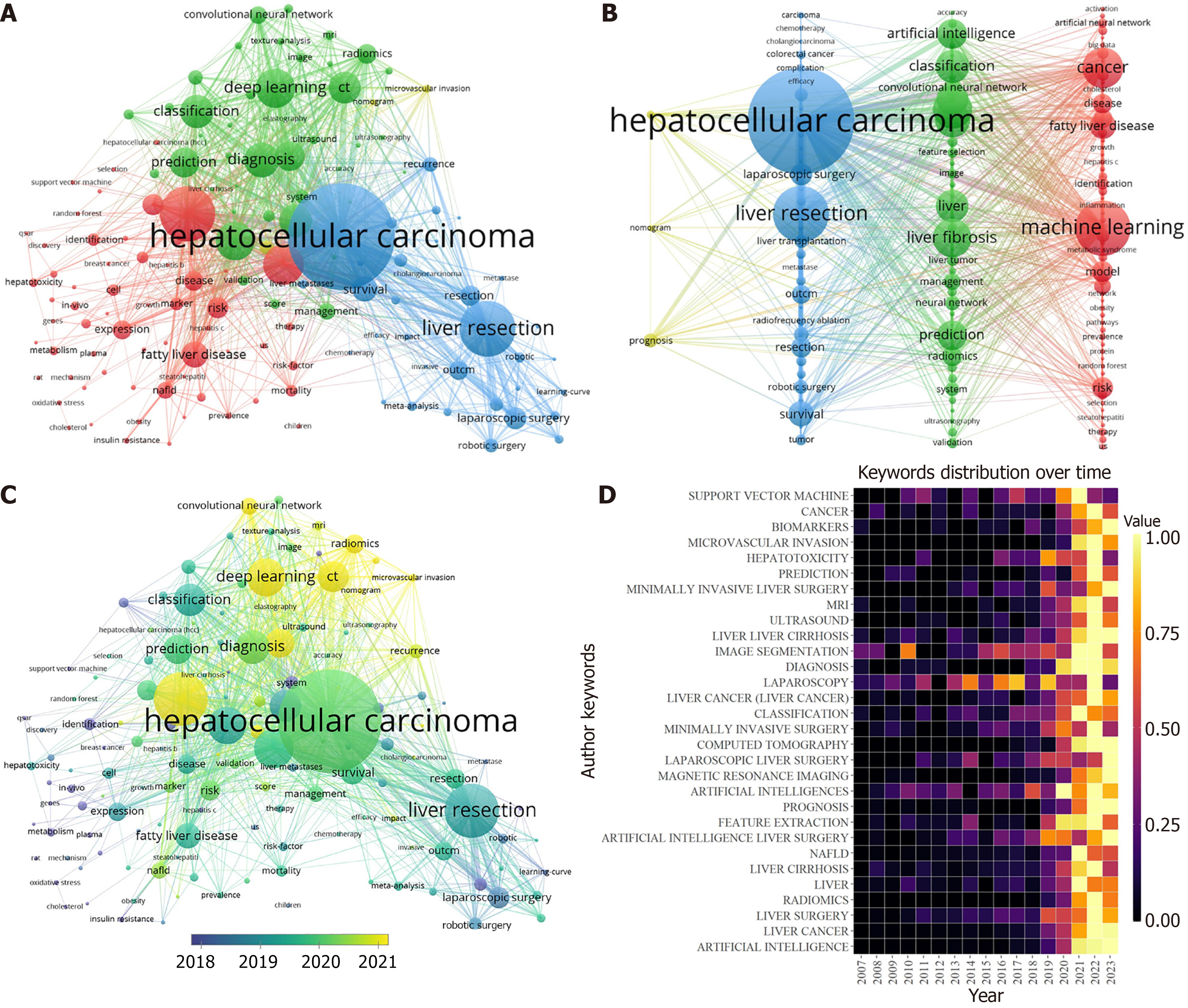
Keyword co-occurrence analysis can help researchers find the key topics and topic structure in the research field to better understand their characteristics. However, keyword bursts refer to the frequent occurrence of a certain keyword in a certain period, through which the emerging hot spots and major events in the research field can be identified. A total of 13667 author keywords were included in our study, of which 138 appeared more than 35 times. Table 6 lists the top 30 keywords that appear most frequently. The most frequent keyword was “hepatocellular carcinoma”, with 1023 keywords, followed by “machine learning” (551 times) and “deep learning” (393 times). We use VoSviewer to produce a network visualization map (Figure 8A and B) and an overlay visualization map (Figure 8C) of keywords related to AI in liver disease research. In Figure 8A and B, the high-frequency keywords (frequency not less than 35) included in this study can be divided into four clusters, and each color indicates a cluster that represents diverse research tendencies. There are four clusters in total: The red cluster focuses on risk prediction of liver disease, such as keywords "machine learning", "model", "risk", "artificial neural network", "fatty liver disease", and "obesity"; the green cluster tends to be associated with liver disease imaging and diagnosis, such as keywords "image", "ultrasonography", "accuracy", "classification", "artificial intelligence", "convolutional neural network", and "prediction"; the blue cluster focuses on the treatment of liver diseases, especially hepatocellular carcinoma (HCC), such as keywords such as "hepatocellular carcinoma", "hepatectomy", "laparoscopic surgery", "robotic surgery" and "chemotherapy"; and the yellow cluster focuses on the prognosis of liver diseases. Since the graph includes keywords with a frequency greater than 50 times, the yellow cluster includes only three keywords: "prognosis", "nomogram" and "microvascular invasion". Figure 8C and D show the change in keywords over time, and the yellow nodes in Figure 8C represent emergent keywords, indicating research hotspots in recent years. The "machine learning", "deep learning", "convolutional neural network", "CT", and "microvascular infiltration" methods receive more attention.
| Rank | Keywords | Count | Rank | Keywords | Count | Rank | Keywords | Count |
| 1 | Hepatocellular carcinoma | 1023 | 11 | Survival | 206 | 21 | CT | 145 |
| 2 | Machine learning | 551 | 12 | Model | 202 | 22 | System | 144 |
| 3 | Deep learning | 393 | 13 | Resection | 200 | 23 | Neural network | 139 |
| 4 | Cancer | 377 | 14 | Outcome | 195 | 24 | Experience | 133 |
| 5 | Diagnosis | 310 | 15 | Disease | 190 | 25 | Robotic surgery | 132 |
| 6 | Classification | 294 | 16 | Expression | 190 | 26 | Liver resection | 124 |
| 7 | Artificial intelligence | 291 | 17 | Liver | 175 | 27 | Identification | 122 |
| 8 | Surgery | 235 | 18 | Management | 169 | 28 | Computed tomography | 120 |
| 9 | Prediction | 219 | 19 | Convolutional neural network | 167 | 29 | Fatty liver disease | 120 |
| 10 | Risk | 210 | 20 | Cirrhosis | 163 | 30 | Cell | 116 |
Over the past two decades, research on AI in liver diseases has exhibited exponential growth. Given China's vast population and the increasing prevalence of liver diseases, its contributions have notable global health implications[7,8]. However, the findings indicate that while the volume of publications from China is substantial, their global impact remains relatively limited, pointing to a need for more in-depth and high-quality research outputs. Several factors might contribute to this phenomenon: (1) In recent years, the Chinese government has robustly supported the development of AI applications, leading to a significant increase in research institutions and publications within China[9,10]; (2) The effectiveness of AI outputs has been closely linked to the quality of input data[11]. The advancement of AI technology hinges on powerful computing capabilities and sophisticated algorithms, areas in which China's development is still progressing; and (3) Language barriers may also play a role, potentially limiting the international citation of Chinese literature. These factors collectively suggest areas for improvement and development to enhance the global influence of China's research in AI applications for liver diseases.
China and the United States rank highest in both publication volume (China: 1568; United States: 1062) and total citations (China: 21137; United States: 25084). They also act as core countries in the international collaboration network, further underscoring their pivotal roles in advancing this field. Additionally, Canada, France, Germany, the United Kingdom, and Japan exhibit outstanding performance in terms of average citations. Specifically, Canada has an average citation of 25.98, France 25.27, Germany 23.02, the United Kingdom 22.70, and Japan 22.16. This reflects these countries' significant contributions to high-impact research, particularly through active participation in international collaborations and scientific innovation.
Cluster analysis of the literature revealed a growing trend in the publication of articles focused on liver cancer[12], robotic liver surgery, liver fibrosis, and nonalcoholic fatty liver disease (NAFLD) in recent years. These topics can be broadly categorized into four clusters, representing key areas in liver disease research: Risk prediction (represented by the red cluster), diagnosis (green cluster), treatment (blue cluster), and prognosis (yellow cluster). As depicted in Figure 8C, initial studies predominantly concentrated on the diagnosis and surgical treatment of liver diseases. Recent trends, however, indicate a shift toward topics such as "machine learning", "deep learning", "convolutional neural networks", "CT imaging", and "microvascular infiltration". This shift in focus points to an evolving research landscape where the application of AI in liver diseases is transitioning from traditional invasive methods to more advanced, noninvasive techniques. This progression underscores the dynamic nature of AI integration in medical research, particularly in the context of liver disease management.
Application of AI in the risk prediction of liver diseases: The etiology of liver diseases, including HCC and NAFLD, is profoundly influenced by individual and temporal factors, posing significant challenges in the development of predictive models for these diseases. Traditional regression analyses often oversimplify these models by excluding variables deemed "nonsignificant", which compromises their accuracy and generalizability. In contrast, AI techniques, which leverage electronic medical records and extensive databases, have shown potential in identifying populations at heightened risk for conditions such as NAFLD and, further, in stratifying this risk with greater precision[13,14]. Risk scoring systems have been developed to evaluate the likelihood of developing HCC in patients with chronic liver disease, and these systems have demonstrated acceptable performance levels[15,16]. However, the clinical application of these risk scores remains limited, primarily due to the restricted size and diversity of the training datasets. This limitation hampers the ability of the models to be generalized externally, thus affecting their practical utility in diverse clinical settings.
Application of AI in the diagnosis of liver diseases: AI integration with various diagnostic methods, such as radiomics, histopathology, and molecular biology, is poised to significantly increase diagnostic accuracy in liver disease[3]. As the largest solid organ in the human body, the liver primarily depends on imaging techniques for initial disease diagnosis. However, imaging results are often qualitative, such as those from ultrasound, or semiquantitative, such as those from CT scans, leading to potential interpretative discrepancies among clinicians. Furthermore, the repetitive task of image analysis can induce physician fatigue[17] and increase the likelihood of errors. Radiomics texture analysis, which is capable of rapidly extracting subtle imaging features that may elude the human eye[18], offers a more comprehensive and objective interpretation, thus aiding in enhancing diagnostic efficiency.
Although radiological examinations are often sufficient for diagnosing HCC, bypassing the need for tissue biopsy, histopathological examination remains the definitive standard for many liver conditions, including the characterization of focal liver lesions, as well as the grading and staging of autoimmune hepatitis and nonalcoholic steatohepatitis. Research indicates that histology reveals extensive information about potential molecular changes and disease prognosis[19-21], providing deeper insights into the pathophysiology and prognosis of liver diseases. Recent advances in sequencing technologies have led to the use of single-cell RNA sequencing (scRNA-seq), which offers a detailed view of the cellular heterogeneity in liver diseases, whereas bulk RNA sequencing provides an overview of the overall pathological state. The synergistic application of these methodologies has enhanced our understanding of the molecular basis of liver diseases and aided in identifying potential therapeutic targets[22]. Currently, single-cell technologies are still evolving, and AI can play a pivotal role in addressing challenges such as data quality, the loss of low-expression data during sequencing, and data noise[23,24]. However, the absence of standardized data formats and norms presents significant challenges in data sharing and comparisons in single-cell research.
Application of AI in the treatment of liver diseases: The application of AI in liver disease treatment primarily focuses on optimizing treatment plan selection and enhancing intraoperative visualization. A critical issue in liver cancer therapy involves accurately predicting the most effective treatment approach or combination for each patient[25], thereby facilitating prompt and informed clinical decisions. The IMBRAVE-150 trial[26] demonstrated that, compared with sorafenib, a combination therapy of atezolizumab and bevacizumab significantly improved survival rates in HCC patients. These findings underscore the potential role of immune checkpoint inhibitors (ICIs) in cancer therapy. However, given that not all HCC patients benefit from ICIs and considering their substantial side effects and costs, the AI-based prediction of treatment response becomes vital[3].
Research has shown that intraoperative complications often arise from errors in visual anatomical assessments[27,28]. DL shows immense promise in computer vision tasks, but its application in real-time surgical guidance is challenging because of the variable intraoperative environment of liver surgeries[29]. Madani et al[29] developed a DL model capable of identifying anatomical landmarks and distinguishing between safe and risky zones during laparoscopic cho
Application of AI in the prognosis of liver diseases: Microvascular infiltration (MVI), a crucial histological characteristic in the treatment and prognosis of liver cancer, is closely linked with postoperative recurrence of the disease. Preoperative identification of MVI status can significantly aid physicians in making more informed medical decisions. Traditionally, the detection of MVI relies on postoperative tissue biopsies, which inherently introduces a delay. Additionally, MVI often occurs interspersed within liver tissues adjacent to the tumor[31], necessitating multipoint sampling around the tumor area to ensure accurate detection. However, such extensive sampling is challenging in clinical practice because of factors such as the need to preserve liver function[32]. With advancements in AI, there is now burgeoning potential for effectively evaluating MVI status via AI algorithms applied to histological whole-slide images of HCC. This technological progress holds promise for enhancing the clinical management of HCC patients by providing a more timely and accurate assessment of MVI[33].
AI has promising prospects in the field of hepatology, but many challenges remain in clinical practice. First, AI holds significant potential in hepatology, yet its implementation in clinical settings is met with several challenges. First, the lack of standardized protocols in AI-mediated medical data analysis is a concern, with the majority of studies being retrospective. This underscores the need for more prospective research to refine and validate AI models. Second, simplistic designs in algorithmic models can inadvertently create healthcare disparities. An example is the MELD, which is used for liver transplant prioritization. MELD is based on three laboratory parameters: The bilirubin level, international normalized ratio, and creatinine level. The issue arises with creatinine levels, which are typically lower in women, possibly owing to lower muscle mass, leading to an underestimation of renal insufficiency in female patients. Addi
This study has certain limitations: (1) We included only English literature in the WoSCC, and the document types included only articles and reviews, which results in a greater probability of missing literature; (2) The search time of our study was up to 2023.08.31, and the time delay of new studies may result in the omission of emerging research hotspots; and (3) Bibliometric analyses may be subject to biases, such as variations in citation practices across different academic fields or countries, which can lead to certain papers being cited more frequently while others are overlooked. Additionally, newly published papers may not have accumulated sufficient citations, resulting in emerging research not being adequately represented.
In conclusion, AI has been extensively applied in various aspects of liver disease management, including risk assessment, diagnosis, treatment, and prognosis. While a significant volume of the literature originates from China, there is a recognized need to increase the quality of these publications. The United States continues to maintain its leadership in this research domain. Emerging trends and future research directions are predominantly focused on areas such as "machine learning", "deep learning", "convolutional neural networks", "CT imaging", and "microvascular infiltration". These trends suggest an increasing emphasis on leveraging AI for the prognostic analysis of liver diseases. Moreover, there has been a noticeable shift in the application of AI within this field, moving from invasive methodologies to more noninvasive approaches. This transition points to the evolving nature of AI technology and its growing integration into less intrusive yet effective medical procedures and analyses.
| 1. | Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1236] [Reference Citation Analysis (4)] |
| 2. | Friedman SL, Sanyal AJ. The future of hepatology. Hepatology. 2023;78:637-648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Calderaro J, Seraphin TP, Luedde T, Simon TG. Artificial intelligence for the prevention and clinical management of hepatocellular carcinoma. J Hepatol. 2022;76:1348-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 188] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 4. | Tao S, Yang D, Zhang L, Yu L, Wang Z, Li L, Zhang J, Yao R, Huang L, Shao M. Knowledge domain and emerging trends in diabetic cardiomyopathy: A scientometric review based on CiteSpace analysis. Front Cardiovasc Med. 2022;9:891428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56688] [Article Influence: 7086.0] [Reference Citation Analysis (135)] |
| 6. | Yasaka K, Akai H, Abe O, Kiryu S. Deep Learning with Convolutional Neural Network for Differentiation of Liver Masses at Dynamic Contrast-enhanced CT: A Preliminary Study. Radiology. 2018;286:887-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 426] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 7. | Xiao J, Wang F, Wong NK, He J, Zhang R, Sun R, Xu Y, Liu Y, Li W, Koike K, He W, You H, Miao Y, Liu X, Meng M, Gao B, Wang H, Li C. Global liver disease burdens and research trends: Analysis from a Chinese perspective. J Hepatol. 2019;71:212-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 423] [Article Influence: 60.4] [Reference Citation Analysis (1)] |
| 8. | Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 981] [Article Influence: 81.8] [Reference Citation Analysis (4)] |
| 9. | Bawack RE, Wamba SF, Carillo KDA, Akter S. Artificial intelligence in E-Commerce: a bibliometric study and literature review. Electron Mark. 2022;32:297-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Huang P, Feng Z, Shu X, Wu A, Wang Z, Hu T, Cao Y, Tu Y, Li Z. A bibliometric and visual analysis of publications on artificial intelligence in colorectal cancer (2002-2022). Front Oncol. 2023;13:1077539. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 11. | Veerankutty FH, Jayan G, Yadav MK, Manoj KS, Yadav A, Nair SRS, Shabeerali TU, Yeldho V, Sasidharan M, Rather SA. Artificial Intelligence in hepatology, liver surgery and transplantation: Emerging applications and frontiers of research. World J Hepatol. 2021;13:1977-1990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
| 12. | Lin J, Chang Y, Hu M, Gu Q, Dai J, Nan J, Wang Z, Chen J, Zhong D, Zhou E, Wang Y, Cai X. Global trends in research of mitophagy in liver diseases over past two decades: A bibliometric analysis. Heliyon. 2023;9:e18843. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 13. | Atabaki-Pasdar N, Ohlsson M, Viñuela A, Frau F, Pomares-Millan H, Haid M, Jones AG, Thomas EL, Koivula RW, Kurbasic A, Mutie PM, Fitipaldi H, Fernandez J, Dawed AY, Giordano GN, Forgie IM, McDonald TJ, Rutters F, Cederberg H, Chabanova E, Dale M, Masi F, Thomas CE, Allin KH, Hansen TH, Heggie A, Hong MG, Elders PJM, Kennedy G, Kokkola T, Pedersen HK, Mahajan A, McEvoy D, Pattou F, Raverdy V, Häussler RS, Sharma S, Thomsen HS, Vangipurapu J, Vestergaard H, 't Hart LM, Adamski J, Musholt PB, Brage S, Brunak S, Dermitzakis E, Frost G, Hansen T, Laakso M, Pedersen O, Ridderstråle M, Ruetten H, Hattersley AT, Walker M, Beulens JWJ, Mari A, Schwenk JM, Gupta R, McCarthy MI, Pearson ER, Bell JD, Pavo I, Franks PW. Predicting and elucidating the etiology of fatty liver disease: A machine learning modeling and validation study in the IMI DIRECT cohorts. PLoS Med. 2020;17:e1003149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Dinani AM, Kowdley KV, Noureddin M. Application of Artificial Intelligence for Diagnosis and Risk Stratification in NAFLD and NASH: The State of the Art. Hepatology. 2021;74:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Kim HY, Lampertico P, Nam JY, Lee HC, Kim SU, Sinn DH, Seo YS, Lee HA, Park SY, Lim YS, Jang ES, Yoon EL, Kim HS, Kim SE, Ahn SB, Shim JJ, Jeong SW, Jung YJ, Sohn JH, Cho YK, Jun DW, Dalekos GN, Idilman R, Sypsa V, Berg T, Buti M, Calleja JL, Goulis J, Manolakopoulos S, Janssen HLA, Jang MJ, Lee YB, Kim YJ, Yoon JH, Papatheodoridis GV, Lee JH. An artificial intelligence model to predict hepatocellular carcinoma risk in Korean and Caucasian patients with chronic hepatitis B. J Hepatol. 2022;76:311-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 16. | Ioannou GN, Tang W, Beste LA, Tincopa MA, Su GL, Van T, Tapper EB, Singal AG, Zhu J, Waljee AK. Assessment of a Deep Learning Model to Predict Hepatocellular Carcinoma in Patients With Hepatitis C Cirrhosis. JAMA Netw Open. 2020;3:e2015626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 17. | Hussein M, Everson M, Haidry R. Esophageal squamous dysplasia and cancer: Is artificial intelligence our best weapon? Best Pract Res Clin Gastroenterol. 2021;52-53:101723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Corrias G, Micheletti G, Barberini L, Suri JS, Saba L. Texture analysis imaging "what a clinical radiologist needs to know". Eur J Radiol. 2022;146:110055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (1)] |
| 19. | Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage P, Nault JC, Zucman-Rossi J. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 576] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 20. | Ziol M, Poté N, Amaddeo G, Laurent A, Nault JC, Oberti F, Costentin C, Michalak S, Bouattour M, Francoz C, Pageaux GP, Ramos J, Decaens T, Luciani A, Guiu B, Vilgrain V, Aubé C, Derman J, Charpy C, Zucman-Rossi J, Barget N, Seror O, Ganne-Carrié N, Paradis V, Calderaro J. Macrotrabecular-massive hepatocellular carcinoma: A distinctive histological subtype with clinical relevance. Hepatology. 2018;68:103-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 192] [Article Influence: 24.0] [Reference Citation Analysis (1)] |
| 21. | Vali Y, Lee J, Boursier J, Petta S, Wonders K, Tiniakos D, Bedossa P, Geier A, Francque S, Allison M, Papatheodoridis G, Cortez-Pinto H, Pais R, Dufour JF, Leeming DJ, Harrison SA, Chen Y, Cobbold JF, Pavlides M, Holleboom AG, Yki-Jarvinen H, Crespo J, Karsdal M, Ostroff R, Zafarmand MH, Torstenson R, Duffin K, Yunis C, Brass C, Ekstedt M, Aithal GP, Schattenberg JM, Bugianesi E, Romero-Gomez M, Ratziu V, Anstee QM, Bossuyt PM; Liver Investigation: Testing Marker Utility in Steatohepatitis (LITMUS) consortium investigators. Biomarkers for staging fibrosis and non-alcoholic steatohepatitis in non-alcoholic fatty liver disease (the LITMUS project): a comparative diagnostic accuracy study. Lancet Gastroenterol Hepatol. 2023;8:714-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 140] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 22. | Jia C, Hu Y, Kelly D, Kim J, Li M, Zhang NR. Accounting for technical noise in differential expression analysis of single-cell RNA sequencing data. Nucleic Acids Res. 2017;45:10978-10988. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 23. | Kim JK, Kolodziejczyk AA, Ilicic T, Teichmann SA, Marioni JC. Characterizing noise structure in single-cell RNA-seq distinguishes genuine from technical stochastic allelic expression. Nat Commun. 2015;6:8687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 166] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Arisdakessian C, Poirion O, Yunits B, Zhu X, Garmire LX. DeepImpute: an accurate, fast, and scalable deep neural network method to impute single-cell RNA-seq data. Genome Biol. 2019;20:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 25. | Hsieh C, Laguna A, Ikeda I, Maxwell AWP, Chapiro J, Nadolski G, Jiao Z, Bai HX. Using Machine Learning to Predict Response to Image-guided Therapies for Hepatocellular Carcinoma. Radiology. 2023;309:e222891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 28] [Reference Citation Analysis (0)] |
| 26. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 5321] [Article Influence: 886.8] [Reference Citation Analysis (29)] |
| 27. | Rogers SO Jr, Gawande AA, Kwaan M, Puopolo AL, Yoon C, Brennan TA, Studdert DM. Analysis of surgical errors in closed malpractice claims at 4 liability insurers. Surgery. 2006;140:25-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 287] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 28. | Way LW, Stewart L, Gantert W, Liu K, Lee CM, Whang K, Hunter JG. Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 2003;237:460-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 460] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 29. | Madani A, Namazi B, Altieri MS, Hashimoto DA, Rivera AM, Pucher PH, Navarrete-Welton A, Sankaranarayanan G, Brunt LM, Okrainec A, Alseidi A. Artificial Intelligence for Intraoperative Guidance: Using Semantic Segmentation to Identify Surgical Anatomy During Laparoscopic Cholecystectomy. Ann Surg. 2022;276:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 206] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 30. | Bhat M, Rabindranath M, Chara BS, Simonetto DA. Artificial intelligence, machine learning, and deep learning in liver transplantation. J Hepatol. 2023;78:1216-1233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 31. | Zhou KQ, Sun YF, Cheng JW, Du M, Ji Y, Wang PX, Hu B, Guo W, Gao Y, Yin Y, Huang JF, Zhou J, Fan J, Yang XR. Effect of surgical margin on recurrence based on preoperative circulating tumor cell status in hepatocellular carcinoma. EBioMedicine. 2020;62:103107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 32. | Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1832] [Article Influence: 83.3] [Reference Citation Analysis (3)] |
| 33. | Chen Q, Xiao H, Gu Y, Weng Z, Wei L, Li B, Liao B, Li J, Lin J, Hei M, Peng S, Wang W, Kuang M, Chen S. Deep learning for evaluation of microvascular invasion in hepatocellular carcinoma from tumor areas of histology images. Hepatol Int. 2022;16:590-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 34. | Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS, Therneau TM. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation. 2018;102:1710-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (1)] |
| 35. | Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, Schnitzler MA, Gheorghian A, Salvalaggio PR, Segev DL. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11:2362-2371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 167] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Uche-Anya E, Anyane-Yeboa A, Berzin TM, Ghassemi M, May FP. Artificial intelligence in gastroenterology and hepatology: how to advance clinical practice while ensuring health equity. Gut. 2022;71:1909-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/