Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110247
Revised: July 9, 2025
Accepted: September 19, 2025
Published online: October 27, 2025
Processing time: 147 Days and 4.8 Hours
Ascites is the most common complication of cirrhosis. Current pharmacological interventions, such as diuretics, often become ineffective in advanced stages due to diuretic resistance. Sodium-glucose co-transporter 2 (SGLT2) inhibitors have demonstrated potential in enhancing urinary sodium excretion and mitigating sodium-fluid retention. This study aims to evaluate the effects of SGLT2 inhibitors on the fractional excretion of sodium (FENa) in patients with cirrhotic ascites.
To determine whether adjunctive therapy with the SGLT2 inhibitor empagliflozin increases FENa compared with standard care alone in patients with cirrhosis and refractory ascites, and to evaluate its short-term safety profile.
The effect of SGLT2 inhibitor empagliflozin on FENa in patients with cirrhosis and refractory ascites is a multicenter, open-label, randomized controlled trial. A total of 70 patients with refractory ascites secondary to cirrhosis will be enrolled and randomly assigned to receive either empagliflozin 10 mg daily plus standard care or standard care alone for 14 consecutive days. The primary outcome is the change in FENa from baseline to day 14. Secondary outcomes include 24-hour urinary sodium excretion, urine volume, ascites volume (assessed by ult
This article reports the study protocol only. No participant data have been collected or analyzed for this manu
This protocol evaluates whether empagliflozin, added to standard therapy, increases sodium excretion and reduces fluid overload in refractory ascites.
Core Tip: Refractory ascites reflects proximal tubular sodium retention and frequent diuretic resistance in cirrhosis. We present a multicenter randomized protocol testing empagliflozin, a sodium-glucose co-transporter 2 inhibitor that promotes proximal tubular natriuresis without renin-angiotensin-aldosterone system stimulation, for refractory cirrhotic ascites. The trial compares empagliflozin plus standard care versus standard care over 14 days. Primary endpoint is change in fractional excretion of sodium; secondary outcomes include 24-hour urinary sodium, urine volume, ascites by ultrasound, body weight, and prespecified clinical safety parameters.
- Citation: Gao Y, Gao YY, Shi RY, Ji D, Wang Y, Xu L, Wang Q, Wu MH, You HL, Bu QS, Dong YX, Zhou LZ, Liu W, Song QK, Han Y, Wei H, Zhang XY, Hu ZJ. Effect of empagliflozin on fractional excretion of sodium in patients with cirrhosis and refractory ascites. World J Hepatol 2025; 17(10): 110247
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/110247.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.110247
Ascites, the most common complication of cirrhosis, significantly affects patients’ prognosis, with an estimated two-year mortality rate approaching 40%[1]. Current therapeutic strategies, primarily involving sodium restriction and diuretic therapy, frequently fail to prevent progression to refractory ascites, which is associated with a median survival time of approximately 6 months[2]. Moreover, the quality of life deteriorates markedly, leading to unplanned hospital rea
The pathophysiological features of ascites involve sodium and water retention, driven by progressively failing circulatory dysfunction. This process is mediated by inappropriate activation of the renin-angiotensin-aldosterone system (RAAS) in response to a reduced effective arterial blood volume[6]. Current interventions, including invasive procedures and liver transplantation, are accompanied by remarkable costs, risks, and restricted applications[7]. Meanwhile, pharmacological interventions (e.g., diuretics) fail to address the RAAS overactivation and only promote transient relief of symptoms.
Sodium-glucose co-transporter 2 (SGLT2) inhibitors, originally approved for the treatment of type 2 diabetes, inhibit the reabsorption of glucose and sodium in the S1 and S2 segments of proximal renal tubules. This action results in the increased urinary excretion of glucose and sodium, accompanied by osmotic diuresis. In contrast to loop diuretics, which stimulate renin secretion[8], SGLT2 inhibitors promote sodium excretion and enhance tubuloglomerular feedback, thereby promoting natriuresis without further activating the RAAS[9]. Recent studies demonstrated that the diuretic effects of SGLT2 inhibitors may synergize with conventional diuretics to potentially alleviate the diuretic resistance, particularly in advanced cirrhosis[10,11].
Preliminary data, including case reports, have demonstrated that SGLT2 inhibitors can reduce ascites and improve serum sodium levels with minimal adverse effects[12-14]. Moreover, recent studies demonstrated that these agents can decrease the need for large-volume paracentesis in patients with refractory ascites and reduce rehospitalization rates[15]. These findings highlight the potential of SGLT2 inhibitors not only as a treatment option for diabetes but also for managing fluid retention in cirrhosis, providing a new direction for the management of ascites that warrants further clinical evaluation[16-18]. Empagliflozin, which is primarily eliminated via the kidneys, is minimally affected by hepatic impairment in terms of its metabolism[19,20]. Additionally, phase III clinical trials involving patients with diabetes and heart failure have reported no liver-related adverse reactions to empagliflozin[21,22], recognizing a safety profile that is equally significant when it is administered to treat cirrhotic ascites.
The effect of empagliflozin on fractional excretion of sodium (FENa) in patients with cirrhosis and refractory ascites (EASTERN) study was initiated to evaluate the impact of empagliflozin on sodium excretion and its safety profile in patients with cirrhosis and refractory ascites. This research represents a critical step toward advancing the management of ascites and may serve as a basis for future studies aimed at reducing rehospitalization rates in patients with cirrhotic ascites in long-term outpatient settings.
Hypothesis: It is hypothesized that in patients with cirrhosis and refractory ascites, the addition of SGLT2 inhibitors to standard care may result in a significant increase in FENa compared with standard treatment alone.
Research question: The primary objective of this research is to assess the effects of SGLT2 inhibitors in augmenting FENa and 24-hour urinary sodium excretion from baseline to day 14 in patients with cirrhosis and refractory ascites.
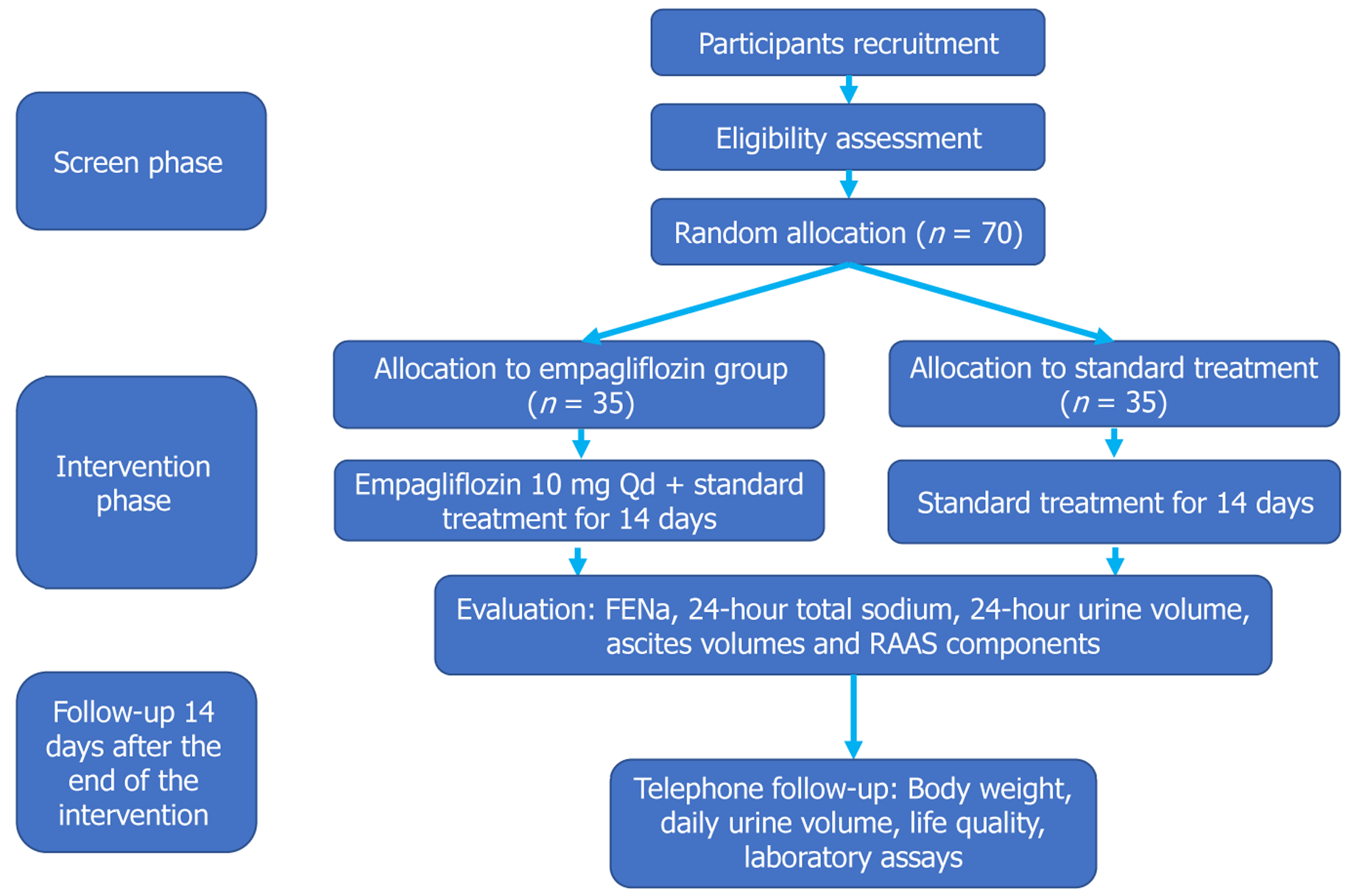
The EASTERN study is a multicenter, open-label, randomized controlled trial. It plans to recruit 70 patients with liver cirrhosis and refractory ascites. Participants will be randomly allocated to either receive empagliflozin in addition to standard care for cirrhosis or standard care alone. The intervention period will last for 14 days or until hospital discharge, whichever occurs first, and will be followed by a 14-day follow-up period. At each study site, group assignments will be determined using pre-prepared block randomization envelopes to ensure a 1:1 allocation ratio between the empagliflozin and standard care groups. No stratification will be used in the randomization process.
The EASTERN study will recruit patients from six centers: Beijing You’an Hospital Affiliated to Capital Medical University, Baoding People’s Hospital, Beijing Friendship Hospital Affiliated to Capital Medical University, Fifth Medical Center of the Chinese PLA General Hospital, Tianjin Second People’s Hospital, and Beijing Ditan Hospital Affiliated to Capital Medical University. All participating institutions are tertiary or tertiary A hospitals located in Beijing, Tianjin, or Baoding, with well-established expertise in the diagnosis, treatment, and research of liver diseases.
Eligible participants should meet all of the following inclusion criteria: (1) Age 18 years or older; (2) Diagnosis of cirrhosis, defined by evidence of hepatocellular dysfunction or portal hypertension based on imaging, biochemical, or hematological tests, or histological findings consistent with cirrhosis; (3) Diagnosis of refractory ascites, defined by one or more of the following: Diuretic-resistant ascites - absence of satisfactory weight loss or fluid mobilisation after ≥ 7 days of combined spironolactone 80-160 mg/day and furosemide 40-80 mg/day, or diuretic-intractable ascites - uncontrolled diuretic-induced complications (e.g., symptomatic hypotension, severe electrolyte imbalance, renal impairment) that preclude escalation to the above doses, or lack of response to therapeutic intermittent paracentesis (4000-5000 mL/per session) combined with human albumin (20-40 g/per session/day) for at least two weeks, diuretic-related complications or adverse reactions that are unmanageable, exclusion of malignant ascites and ascites due to pre-sinusoidal portal hypertension; and (4) Ability to provide informed consent and sign the consent form independently.
Participants will be excluded if they meet any of the following conditions: (1) Systolic blood pressure < 95 mmHg or diastolic blood pressure < 60 mmHg; (2) Estimated glomerular filtration rate < 45 mL/minutes/1.73 m2; (3) History of esophageal or gastric variceal bleeding within 3 months prior to screening; (4) History of grade II or higher hepatic encephalopathy within 3 months prior to screening; (5) Presence of advanced liver cancer; (6) History of alcohol or drug abuse within 6 months prior to screening; (7) Alcohol consumption within 2 weeks prior to screening; (8) Use of SGLT2 inhibitors within 3 months prior to screening; (9) Model for end-stage liver disease score > 20 or an expected median survival < 6 months; (10) Anemia with hemoglobin < 7 g/dL; (11) Use of diuretics other than spironolactone or furo
Before initiating patient recruitment, each participating site must obtain formal approval from its respective hospital’s research ethics committees, such as the institutional review boards. This approval is essential to ensure adherence to ethical standards and regulatory guidelines. During the recruitment phase, potential participants, or their legal surrogate, will be assisted by a designated trial coordinator at each site to complete a detailed questionnaire assessing their medical conditions. Subsequently, each candidate will undergo a comprehensive physical examination by an attending hepatologist. Diagnostic imaging examinations, such as ultrasound or computed tomography scans, will be conducted to con
To ensure full adherence to ethical and legal standards, no subject will be enrolled in the study without fulfilling all required conditions. Written informed consent will be obtained from each participant or their legal surrogate by the attending physician. This consent process will cover all aspects of the study, including the potential risks and benefits of the treatment, participants’ data privacy rights, the voluntary nature of participation, and the procedures for data anonymization to ensure confidentiality. After receiving comprehensive information about the study, each participant will be allotted a minimum of 6 hours and up to 24 hours to decide whether to participate. This process is designed to ensure that participants can make an informed decision about their involvement in the study.
As part of the informed consent process for the EASTERN study, participants will be asked to provide additional permission for the use of their data and biological specimens beyond the primary scope of evaluating the effects of SGLT2 inhibitors on sodium excretion in patients with cirrhosis and ascites or pleural effusion. The protocol has been approved by the Ethics Committee of Beijing You’an Hospital, Capital Medical University (No. LL-2024-151-K), and subsequently approved by the ethics committee of each participating sub-center following approval by You’an Hospital. All procedures related to the sample collection, storage, and future use of participant data and biological specimens will strictly adhere to the standard protocol items: Recommendations for interventional trials guidelines and the principles of the Declaration of Helsinki. In addition, compliance with good clinical practice standards, relevant local regulatory requirements, and applicable laws will be maintained to ensure the protection of participants’ rights, safety, and well-being. Before enro
The comparator group will include patients with decompensated cirrhosis and refractory ascites who will receive standard medical management in accordance with current clinical guidelines. This standard treatment includes sodium-restricted diets, diuretic therapy to control fluid retention, and, when indicated, therapeutic paracentesis for fluid removal along with albumin intravenous infusion[1].
All interventions will be conducted in an open-label manner, and participants will be fully aware of the treatment they receive. On the first day of the study period, patients in the empagliflozin group will initiate treatment with a daily oral dose of 10 mg empagliflozin, to be taken each morning in a fasting state. This regimen will continue for a total of 14 days. If a patient is discharged before completing the 14-day period, the administration of empagliflozin will cease on the day of discharge. At the end of the 14-day intervention or prior to discharge, ultrasound assessments of ascites volumes will be conducted. Each participant will be provided with a 4000 mL graduated cylinder to collect all urine produced from 8 AM until 8 AM the following day. Urinary volume will be recorded every 24 hours. For biochemical analysis, a 10 mL aliquot will be drawn each morning from the total collected volume for quantitative assays, including urinary ele
For participants in the empagliflozin group, the intervention will be discontinued either upon completion of the 14-day treatment course or at the time of hospital discharge, whichever occurs first. A telephone follow-up will be conducted to assess the patient’s life status, daily urine output, and changes in body weight 14 days after discharge. Patients will be instructed to return to the hospital for a routine follow-up visit within 14 ± 7 days after discharge. This visit will include assessments of liver function, renal function, blood biochemistry, urinary electrolytes, urine glucose quantification, and routine urinalysis. All tests conducted during the follow-up visit will be provided at no cost to the patient.
In the EASTERN trial, several criteria have been established for discontinuing or modifying the allocated interventions to ensure patient safety. If participants experience any of the following conditions during the study, the research will be terminated and active interventions will be taken to ensure patient safety, including: (1) Severe hypotension: If a par
Each participant has the right to refuse treatment or withdraw from the trial at any time for any reason. Participants may also be required to withdraw from the trial under certain conditions: (1) Poor compliance with the study protocol by the participant; (2) Occurrence of unacceptable adverse reactions or serious adverse events (SAEs); (3) Emergence of serious complications during the trial that require urgent interventions; (4) Voluntary withdrawal of consent by the participant; and (5) Any other special circumstances, as determined by the investigator, that warrant participant with
Prior to enrollment, it is crucial to engage in detailed discussions with potential participants to explain the mechanism of action of SGLT2 inhibitors, their potential benefits, and common side effects. The medical staff responsible for patient enrollment in the EASTERN study must ensure that all participants fully understand the study protocol and receive appropriate counseling to address any questions or concerns. It is important to emphasize that SGLT2 inhibitors are widely used in clinical practice and have a well-established safety profile in the management of diabetes. Furthermore, the use of empagliflozin for a short duration of 14 days in a controlled hospital setting among cirrhotic patients is expected to pose minimal safety concerns. The brief treatment period and close clinical monitoring are designed to further ensure patient safety. A protocol will be implemented to monitor urinary glucose levels as an indirect measure of treatment adherence. The presence of increased urinary glucose level reflects the pharmacological effect of SGLT2 inhibitors and can be used to confirm that participants in the intervention group are taking the medication as prescribed. To ensure the integrity of the study, urinary glucose levels will also be assessed in the control group to confirm that they have not inadvertently or intentionally received SGLT2 inhibitors. This measure is essential to maintain the validity of the study outcomes, as any urinary glucose elevation in the control group may indicate unsanctioned medication use.
The EASTERN study is conducted in a hospital setting, enabling cirrhotic patients to receive appropriate medical care based on their clinical condition. However, the administration of certain medications is prohibited, as they may significantly influence the study endpoints. These include: (1) An increase in diuretic dosage exceeding 100% during the study period; (2) Use of tolvaptan; and (3) Use of vasopressin or its analogs, such as terlipressin or selepressin. Addi
In the post-trial phase, all participants will receive standard care for cirrhosis as recommended by their healthcare providers. Regular follow-up visits will be scheduled for two weeks post-trial to evaluate the outpatient treatment effects and to manage any ongoing complications, and the related tests during these outpatient follow-up visits will be free of charge.
The primary outcome of this study is the change in the FENa from baseline to day 14. Comparison of FENa will be conducted on day 1, day 3, day 7, day 10, and day 14. Secondary outcomes will concentrate on clinical improvements and safety profiles, including monitoring changes in 24-hour total urinary sodium and urine volume on days 1, 3, 7, 10, and 14 to assess urinary sodium excretion and overall urine output. Ascites volume will be measured by ultrasound at baseline and again on day 14 or the day of discharge to evaluate changes in fluid accumulation. Changes in body weight from baseline to day 14 or discharge will also be documented. Safety assessments will include monitoring blood pressure, liver and renal function tests, blood glucose level, and other relevant clinical parameters to evaluate the safety profile of empagliflozin in this patient population. Exploratory endpoints concentrate on the RAAS components and related neurohumoral changes after administration of empagliflozin. Levels of norepinephrine, plasma renin activity, total renin, aldosterone, and angiotensin II will be measured at baseline and on day 14 or on the discharge day.
Upon enrollment, baseline demographic and clinical characteristics of participants, including medical history, severity of cirrhosis, extent of ascites, and baseline biochemical results, will be collected. These initial evaluations ensure that all participants meet the study inclusion criteria without any of the exclusion criteria, impacting their eligibility. Treatment with empagliflozin or continuation of standard care is initiated immediately following randomization. Standard care adheres to the current guidelines for managing decompensated cirrhosis with ascites[1]. Protocol-mandated assessments and laboratory tests are outlined in Table 1. The overall study flowchart, from screening to follow-up, is depicted in Figure 1.
| Study period | Screening period (14-day) | Patient enrollment (day 0) | Day 1 | Day 3 | Day 7 | Day 10 | Day 141 | Day 282 (end of follow-up) |
| Informed consent | √ | |||||||
| Demographic data | √ | |||||||
| Medical history | √ | |||||||
| Inclusion/exclusion criteria | √ | |||||||
| Vital signs | √ | √ | √ | √ | √ | √ | √ | √ |
| Blood routine + reticulocytes | √ | √ | √ | |||||
| HbA1c3 | √ | |||||||
| Urine routine | √ | √ | √ | √ | √ | √ | ||
| Urine electrolytes + urine creatinine + urine glucose4 | √ | √ | √ | √ | √ | √ | √ | |
| Liver and kidney function + blood glucose + cardiac enzymes + blood lipids | √ | √ | √ | √ | ||||
| AFP and PIVKA-II | √ | |||||||
| Abdominal ultrasound + ascites | √ | √ | √ | |||||
| Enhanced abdominal CT5 | √ | |||||||
| ECG | √ | |||||||
| Echocardiography | √ | |||||||
| 24-hour urine measurement | √ | √ | √ | √ | √ | √ | ||
| Blood sample collection | √ | √ | ||||||
| RAAS assays6 | ||||||||
| Urine sample collection | √ | √ | ||||||
| Concomitant medication | √ | √ | √ | √ | √ | √ | √ | √ |
| Adverse events | √ | √ | √ | √ | √ | √ | ||
| Body weight | √ | √ | √ | √ | √ | √ | √ |
Based on our preliminary pilot data and historical data, the FENa in patients with cirrhotic ascites is approximately 0.5[23,24]. With empagliflozin intervention, we anticipate an increase in FENa to approximately 0.8. Assuming a standard deviation of 0.4 in both the control and empagliflozin groups, we calculated the required sample size using a two-sample t-test with a two-sided significance level of 0.05 (α = 0.05) and 80% power to detect the expected difference. The analysis indicates that 32 patients per group are required, accounting for a total of 64 participants, to adequately power the study. To account for potential loss to follow-up, a total of 70 patients will be enrolled.
Patients will be identified in the hepatology departments of each participating site and assessed for eligibility by a medically qualified individual. Eligible patients will be referred to the research coordinator and provided with detailed information about the study. Sufficient time will be given for participants to consider their involvement. Informed written consent will be obtained by a trained research coordinator at each study site after participants have had the opportunity to review the study information and ask questions.
Eligible participants will be randomly assigned in a 1:1 ratio to either the empagliflozin group (standard care combined with empagliflozin) or the blank control group (standard care alone). Block randomization with a block size of 4 will be employed to ensure balanced allocation of participants across study groups. To manage the randomization process, 20 Large sealed envelopes are prepared, each containing four smaller sealed envelopes. Each smaller envelope contains allocation information and is numbered in a random order and contains two “A” (empagliflozin group) and two “B” (blank control group), respectively. The approach ensures that the randomization process remains unbiased and free from investigator influence or interference.
Although this study utilizes a relatively simple block randomization approach, the concealment mechanism ensures that clinical doctors and researchers are unaware of participants’ group allocation prior to randomization. The use of pre-prepared opaque large envelopes containing four smaller opaque envelopes effectively maintains confidentiality during the randomization process. After the randomization, the research coordinators, clinical doctors, and participants will be informed of the group allocation.
In this study, the domestic formulation of empagliflozin, commercially known as Saikefei and manufactured by Chia Tai Tianqing Pharmaceutical Group Co., Ltd., will be used. This product holds a good manufacturing practice certification issued by the State Food and Drug Administration of China and has passed the national quality and efficacy consistency evaluation for generic drugs. Patients assigned to the empagliflozin group will receive treatment on the first day of the study period. A 10 mg dose of empagliflozin will be administered orally each morning under the supervision of ward nurses to ensure adherence. The medication will be stored and dispensed in accordance with the hospital’s drug safety protocols to ensure proper handling and maintain the integrity of the treatment regimen.
To ensure effective and safe treatment, this study is conducted as an open-label trial. All participants, healthcare providers, and outcome assessors will be aware of the treatment assignments following randomization, whether to the empagliflozin plus standard care group (intervention) or the standard care alone group (comparator). However, the data analysts will remain blinded to the allocation, except when unblinding is necessary for analysis or data cleaning as directed by the independent data monitoring committee (IDMC).
Sonographers who acquire the ultrasound images are unaware of treatment allocation. Laboratory staff, the central sta
Data will be collected using specially designed paper-based case report forms (CRFs). Once completed, these forms will be entered into a database by designated personnel following data inspection. The accompanying table provides a detailed illustration of both the schedule and the specific nature of data collection required throughout the study period.
Participants will be contacted by phone call or short message service to invite them to the follow-up assessment. They may attempt to complete the follow-up either via telephone or through WeChat, using the designated contact points outlined in Table 1. A site research coordinator will conduct a comprehensive follow-up call approximately two weeks after discharge to assess urine output, body weight, activities of daily living, and any adverse events. During this call, a follow-up visit will also be scheduled. All examinations conducted during the visit will be detailed in Table 1.
Data for the trial are gathered and stored in compliance with Good Clinical Practice guidelines {China National Medical Products Administration, National Health Committee [Notice on the Publication of Good Clinical Practice (No. 57 of 2020), April 26, 2020 (in Chinese); available from: https://www.nmpa.gov.cn/directory/web/nmpa/xxgk/ggtg/ypggtg/ypqtggtg/20200426162401243.html (accessed July 6, 2025)]}. The data management plan outlines methods to ensure the accuracy of trial data. The trial manager, statistician, and programmer will work collaboratively to develop standardized procedures for coding and validation. The study database receives final approval only after the con
Stringent measures are implemented to protect participants’ privacy. All data used for statistical analysis and subsequent publication will be anonymized to ensure confidentiality. Identifiable information will be replaced with subject numbers in all documentation provided to the investigator. Furthermore, a consolidated table linking subjects’ names to their numbers will be securely maintained by the data management team and the principal investigator and will not be dis
Laboratory tests are conducted according to the standard blood collection procedures of Beijing You’an Hospital. In the study, plasma and urine samples collected as specified in Table 1 will be labeled and stored in a -80 °C freezer designated for scientific research.
Primary outcomes: A linear mixed-effects model will be utilized to compare changes in FENa from baseline to treatment between the empagliflozin group and the blank control group.
Secondary outcomes: A linear mixed-effects model will also be employed to analyze changes in 24-hour urinary sodium excretion and urine volume at the specified time points (baseline, day 1, day 3, day 7, day 10, and day 14). This approach enables the modeling of time as a fixed effect and participants as random effects, thereby accounting for intra-individual correlations across repeated measurements. Linear mixed-effects model analysis of changes in body weight will also be applied over the course of the study at the specified time points (baseline, day 1, day 3, day 7, day 10, and day 14). All potential confounders will also be adjusted for, such as age, gender, baseline disease severity, and any other relevant baseline characteristics identified during the analysis phase. Different variance-covariance structures will be evaluated to identify the model that best fits the data. The Wilcoxon signed-rank test will be applied to compare the ordinal levels of ascites volume at two time points: At baseline (pre-treatment) and at day 14 or day of discharge (post-treatment).
Exploratory endpoints: The exploratory endpoint of this study involves assessing the impact of empagliflozin on components of the RAAS in patients with cirrhosis, diabetes, and ascites. The aim is to compare the levels of RAAS components at the beginning and end of the in-hospital intervention. These components include plasma renin concentration, plasma renin activity, angiotensin-II level, and aldosterone concentration. In addition, the refractory-ascites category (resistant vs intractable) will be included as a fixed covariate. Interaction terms will be explored to assess the consistency of the treatment effect. An exploratory subgroup analysis will compare primary and secondary outcomes between the two categories.
Given the short duration of the intervention and follow-up, each lasting only 2 weeks, and the relatively small sample size of 70 participants, interim analyses are not planned for this study. The expected recruitment process, projected to be completed within one year, further supports the decision to forgo interim assessments.
The rationale for selecting glycated hemoglobin (HbA1c) as the basis for subgroup analysis originates from its clinical significance in reflecting recent glycemic control and its potential to influence the therapeutic effects of empagliflozin on patients with cirrhosis and ascites. The analysis will compare outcomes between the two specific subgroups: Participants with HbA1c levels greater than 6.5%, indicating recent hyperglycemia or poorly controlled diabetes; participants with HbA1c levels less than or equal to 6.5%, indicating recent normoglycemia or well-controlled diabetes.
In this study, the protocol’s non-adherence will be assessed and documented rigorously. A per-protocol analysis will be conducted to assess the impact of adherence on the study outcomes. For missing data, multiple imputation techniques will be employed under the assumption that data are missing at random. Specifically, if more than 15% of FENa data points are missing, multiple imputation will be applied to perform sensitivity analysis of the primary outcome.
The anonymized trial dataset and associated statistical code will be available to all study investigators and to external researchers following the completion of the study. Access to the data will be granted from 6 months after the publication of the main trial results to allow for further analyses and initial publications by other study teams. External researchers interested in accessing the data will be required to reach a data sharing agreement that specifies the terms of use, confidentiality obligations, and the intended use of the data. Requests for data access should be submitted in writing and include a detailed research proposal. These requests will be reviewed by the chief investigator in consultation with the co-investigator group to ensure scientific merit and alignment with ethical guidelines.
This trial is coordinated by the trial management group (TMG) of Beijing You’an Hospital. The TMG consists of a chief investigator, a clinical trial statistician, and other key members of the trial management team. Additionally, ward physicians involved in patient enrollment at the clinical trials unit may attend meetings as needed. Each participating site, including Beijing You’an Hospital, has a designated local principal investigator who reports directly to the TMG. The TMG convenes at least once every three months, or more frequently, if necessary, to review trial progress, address emerging issues, and ensure adherence to the study protocol. The TMG is responsible for comprehensive oversight of the trial, including the management of operational activities, protecting participant safety, and ensuring that sufficient research resources and support are maintained throughout the study. The TMG will also review clinical trial data and, when appropriate, make decisions regarding trial continuation or termination based on safety and efficacy considerations.
The IDMC for this trial is composed of a senior clinical expert and a statistician, both selected for their expertise in overseeing clinical trials. The IDMC is responsible for the ongoing evaluation of the trial’s progress, with particular attention to participant safety, key clinical endpoints, and the overall scientific integrity of the study. Its duties include conducting confidential data analyses beginning six months after trial initiation and making evidence-based recommendations regarding whether to continue, modify, or terminate the study. The IDMC meets at least twice per year, as outlined in the IDMC charter, with the flexibility to convene more frequently if needed. All recommendations from the IDMC are reported directly to the TMG, subsequently communicating them to the trial’s chief investigator.
SAE in the EASTERN study is defined as any event that causes significant harm to a participant or requires medical intervention to prevent serious harm, regardless of whether it is directly related to the study medication, including empagliflozin. Non-SAEs include any undesirable medical occurrences in a participant, including abnormal laboratory findings that lack clinical significance. All SAEs must be reported within 24 hours of the site investigator becoming aware of the event. A comprehensive report must be submitted to the chief investigator and the appropriate regulatory authority, such as the state food and drug administration. Upon SAE notification, the ethics committee responsible for pharmaceutical oversight at the coordinating center will initiate an investigation to determine the cause and implications of the event. All SAEs must be thoroughly documented in the participant’s medical records or trial file. This docu
In the EASTERN study, the CRFs are initially reviewed and verified by two independent investigators at each par
Any protocol modifications that may impact the study’s conduct, participant safety, potential benefits, or scientific validity, including changes to study objectives, design, eligibility criteria, sample size, procedures, or significant administrative details, will require a formal amendment. Such amendments must be approved by the trial steering committee and the ethics committee prior to implementation. Amendments arising during trial execution must be initiated by the chief investigator and subsequently discussed with the multi-centre coordinating committee. These proposed amendments should be documented and signed by the chief investigator and the involved centers, and they can only be implemented following approval from the ethics committee.
The findings of this study will be disseminated to healthcare providers and the scientific community through high-impact, peer-reviewed journals, as well as presentations at national and international hepatology and gastroenterology conferences. Additionally, a series of seminars will be organized at the end of the study. Influential experts in the field will be invited to these seminars, where the practical application of the research findings in clinical practice and their potential integration into current guidelines or updated consensus will be discussed.
This article reports the study protocol only. No participant data have been collected or analyzed for this manuscript. Recruitment is planned to start in February 2025, with a target sample size of 70. The trial is prospectively registered at https://www.chictr.org.cn/ (ChiCTR2500095222); current protocol version: [v1.0, Dec 11, 2024].
Ascites, the most prevalent complication among patients with cirrhosis, presents substantial challenges in therapeutic management, particularly due to the remarkable occurrence of diuretic resistance even when renal function remains within normal ranges. Despite adherence to diuretic regimens, a significant proportion of patients progressively become less responsive to such treatments, namely “diuretic resistance”. This is primarily attributed to the increased sodium reabsorption in the proximal tubule among patients with cirrhotic ascites, leading to hypotonic tubular fluid reaching the distal tubule and thereby reducing the effectiveness of diuretics acting on this segment. SGLT2 inhibitors, inhibiting sodium reabsorption in the proximal tubule, may exert a synergistic effect with traditional diuretics acting on the distal nephron.
The EASTERN study aims to explore whether the addition of the SGLT2 inhibitor (empagliflozin) to a standard therapy can further enhance urinary sodium excretion in hospitalized patients with cirrhotic ascites. This field has recently attracted hepatologists’ attention, and several small-scale clinical studies have already been published[15,20]. Clinical events were not selected as primary outcomes, as they are difficult to assess in a short-term, open-label study with a limited sample size. Alternatively, FENa is employed as a direct biochemical marker of sodium excretion, pro
Several limitations of the trial design are acknowledged, including the potential for selection bias inherent in open-label studies. To mitigate this, objective biochemical endpoints are prioritized over subjective clinical outcomes. Additionally, the relatively short follow-up period may constrain the assessment of the long-term therapeutic benefits of empagliflozin. Nevertheless, if a small-molecule agent demonstrates consistent enhancement of urinary sodium excretion over a 14-day period, it provides a strong rationale for subsequent longer-term trials investigating its role in the management of cirrhotic ascites. Furthermore, the evaluation of RAAS components and subgroup analyses stratified by HbA1c levels may yield valuable mechanistic insights, potentially laying the groundwork for future studies aimed at determining whether prolonged SGLT2 inhibitor therapy can reduce rehospitalization rates in patients with cirrhotic ascites in the outpatient setting.
This multicenter, open-label randomized trial will determine whether short-term administration of empagliflozin safely augments natriuresis and reduces fluid overload in patients with cirrhosis and refractory ascites. Positive findings will lay the groundwork for long-term outcome-oriented studies and may introduce a novel pharmacologic option for mana
| 1. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69:406-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1777] [Cited by in RCA: 1985] [Article Influence: 248.1] [Reference Citation Analysis (2)] |
| 2. | Macken L, Joshi D, Messenger J, Austin M, Tibble J, Mason L, Verma S. Palliative long-term abdominal drains in refractory ascites due to end-stage liver disease: A case series. Palliat Med. 2017;31:671-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Ge PS, Runyon BA. Treatment of Patients with Cirrhosis. N Engl J Med. 2016;375:767-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 244] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 4. | Volk ML, Tocco RS, Bazick J, Rakoski MO, Lok AS. Hospital readmissions among patients with decompensated cirrhosis. Am J Gastroenterol. 2012;107:247-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 371] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 5. | Hudson B, Round J, Georgeson B, Pring A, Forbes K, McCune CA, Verne J. Cirrhosis with ascites in the last year of life: a nationwide analysis of factors shaping costs, health-care use, and place of death in England. Lancet Gastroenterol Hepatol. 2018;3:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 6. | Arroyo V, Fernandez J. Pathophysiological basis of albumin use in cirrhosis. Ann Hepatol. 2011;10 Suppl 1:S6-14. [PubMed] |
| 7. | Moore CM, Van Thiel DH. Cirrhotic ascites review: Pathophysiology, diagnosis and management. World J Hepatol. 2013;5:251-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Felker GM, Ellison DH, Mullens W, Cox ZL, Testani JM. Diuretic Therapy for Patients With Heart Failure: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75:1178-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 285] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 9. | Verma A, Patel AB, Waikar SS. SGLT2 Inhibitor: Not a Traditional Diuretic for Heart Failure. Cell Metab. 2020;32:13-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and Cardiovascular Effects of SGLT2 Inhibition in Combination With Loop Diuretics in Patients With Type 2 Diabetes and Chronic Heart Failure: The RECEDE-CHF Trial. Circulation. 2020;142:1713-1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 191] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 11. | Griffin M, Rao VS, Ivey-Miranda J, Fleming J, Mahoney D, Maulion C, Suda N, Siwakoti K, Ahmad T, Jacoby D, Riello R, Bellumkonda L, Cox Z, Collins S, Jeon S, Turner JM, Wilson FP, Butler J, Inzucchi SE, Testani JM. Empagliflozin in Heart Failure: Diuretic and Cardiorenal Effects. Circulation. 2020;142:1028-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 321] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 12. | Montalvo-Gordon I, Chi-Cervera LA, García-Tsao G. Sodium-Glucose Cotransporter 2 Inhibitors Ameliorate Ascites and Peripheral Edema in Patients With Cirrhosis and Diabetes. Hepatology. 2020;72:1880-1882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Kalambokis GN, Tsiakas I, Filippas-Ntekuan S, Christaki M, Despotis G, Milionis H. Empagliflozin Eliminates Refractory Ascites and Hepatic Hydrothorax in a Patient With Primary Biliary Cirrhosis. Am J Gastroenterol. 2021;116:618-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Miyamoto Y, Honda A, Yokose S, Nagata M, Miyamoto J. Weaning from concentrated ascites reinfusion therapy for refractory ascites by SGLT2 inhibitor. Clin Kidney J. 2022;15:831-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 15. | Gao Y, Wei L, Zhang DD, Chen Y, Hou B. SGLT2 Inhibitors: A New Dawn for Recurrent/Refractory Cirrhotic Ascites. J Clin Transl Hepatol. 2021;9:795-797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Gao Y, Liu X, Gao Y, Duan M, Hou B, Chen Y. Pharmacological Interventions for Cirrhotic Ascites: From Challenges to Emerging Therapeutic Horizons. Gut Liver. 2024;18:934-948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Siafarikas C, Kapelios CJ, Papatheodoridi M, Vlachogiannakos J, Tentolouris N, Papatheodoridis G. Sodium-glucose linked transporter 2 inhibitors in liver cirrhosis: Beyond their antidiabetic use. Liver Int. 2024;44:884-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 18. | Macha S, Rose P, Mattheus M, Cinca R, Pinnetti S, Broedl UC, Woerle HJ. Pharmacokinetics, safety and tolerability of empagliflozin, a sodium glucose cotransporter 2 inhibitor, in patients with hepatic impairment. Diabetes Obes Metab. 2014;16:118-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Shen I, Stojanova J, Yeo M, Olsen N, Lockart I, Wang M, Roggeveld J, Heerspink HJL, Greenfield JR, Day R, Danta M. A potential novel treatment for cirrhosis-related ascites: Empagliflozin is safe and tolerable in advanced chronic liver disease. Br J Clin Pharmacol. 2024;90:2529-2538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 20. | Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE; EMPA-REG OUTCOME Investigators. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373:2117-2128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7124] [Cited by in RCA: 8728] [Article Influence: 793.5] [Reference Citation Analysis (2)] |
| 21. | Anker SD, Butler J, Filippatos G, Ferreira JP, Bocchi E, Böhm M, Brunner-La Rocca HP, Choi DJ, Chopra V, Chuquiure-Valenzuela E, Giannetti N, Gomez-Mesa JE, Janssens S, Januzzi JL, Gonzalez-Juanatey JR, Merkely B, Nicholls SJ, Perrone SV, Piña IL, Ponikowski P, Senni M, Sim D, Spinar J, Squire I, Taddei S, Tsutsui H, Verma S, Vinereanu D, Zhang J, Carson P, Lam CSP, Marx N, Zeller C, Sattar N, Jamal W, Schnaidt S, Schnee JM, Brueckmann M, Pocock SJ, Zannad F, Packer M; EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. 2021;385:1451-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3009] [Cited by in RCA: 3195] [Article Influence: 639.0] [Reference Citation Analysis (0)] |
| 22. | Angeli P, Gatta A, Caregaro L, Menon F, Sacerdoti D, Merkel C, Rondana M, de Toni R, Ruol A. Tubular site of renal sodium retention in ascitic liver cirrhosis evaluated by lithium clearance. Eur J Clin Invest. 1990;20:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Hattori K, Yauchi T, Minato Y, Hasumura Y, Takeuchi J, Shiigai T. A lithium clearance study of sodium reabsorption at the proximal tubule in liver cirrhosis with ascites. Gastroenterol Jpn. 1989;24:535-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 24. | Kalambokis G, Tsiakas I, Filippas-Ntekouan S, Christaki M, Milionis H. Empagliflozin controls cirrhotic refractory ascites along with improvement of natriuresis and circulatory, cardiac, and renal function: A pilot study. Eur J Intern Med. 2024;130:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/