Published online Oct 27, 2025. doi: 10.4254/wjh.v17.i10.110107
Revised: July 3, 2025
Accepted: September 18, 2025
Published online: October 27, 2025
Processing time: 151 Days and 17.3 Hours
Chronic hepatitis B (CHB) remains a significant global health challenge, affecting more than 250 million individuals worldwide. A functional cure, defined as the loss of hepatitis B surface antigen (HBsAg) and suppression of hepatitis B virus (HBV) DNA to undetectable levels, represents the optimal therapeutic endpoint for managing CHB. However, the complex pathogenesis of CHB, which includes HBV DNA integration, persistence of covalently closed circular DNA, and impai
Core Tip: Achieving a functional cure for chronic hepatitis B, defined as sustained hepatitis B surface antigen loss with undetectable hepatitis B virus (HBV) DNA, remains challenging due to the persistence of covalently closed circular DNA (cccDNA) and immune dysfunction. While standard therapies, such as nucleos(t)ide analogues and pegylated interferon, yield low seroclearance rates, novel therapeutics targeting various steps in the life cycle of HBV-such as cccDNA silencing, RNA degradation, capsid modulation, and immune restoration- show promise. Combinatorial approaches, including small interfering RNA, checkpoint inhibitors, and therapeutic vaccines, are being evaluated. Identifying predictive biomarkers and optimizing the timing for treatment cessation may improve treatment outcomes.
- Citation: Marrapu S, Soni JR, Kamal K, Kumar R. Hepatitis B functional cure: Current and future perspective. World J Hepatol 2025; 17(10): 110107
- URL: https://www.wjgnet.com/1948-5182/full/v17/i10/110107.htm
- DOI: https://dx.doi.org/10.4254/wjh.v17.i10.110107
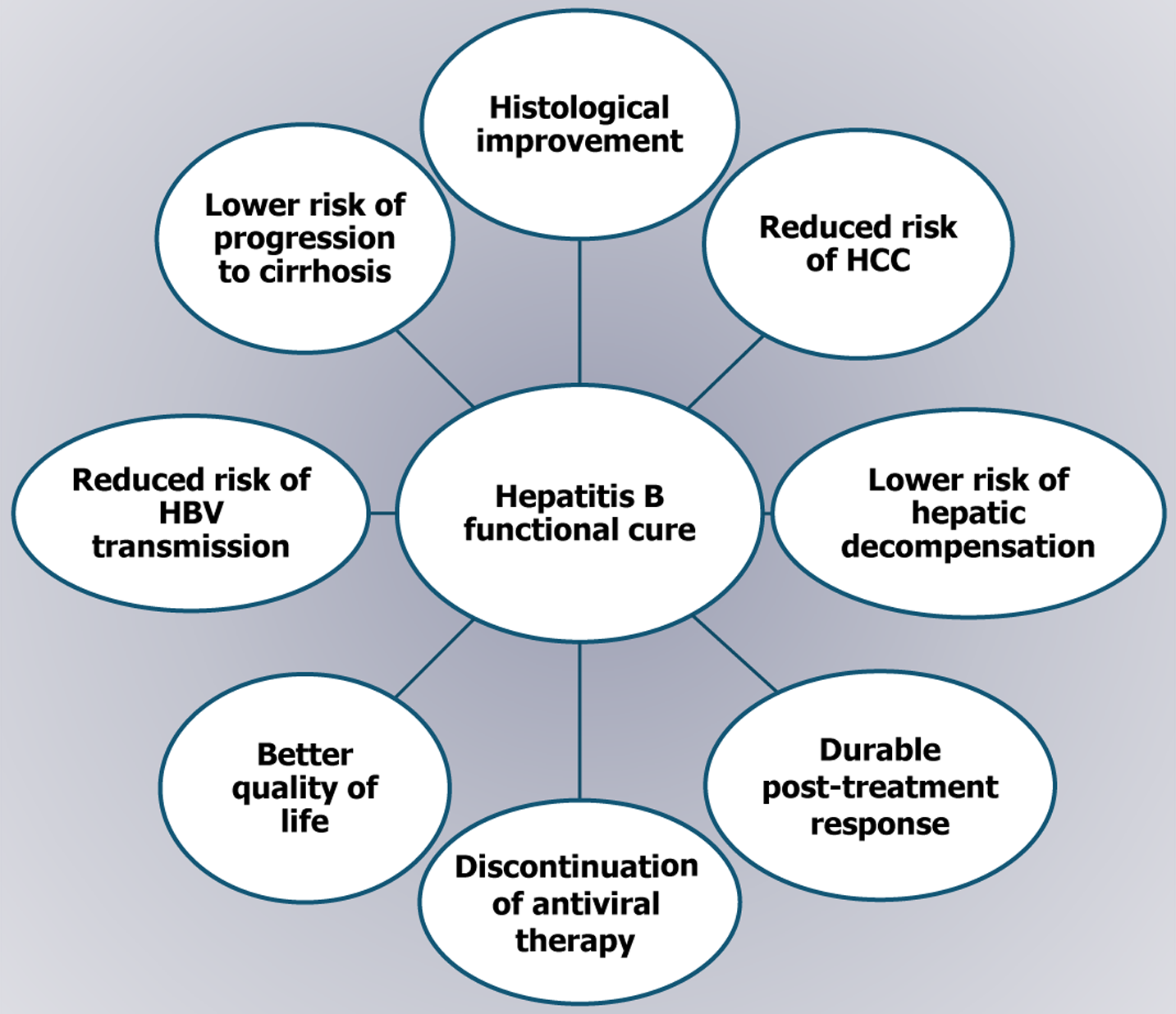
Chronic hepatitis B (CHB) poses a major global health burden, leading to cirrhosis and hepatocellular carcinoma (HCC)[1]. According to the World Health Organization’s hepatitis report for 2024, the global seroprevalence of hepatitis B surface antigen (HBsAg) was 3.8%, with approximately 254 million individuals living with CHB in 2022. Hepatitis B caused an estimated 1.1 million deaths in 2022, primarily from cirrhosis and HCC, which is the second deadliest cancer worldwide[1,2]. The nature of chronic hepatitis B virus (HBV) infection is dynamic, shaped by interactions among the virus, hepatocytes, and host immunity[1,3,4]. While a complete virological cure remains elusive, the loss of HBsAg is considered the optimal therapeutic endpoint. This outcome, considered a functional cure, is defined as sustained undetectable viremia with durable HBsAg loss of at least 24 weeks after the end of therapy, with or without seroconversion to anti-HBsAg antibody[3]. However, a true cure is defined as the elimination of both HBsAg and covalently closed circular DNA (cccDNA)[4]. In recent years, however, achieving a functional cure has become a realistic and meaningful goal in HBV treatment, offering several advantages, including improved survival, reduced HCC risk, and decreased liver-related mortality, comparable to outcomes seen with spontaneous viral clearance[5] (Figure 1). The current standard of care, including nucleos(t)ide analogues (NAs) and immunomodulators like pegylated interferon (PEG-IFN), can induce functional cure, although success rates remain low. However, advances in HBV research and the success of curative therapies for hepatitis C have spurred the development of promising new treatments for CHB, many of which are now in clinical trials. This review aims to provide an overview of current evidence on the functional cure of hepatitis B, limitations of current therapies and an outline of future treatment directions.
The persistence of HBV infection is primarily driven by the stability of cccDNA in hepatocytes and its ability to evade immune clearance[6]. After HBV enters hepatocytes via the sodium taurocholate co-transporting polypeptide (NTCP) receptor, its relaxed circular DNA is transported to the nucleus and repaired by host enzymes to form transcriptionally active cccDNA, which associates with host histones to form a viral minichromosome that serves as a template for viral RNAs, including pregenomic RNA[7,8]. While liver-specific transcription factors and chromatin activating enzymes boost cccDNA-driven gene expression, host restriction complexes, noncoding RNAs, and protein modifiers such as protein arginine methyltransferase-1 inhibit it. This balance governs the long term persistence of HBV[9]. HBV further promotes the persistence of cccDNA by altering cell cycle control via HBV X (HBx)-protein, preventing dilution during mitosis[10,11]. HBV-induced immune dysfunction plays a central role in viral persistence. Kupffer cells and dendritic cells exhibit impaired antigen presentation, diminished interferon (IFN) production, and defective Toll like receptor (TLR) 7/9 signaling[12]. Natural killer (NK) cells show reduced cytotoxic activity and IFN gamma (IFN-γ) secretion, and contribute to the depletion of virus-specific CD8+ T cells[12,13]. HBV proteins, such as HBsAg and hepatitis B envelope antigen (HBeAg), inhibit innate immune signaling by blocking pattern recognition receptor (PRR) pathways. The adaptive immune response is also compromised: Both CD8+ and CD4+ T cells exhibit features of functional exhaustion, characterized by the high expression of inhibitory receptors, mitochondrial dysfunction, and reduced cytokine production[14]. B cells exhibit the expansion of programmed cell death protein-1 (PD-1) atypical memory subsets with impaired antibody responses[15]. Persistent high levels of viral antigens, particularly HBsAg and hepatitis B core antigen (HBcAg), con
| Category | Barrier | Description /mechanism |
| Viral factors | cccDNA persistence | cccDNA forms a stable nuclear minichromosome, serving as a reservoir for HBV transcription |
| High antigen load | Continuous production of HBsAg and HBcAg from cccDNA promotes immune tolerance and T cell exhaustion | |
| HBV-mediated immune suppression | HBsAg and HBeAg interfere with PRR signaling, hindering innate immune responses via IRF3 and NF-κB inhibition | |
| HBx-induced cell cycle modulation | HBx protein disrupts hepatocyte proliferation and prevents cccDNA dilution during cell division | |
| Host factors | Epigenetic control of cccDNA | Host enzymes (e.g., HDAC1, Smc5/6) modify cccDNA chromatin, affecting its transcriptional activity |
| Effective innate immunity | Kupffer cells, dendritic cells, and monocytes show impaired PRR signaling and reduced IFN production | |
| NK cell dysfunction | NK cells display reduced cytotoxicity, lower IFN-γ secretion, and contribute to CD8+ T cell depletion | |
| T cell exhaustion | HBV-specific CD4+ and CD8+ T cells overexpress inhibitory receptors (PD-1, CTLA-4), resulting in functional impairment | |
| B cell dysfunction | Atypical memory B cells (e.g., PD-1+, FcRL5+) fail to generate effective anti-HBs antibodies | |
| Expansion of immunosuppressive cells | Regulatory T cells and myeloid-derived suppressor cells suppress antiviral immune responses | |
| Therapeutic gaps | No cccDNA-targeting therapies | Current antivirals suppress replication but do not eliminate or silence cccDNA effectively |
| Limited immunotherapeutic tools | PEG-IFN and NAs yield low HBsAg clearance; novel immunotherapies are investigational |
Although HBsAg seroclearance can occur spontaneously, antiviral therapies such as PEG-IFN or NAs are more likely to induce it. Table 2 summarizes published studies on HBsAg seroclearance in CBH patients.
| Ref. | Year | Study type | n | Cohort and treatment | HBsAg seroclearance rate |
| Lau et al[36] | 2005 | RCT | 814 | HBeAg+ CHB on PEG-IFN α | 2.95% at 6 months post-treatment |
| Marcellin et al[39] | 2013 | RCT | 537 | HBeAg- CHB on PEG-IFN α | 3% (6 months), 9% (3 years), 12% (5 years) |
| Bourlière et al[53] | 2017 | RCT | 185 | HBeAg- CHB: NA vs NA + PEG-IFN | 3.2% vs 7.8% at 96 weeks |
| Ahn et al[55] | 2018 | RCT | 740 | TDF + PEG-IFN vs TDF mono | 9.1% vs 0% at 72 weeks |
| Zhou et al[32] | 2019 | Meta-analysis | 48972 | Untreated CHB | 1.17% per year |
| Yeo et al[33] | 2019 | Meta-analysis | 42588 | Untreated CHB | 1.02% per year |
| Yip et al[45] | 2019 | Retrospective | 20263 | CHB on TDF/ETV | 2.1% (mean FU: 4.8 years) |
| Yeo et al[34] | 2020 | Prospective cohort | 11262 | Untreated CHB | 1.31% per year |
| Yang et al[52] | 2020 | RCT | 144 | CHB on ETV vs ETV + PEG-IFN | 1.8% vs 4.1% |
| Fonseca et al[56] | 2020 | Meta-analysis | 8719 | CHB on IFN+NA vs IFN vs NA (48-130 weeks) | 4.8% to 6.5% vs 3.6% to 6.5% vs 0.27% to 0.58% |
| Song et al[43] | 2021 | Meta-analysis | 1029 | IHCs on PEG-IFN | 47% at 48 weeks |
| Hsu et al[47] | 2021 | Retrospective | 4769 | CHB on TDF/ETV | 0.22% per year |
| Chan et al[48] | 2024 | RCT | 1298 | CHB on TAF | ≤ 1.2% at 5 years |
Multiple cohort studies from Asian and European countries reported variable annual spontaneous HBsAg seroclearance rates in CHB patients. In Asian cohorts, seroclearance rates ranged from 0.12%-2.38% per year[18-23], while Western cohorts showed rates of 0.54%-1.98% per year[24-31]. Among cirrhotic patients, the annual clearance rate was 0.8% in European patients and 1.5% in Taiwanese patients[23,30]. A meta-analysis by Zhou et al[32] (48972 CHB patients, 352381 person-years) reported a pooled clearance rate of 1.17%. This was higher in HBeAg-negative than HBeAg-positive patients, and in those aged ≥ 40 years (1.7%) compared to < 40 years (1.1%). Inactive carriers experienced a rate of 1.24%, while cirrhotic patients showed rates of 0.8%-1.4%. Another meta-analysis of 34 studies by Yeo et al[33] found a 1.02% annual clearance rate among untreated patients. Yeo et al[34] studied a large longitudinal cohort of 11264 patients, re
PEG-IFN: PEG-IFN has demonstrated modest efficacy in achieving HBsAg clearance. In a multicenter randomized controlled trial (RCT), 266 HBeAg-positive patients treated with PEG-IFN-α2b for 52 weeks reported a 7% HBsAg loss rate at one year, with no additional benefit from combining lamivudine[35]. Another study reported a 2.95% HBsAg seroconversion rate with PEG-IFN-α2a in HBeAg-positive patients[36]. In HBeAg-negative CHB, a multicenter trial showed 3% HBsAg loss at 6 months, increasing to 9% at year 3 and 12% at year 5[37-39]. A retrospective study in 233 HBeAg-negative patients reported cumulative HBsAg loss rates of 4.7%, 9.4%, and 14.2% at 3, 5, and 10 years, resp
NAs: NAs are effective in suppressing HBV replication and reducing disease progression, but they rarely achieve HBsAg clearance. Long-term studies indicate that only 2%-5% of patients achieve HBsAg loss after up to 10 years of continuous NA therapy[44]. Large retrospective analyses report clearance rates of 2.1% over 4.8 years[45], 4.58% over 6 years[46], and 2.11% at 10 years[47]. Even in tenofovir alafenamide (TAF)-treated cohorts, HBsAg loss remained ≤ 1.2% over 5 years[48]. Across studies, clearance rates consistently ranged from 1.4% to 5.1%, underscoring the limited capacity of NAs to achieve a functional cure[45,46,49-51]. The consistently low HBsAg clearance rates with NAs, even after a decade of therapy, reflect their inability to eliminate cccDNA or restore effective immune control-highlighting the need for novel agents targeting functional cure.
PEG-IFN plus NAs: Individual trials have shown modest but improved HBsAg clearance with combination therapy using PEG-IFN and NAs compared to monotherapy. In a study by Yang et al[52], the addition of PEG-IFN to entecavir (ETV) resulted in a 4.1% clearance rate, compared to 1.8% with ETV alone. Similarly, Bourlière et al[53] reported 7.8% clearance with NAs + PEG-IFN compared to 3.2% with NAs monotherapy. The highest efficacy was observed with tenofovir disoproxil fumarate (TDF) plus PEG-IFN for 48 weeks, resulting in a 10.4% clearance rate at week 120[54,55]. A meta-analysis comparing combination therapy to IFN alone showed only a modest difference in HBsAg loss (4.8% vs 3.6%)[56]. However, compared to NAs monotherapy, combination therapy was significantly superior (6.55% vs 0.58%), particularly when PEG-IFN was administered at standard doses for ≥ 48 weeks[56]. Among various regimens, switch-to-IFN strategies yielded the highest end-of-treatment HBsAg loss, although this benefit diminished over time. Sensitivity analyses revealed that clearance peaked at one year post-treatment, followed by a gradual decline[56]. In summary, while combination therapy with PEG-IFN and NAs offers a modest enhancement in HBsAg clearance, especially with pro
Newer agents: Several newer antiviral agents are under clinical investigation for their potential to enhance HBsAg seroclearance by targeting different stages of the viral replication cycle. Bepirovirsen, an antisense oligonucleotide (ASO) designed to degrade HBV mRNA and pregenomic RNA, has shown promising initial efficacy. In phase 2 trials, it achieved HBsAg seroclearance in approximately 26%-29% of patients after 24 weeks of monotherapy or in combination with NAs. However, the durability of this response is limited; only 12%-14% of patients maintained HBsAg loss 24 weeks after treatment discontinuation, underscoring the challenge of achieving a sustained off-treatment response[57]. These findings highlight that while ASOs can effectively suppress antigen production, they may not be sufficient to achieve a functional cure without concurrent immune restoration. Xalnesiran, a small interfering RNA (siRNA) targeting the S gene of HBV, represents another novel approach aimed at reducing HBsAg levels by promoting RNA degradation. In a recent phase 2 study, when combined with NAs and either PEG-IFN-α2a or ruzotolimod (a TLR 7 agonist), Xalnesiran achieved HBsAg seroclearance rates ranging from 3% to 23%, with higher efficacy noted in the PEG-IFN combination group[58]. Despite these encouraging short-term outcomes, post-treatment follow-up revealed a decline in response rates, mirroring the limited durability seen with bepirovirsen. These observations suggest that while RNA-targeting therapies can induce significant reductions in viral antigenemia, their long-term effectiveness may require combinatorial strategies, including immunomodulatory agents, to sustain virological control.
To summarize, the data suggest that while spontaneous or NAs-induced functional cure remains uncommon, the strategic use of PEG-IFN-especially in combination with NAs-offers a more promising route to achieving HBsAg loss. PEG-IFN plus NAs consistently outperforms monotherapy, with seroclearance rates of up to 12% at 5 years compared to < 2% for NAs alone. Notably, inactive HBsAg carriers show high response rates (up to 47%), highlighting a potential target group.
HBsAg seroclearance is influenced by both host and viral factors (Table 3). Spontaneous clearance is more likely in older males, those with HBeAg-negative status, and genotype C infection, while high HBV DNA (> 20000 IU/mL) and qHBsAg ≥ 1000 IU/mL reduce the likelihood of clearance[59]. In PEG-IFN treated patients, favorable predictors include younger age, female sex, genotype A, combination therapy, low baseline HBsAg/HBV DNA, early HBsAg decline, and ALT elevation at week 12[60]. In NAs treated patients, predictors of functional cure include early HBeAg seroconversion, initiation of high-barrier NAs before seroconversion, and HBsAg < 1000 IU/mL at 18 months post-seroconversion[61]. In HBeAg-negative CHB, older age, shorter consolidation, and baseline/end-of-treatment HBsAg ≥ 1000 IU/mL increase relapse risk[62], while HBsAg < 100 IU/mL and or an end-treatment ALT/qHBsAg ratio ≥ 0.2 strongly predict clearance[63]. A low HBsAg glycan isomer level (≤ 3.5 Log ng/mL) is associated with a fivefold higher 10 year clearance pro
| Category | Subcategory | Predictor variables |
| Spontaneous clearance | Host factors | Male sex; older age (hazard ratio 1.16-1.21) |
| Viral factors | HBeAg-negative status; genotype C infection; lower HBV DNA (≤ 20000 IU/mL); low quantitative HBsAg (≤ 1000 IU/mL) | |
| PEG-IFN therapy | Host factors | Younger age; female sex; ALT elevation at week 12 |
| Treatment factors | Combination therapy (PEG-IFN + NAs) more effective than PEG-IFN alone | |
| Viral factors | Genotype A; low baseline HBsAg and HBV DNA; early HBsAg decline (at week 12 or 24) | |
| NAs therapy | Treatment factors | Early HBeAg seroconversion (before age 18); initiating high-barrier NA before seroconversion |
| Viral factors | Low HBsAg (< 1000 IU/mL) at 18 months post-seroconversion | |
| NA discontinuation in HBeAg-negative CHB | Treatment factors | End-of-treatment HBsAg < 100 IU/mL; ALT/qHBsAg ratio ≥ 0.2 |
| Viral factor | Low HBsAg glycan isomer (≤ 3.5 Log ng/mL) associated with 5-fold higher 10-year clearance | |
| Prediction models | Nomogram | BMI, HBeAg status, qHBsAg, HBV DNA (C-index 0.91) |
| HepBLOSS-1 & HepBLOSS-2 | Age, sex, HBsAg, HBV DNA; AUROC 0.81-0.89; > 50% 10-year clearance in high-risk groups | |
| HBRN-SQuARe | Sex, age, race, qHBsAg; AUROC 0.99 (1 year), 0.95 (3 years) in untreated HBeAg-negative CHB |
Several prediction models have shown high accuracy. A nomogram using body mass index, HBeAg status, qHBsAg, and HBV DNA achieved a C-index of 0.913, validated externally (C-index 0.886)[65]. Additionally, HepBLOSS-1 (HBV DNA, age, sex, HBsAg) and HepBLOSS-2 (excluding sex) predicted 5-, 10-, and 15-year seroclearance in untreated HBeAg-negative patients, with an area under the receiver operating characteristics (AUROC) curve of 0.81-0.89 and > 50% 10-year clearance in high scorers[66]. Recently, the HBRN-SQuARe model, incorporating sex, ∆qHBsAg, age, and race, predicted HBsAg loss with AUROC of 0.99 and 0.95 at 1 and 3 years, respectively, in a multiethnic cohort of 1240 patients[67]. In summary, HBsAg clearance is influenced by multiple host and viral factors. Predictors vary by treatment type, with early seroconversion and HBsAg decline being favorable.
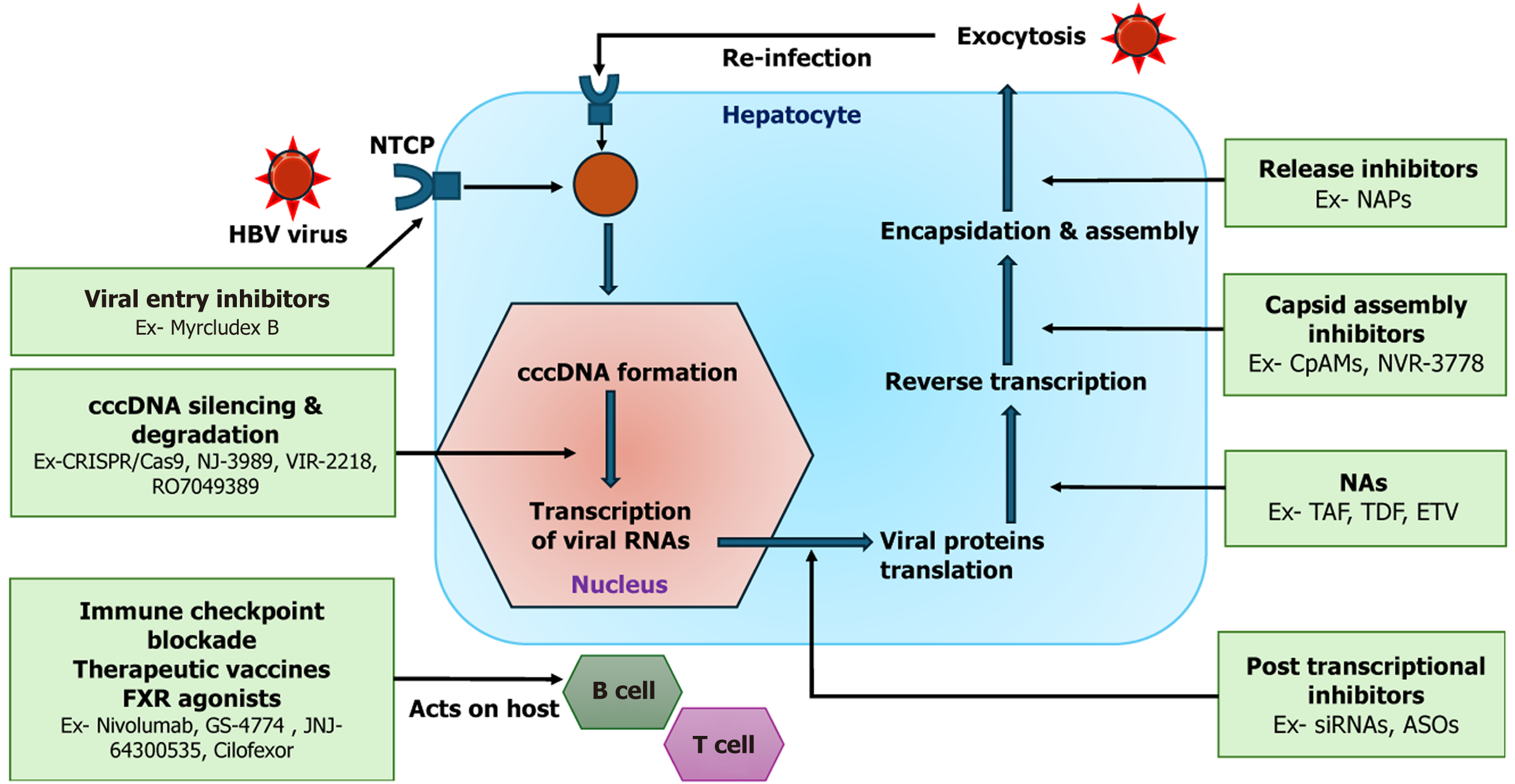
The development of curative therapy for hepatitis C virus (HCV) stands in sharp contrast to the ongoing challenge of curing HBV infection, primarily due to fundamental differences in viral biology and persistence mechanisms. Direct-acting antivirals have revolutionized HCV treatment by targeting key viral enzymes involved in replication. Since HCV does not integrate into the host genome or establish a stable nuclear reservoir, viral eradication is achievable in over 95% of treated patients, resulting in a true virological cure. In contrast, curing CHB requires addressing cccDNA persistence and restoring effective immune responses[3,5]. While NAs effectively suppress viral replication, they do not eliminate cccDNA. PEG-IFN can target cccDNA to a limited extent but achieves only modest HBsAg clearance, with its efficacy constrained by immune evasion, hepatic flares, and poor tolerability, restricting its broader application[68-70]. To overcome these limitations, novel therapies targeting multiple stages of the HBV life cycle, including viral entry, replication, and assembly, as well as cccDNA disruption and immune stimulation, are being developed[71,72]. These innovative strategies represent a paradigm shift from viral suppression to functional cure. While most of the evidence on newer molecules comes from preclinical studies (in vitro or animal models), some agents have progressed to early-phase clinical trials, including phase 1 and phase 2. Figure 2 illustrates the HBV life cycle within hepatocytes and highlights current and emerging therapeutic intervention points.
Targeting cccDNA represents a key strategy in the pursuit of a functional cure; however, therapeutic progress is limited by an incomplete understanding of the formation, stability, and regulation of cccDNA. While approaches to silence or eliminate cccDNA are advancing, they remain largely experimental. Endonuclease-based genome editing tools like zinc finger nucleases, transcription activator-like effector nucleases, and clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated proteins (Cas) systems have shown potential in disrupting cccDNA in vitro[72]. Among these, CRISPR-Cas9 has demonstrated the ability to cleave more than 90% of HBV DNA. However, a small proportion (approximately 7%) may undergo functional repair, necessitating the use of multiplexed guide RNAs to ensure complete inactivation of viral genes[73]. Key barriers to the clinical translation of this novel approach include hepatocellular delivery efficiency, off target risks to the host genome, and the cleavage of chromosomally integrated HBV DNA and DNA recombination. Despite these concerns, endonuclease-mediated mutagenesis remains the only experimentally proven method for permanently inactivating cccDNA in tissue. Transcriptional regulation of cccDNA involves both viral factors (e.g., HBx, HBcAg) and host pathways. A key mechanism includes HBx-mediated degradation of the structural maintenance of chromosomes 5/6 (SMC5/6), which otherwise represses HBV transcription. Nitazoxanide, an antiparasitic agent, has been shown to preserve SMC5/6 and inhibit HBV replication, including cccDNA activity. In a pilot study of 9 CHB patients treated with nitazoxanide for 48 weeks, 89% achieved undetectable HBV DNA, HBeAg loss occurred in 2 patients, and HBsAg clearance in 33%, though larger studies are needed to validate these findings[74,75]. Pevonedistat, a ubiquitin-like protein inhibitor, suppresses HBV cccDNA transcription by inhibiting the HBx protein-mediated degradation of the SMC5/6 complex[76]. Epigenetic modifiers offer another promising strategy by reversing pro-viral chromatin states. Agents such as AGK2 and GS-5801 (a lysine demethylase inhibitor) have shown antiviral activity in preclinical models, but clinical efficacy remains limited[77]. Lastly, host apolipoprotein B mRNA-editing catalytic polypeptide-like 3 (APOBEC3) family deaminases can induce cccDNA degradation following IFN stimulation, although the clearance is partial and variable[78]. Thus, both epigenetic modifiers and APOBEC3 based approaches offer novel and mechanistically distinct strategies for cccDNA silencing or degradation, but are currently limited by efficacy, delivery, and safety challenges.
Capsid assembly inhibitors are promising adjuncts to NAs in CHB, aiming for deeper viral suppression to aid immune function restoration[79,80]. These agents target the HBV core protein, a key component in the viral replication cycle, and are primarily categorized into two types based on their mechanism of action. The first group includes core protein allosteric modulators.
Such as GLS4 and RO7049389, which induce the formation of defective or misassembled capsids that are incapable of supporting viral replication. The second group includes capsid assembly modulators such as AT-130, NVR-3778, and JNJ-6379, which promote the assembly of empty capsids lacking viral genomic material[71,79,80]. Both classes effectively inhibit the encapsidation of pregenomic RNA, thereby reducing the production of HBV DNA particles. More importantly, they prevent the recycling of nucleocapsids back into the nucleus, a key step in the replenishment of cccDNA during successive infection cycles. By blocking this step, capsid assembly inhibitors help to limit the persistence of HBV within hepatocytes. Clinical studies evaluating RO7049389 and the combination of NVR-3778 with PEG-IFN have demonstrated enhanced antiviral activity, with significant reductions in HBV DNA and RNA levels; however, these interventions did not produce a meaningful decline in HBsAg levels[81,82]. These findings suggest that while capsid assembly inhibitors are effective at disrupting viral replication and cccDNA amplification, their impact on key functional cure markers such as HBsAg remains limited.
Persistent high levels of HBV antigens contribute to immune dysfunction and chronicity in hepatitis B[13,14,17]. The
HBsAg is secreted in massive quantities as non-infectious subviral particles (SVPs), making up more than 99.9% of circulating HBV particles. Inhibiting its release may reduce antigen load and promote immune reactivation[89]. Nucleic acid polymers (NAPs) developed by Replicor Inc, such as RNA-based REP-2139 and REP-2165, and DNA-based REP-2055 and REP-2031, block SVP assembly or secretion. In a trial, REP-2139 followed by PEG-IFN achieved sustained HBsAg loss and anti-HBs seroconversion in 42% of HBV/hepatitis D virus (HDV) co-infected patients, lasting more than a year[90]. However, REP-2139 was associated with heavy metal-like toxicity due to accumulation in plasma and liver, prompting the development of REP-2165, which demonstrated comparable efficacy with reduced hepatic build-up[91]. A phase 2 study combining NAPs with TDF and PEG-IFN showed enhanced efficacy: 60% of patients achieved HBsAg ≤ 0.05 IU/mL, and 35% maintained a functional cure 48 weeks post-treatment[92]. Notably, over 90% of patients experienced ALT flares, especially those who lost HBsAg, indicating that immune-mediated responses contributed to viral control. To summarize, NAPs significantly reduce circulating HBsAg by blocking SVP release, thereby promoting immune reac
The identification of NTCP as the entry receptor for HBV and HDV has enabled novel therapeutic strategies aimed at preventing the entry of the virus and hepatocyte reinfection particularly relevant in HBV/HDV co-infection, where HDV relies on HBV surface proteins and shares the NTCP receptor. As HDV is replication defective and requires HBsAg for assembly and propagation, the loss of HBsAg in the context of HBV infection signifies not only a functional cure of HBV but also facilitates the suppression or potential eradication of HDV. This dual therapeutic advantage underscores the significance of achieving HBsAg clearance in co-infected patients, making it a critical therapeutic goal[93]. Myrcludex B (MyrB; bulevirtide), a myristoylated peptide derived from the pre-S1 domain of large HBsAg, is a key entry inhibitor. While MyrB monotherapy has been shown to reduce HDV RNA, it has a limited effect on HBsAg levels[94,95]. In an open-label phase 2 study, combining MyrB with PEG-IFN led to HBsAg loss in 27% of patients at 24 weeks, compared to 0% in monotherapy arms[95]. However, MyrB use is associated with elevated bile salts, a predictable outcome of NTCP inhibition. Additional entry inhibitors, such as ezetimibe and cyclosporin derivatives, are under investigation and may act independently of NTCP[96,97].
Spontaneous HBV clearance highlights the importance of a robust innate and adaptive immune response, making immunomodulatory strategies a promising avenue for restoring HBV-specific immunity[71]. PRRs such as TLRs and retinoic acid-inducible gene (RIG)-I detect HBV and trigger antiviral cytokines, promoting NK cell activity and adaptive immune restoration. Pharmacologic activation of these pathways is under exploration.
PRR agonists: RO7020531, a TLR7 agonist, demonstrated good safety in healthy individuals and is currently under investigation in combination with capsid inhibitors in CHB patients[98]. Vesatolimod (GS-9620), another TLR7 agonist, showed strong antiviral activity in animal models, but its efficacy in humans was limited-likely due to lower dosing strategies[99]. Selgantolimob (GS-9688), a TLR8 agonist, produced modest outcomes in CHB patients, with no patient achieving > 1 Log decline in HBsAg, and only 6% achieving a ≥ 0.5 Log reduction. However, when combined with TAF, GS-9688 resulted in a > 0.3 Log IU/mL reduction in HBsAg[100]. RIG-I, a cytoplasmic sensor of double-stranded RNA, recognizes the epsilon encapsidation signal in HBV pregenomic RNA, triggering the production of interferon lambda (IFN-λ), which inhibit HBV replication and activate innate immune responses[101,102]. Additionally, RIG-I disrupts the interaction between the epsilon signal and HBV polymerase, further suppressing viral replication. The RIG-I agonist SB 9200 (Inarigrivir) demonstrated dose-dependent reductions in HBV DNA and RNA, with a 0.5 Log HBsAg decline observed in 22% of patients. However, the drug was discontinued due to severe adverse events, including hepatocellular dysfunction and one death reported in the phase 2 CATALYST trial[71]. Recent studies also suggest that PEG-IFN may restore PRR expression, supporting the rationale for combination strategies in CHB treatment[103,104].
Immune checkpoint inhibitors: Immune checkpoint inhibitors (ICIs) have garnered attention as potential immunotherapeutic agents in CHB, inspired by their growing use in cancer therapy. Immune checkpoint receptors such as PD-1, cytotoxic T-lymphocyte antigen-4, and T-cell immunoglobulin and mucin-domain containing-3 contribute to HBV-specific T cell exhaustion. In CHB patients, elevated PD-1 expression on HBV-specific CD8+ T cells correlates with higher viral loads and HBeAg levels[105]. Multiple PD-1/programmed death-ligand 1 (PD-L1) inhibitors, including nivolumab, serplulimab, and envafolimab, are currently in phase II trials[106,107]. In a phase Ib trial, nivolumab (0.3 mg/kg), with or without GS-4774 (therapeutic vaccine), induced modest HBsAg declines in HBeAg-negative CHB patients[106]. In a recent Phase IIb study, subcutaneous administration of ASC22 (envafolimab), a humanized, single-domain PD-L1 anti
IFN-λ: IFN-λ is a complex and highly regulated host innate immune defense system that can impact HBV replication and cccDNA[109]. It offers antiviral effects similar to IFN-α but with reduced systemic toxicity due to its limited receptor distribution[110]. In the LIRA-B2a trial, PEG-IFN-λ had similar on-treatment HBeAg seroconversion rates but failed non inferiority at 24 weeks post-treatment. However, combination therapy, ETV followed by ETV plus PEG-IFN-λ (LIRA-B2b trial) improved immune profiles and preserved CD8+ T cell function, showing promise for future use[111].
Therapeutic vaccines: Despite the availability of an effective prophylactic vaccine, no therapeutic vaccine for CHB has yet been approved. Therapeutic vaccines aim to restore impaired HBV specific T cell responses and induce de novo adaptive immunity. Several candidates such as TG1050, VTP-300, GS-4774, and BRII-179 are currently in phase II clinical trials, with encouraging results in preclinical models[112-115]. In a phase II trial by Lok et al[112], GS-4774 a heat-inactivated yeast-based vaccine expressing HBsAg, HBcAg, and HBx failed to induce HBsAg decline or clearance at weeks 24 or 48. However, when combined with TDF, it boosted IFN-γ, TNF-α, and IL-2 production by HBV specific CD8+ T cells[113]. BRII-179 (VBI-2601), a protein-based vaccine expressing all three envelope proteins, was evaluated in a phase 2a trial in combination with the siRNA agent elebsiran (BRII-835), with or without IFN-α. While siRNA monotherapy alone yielded limited immune recovery, adding BRII-179 Led to the generation of durable neutralising anti-HBs antibodies in approximately 40% of participants, along with expansion of IL-2-producing Pre-S1/Pre-S2-specific CD4+ T cells. Although no group achieved sustained HBsAg loss, this data confirm that deep antigen reduction can “unmask” HBV-specific humoral immunity when paired with a broad-spectrum vaccine[114]. VTP-300, a vector-based vaccine, achieved a 0.7-1.4 Log10 IU/mL reduction in HBsAg among patients with baseline HBsAg < 50 IU/mL, with two participants reaching undetectable levels, suggesting potential synergy when combined with siRNA[115]. A key challenge across these platforms is the generation of immune responses against the vaccine vector itself, which may blunt HBV specific imm
To summarize, Immune modulation therapies in CHB-including PRR agonists, ICIs, IFN-λ, and therapeutic vaccines aim to restore HBV specific immunity. While some agents show immunologic activity, most have demonstrated limited HBsAg reduction, with future success likely dependent on optimized combinations and improved patient selection.
Combination therapies: Given the distinct mechanisms of action of NAs and PEG-IFN, combination strategies might enhance treatment outcomes. These include de-novo (simultaneous initiation), add-on (adding PEG-IFN to ongoing NAs therapy), and switch-to approaches (transitioning from NAs to PEG-IFN). Emerging data suggest that sequential stra
NAs are the cornerstone of CHB treatment, yet decisions regarding therapy cessation remain complex[118]. Discontinuation is generally advised in patients achieving HBeAg seroconversion or HBsAg loss[119]. For non-cirrhotic HBeAg positive patients, all major guidelines support stopping NAs after seroconversion and at least one year of virological suppression[5,120], with the Asian Pacific Association for the Study of the Liver (APASL) recommending a longer 3-year consolidation to reduce relapse risk[121]. However, guidelines diverge significantly for HBeAg-negative CHB. Since HBsAg decline is gradual, lifelong therapy is often needed. However, the 2008 APASL guideline proposed stopping therapy after at least two years of undetectable HBV DNA, confirmed thrice over 18 months. Supporting this, Hadzi
HBsAg loss post-therapy may stem from restored immune function control, including revived CD8+ T cell function and cytokine upregulation[125]. HBsAg level, especially its decline during therapy and low levels at cessation, strongly predicts HBsAg loss. In a study of 1075 HBeAg-negative patients, HBsAg seroclearance rose from 0.15% annually on therapy to 1.78% annually post-therapy, reaching 13% at 6 years. Predictors included early HBV DNA suppression, HBsAg decline > 1 Log10, end-of-treatment HBsAg < 100 IU/mL, and no retreatment despite relapse[126]. The RETRACT-B study (n = 1552) found cumulative HBsAg loss rates of 3.2% at 12 months and 13% at 48 months post-treatment, with a higher likelihood in patients with end of treatment HBsAg < 100 IU/mL (HR 22.5). HBsAg loss exceeded 30% at 48 months in Whites individuals with < 1000 IU/mL and Asians with < 100 IU/mL at cessation; others had < 10% probability[127]. In the CREATE study (n = 572), 4.2% achieved HBsAg loss, with non-Asians faring better (15% vs 1.5%). Lower levels of HBcrAg (< 2 Log U/mL) and HBsAg (< 100 IU/mL) predicted favorable outcomes[128].
Key concerns with cessation of NAs include ALT flares, decompensation, and HCC. In RETRACT-B study, hepatic decompensation occurred in 1.22% (4.3% in cirrhotics) study subjects, and HCC in 2.2% with cirrhosis vs 0.7% without[127]. Another study (n = 691) reported comparable HCC rates post-cessation and during therapy, with clinical relapse in 60.6%[126]. Smaller studies reported clinical relapse in 57.9%-86.8% within 6months of tenofovir withdrawal[129], and virological and clinical relapse rates of 70% and 43.6% respectively[130]. Based on these findings, treatment cessation may enhance the chance of functional cure and is generally safe in non-cirrhotic patients when supported by rigorous follow-up.
Hepatitis B treatment has evolved significantly over the past few decades, yet finding a functional cure still presents daunting obstacles. The incorporation of cccDNA into the host genome and the modulation of HBV specific immunity remain major obstacles in antiviral therapy approaches. Research on promising medications is progressing rapidly, but may take time before they are translated into clinical settings. Furthermore, clinical trials of several emerging drugs have shown that monotherapy is not as effective as intended. Therefore, multi-target and multi-drug combination therapy that includes a mix of PEG-IFN, NAs, or other drugs constitutes a promising strategy to achieve functional cure in a large proportion of CHB patients.
| 1. | Marrapu S, Kumar R. Chronic hepatitis B: Prevent, diagnose, and treat before the point of no return. World J Hepatol. 2024;16:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 2. | Polaris Observatory Collaborators. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis B in 2022: a modelling study. Lancet Gastroenterol Hepatol. 2023;8:879-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 243] [Article Influence: 81.0] [Reference Citation Analysis (1)] |
| 3. | Lok ASF. Toward a Functional Cure for Hepatitis B. Gut Liver. 2024;18:593-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 4. | Lok AS, Zoulim F, Dusheiko G, Ghany MG. Hepatitis B cure: From discovery to regulatory approval. Hepatology. 2017;66:1296-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 255] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 5. | Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, Brown RS Jr, Bzowej NH, Wong JB. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560-1599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2290] [Cited by in RCA: 3090] [Article Influence: 386.3] [Reference Citation Analysis (1)] |
| 6. | Naidu S, Margeridon S. Chronic Hepatitis B Virus Persistence: Mechanisms and Insights. Cureus. 2025;17:e78944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 7. | Diogo Dias J, Sarica N, Neuveut C. Early Steps of Hepatitis B Life Cycle: From Capsid Nuclear Import to cccDNA Formation. Viruses. 2021;13:757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Wei L, Ploss A. Mechanism of Hepatitis B Virus cccDNA Formation. Viruses. 2021;13:1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 91] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 9. | Mohd-Ismail NK, Lim Z, Gunaratne J, Tan YJ. Mapping the Interactions of HBV cccDNA with Host Factors. Int J Mol Sci. 2019;20:4276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Tu T, Zehnder B, Wettengel JM, Zhang H, Coulter S, Ho V, Douglas MW, Protzer U, George J, Urban S. Mitosis of hepatitis B virus-infected cells in vitro results in uninfected daughter cells. JHEP Rep. 2022;4:100514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 11. | Chen HY, Tang NH, Lin N, Chen ZX, Wang XZ. Hepatitis B virus X protein induces apoptosis and cell cycle deregulation through interfering with DNA repair and checkpoint responses. Hepatol Res. 2008;38:174-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Li A, Yi Z, Ma C, Sun B, Zhao L, Cheng X, Hui L, Xia Y. Innate immune recognition in hepatitis B virus infection. Virulence. 2025;16:2492371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Tang J, Wu ZY, Dai RJ, Ma J, Gong GZ. Hepatitis B virus-persistent infection and innate immunity defect: Cell-related or virus-related? World J Clin Cases. 2018;6:233-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Ye B, Liu X, Li X, Kong H, Tian L, Chen Y. T-cell exhaustion in chronic hepatitis B infection: current knowledge and clinical significance. Cell Death Dis. 2015;6:e1694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 208] [Cited by in RCA: 299] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 15. | Oliviero B, Cerino A, Varchetta S, Paudice E, Pai S, Ludovisi S, Zaramella M, Michelone G, Pugnale P, Negro F, Barnaba V, Mondelli MU. Enhanced B-cell differentiation and reduced proliferative capacity in chronic hepatitis C and chronic hepatitis B virus infections. J Hepatol. 2011;55:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Kong X, Sun R, Chen Y, Wei H, Tian Z. γδT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol. 2014;193:1645-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Tout I, Loureiro D, Mansouri A, Soumelis V, Boyer N, Asselah T. Hepatitis B surface antigen seroclearance: Immune mechanisms, clinical impact, importance for drug development. J Hepatol. 2020;73:409-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 127] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 18. | Liaw YF, Sheen IS, Chen TJ, Chu CM, Pao CC. Incidence, determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology. 1991;13:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 179] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Chu CM, Liaw YF. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45:1187-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 20. | Furusyo N, Hayashi J, Sawayama Y, Kishihara Y, Kashiwagi S. Hepatitis B surface antigen disappearance and hepatitis B surface antigen subtype: a prospective, long-term, follow-up study of Japanese residents of Okinawa, Japan with chronic hepatitis B virus infection. Am J Trop Med Hyg. 1999;60:616-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Kato Y, Nakao K, Hamasaki K, Kato H, Nakata K, Kusumoto Y, Eguchi K. Spontaneous loss of hepatitis B surface antigen in chronic carriers, based on a long-term follow-up study in Goto Islands, Japan. J Gastroenterol. 2000;35:201-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Kim JH, Lee JH, Park SJ, Bae MH, Kim JH, Kim DY, Kim JK, Choi MS, Koh KC, Paik SW, Yoo BC. Factors associated with natural seroclearance of hepatitis B surface antigen and prognosis after seroclearance: a prospective follow-up study. Hepatogastroenterology. 2008;55:578-581. [PubMed] |
| 23. | Chen YC, Chu CM, Yeh CT, Liaw YF. Natural course following the onset of cirrhosis in patients with chronic hepatitis B: a long-term follow-up study. Hepatol Int. 2007;1:267-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 24. | Sampliner RE, Hamilton FA, Iseri OA, Tabor E, Boitnott J. The liver histology and frequency of clearance of the hepatitis B surface antigen (HBsAg) in chronic carriers. Am J Med Sci. 1979;277:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 82] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Alward WL, McMahon BJ, Hall DB, Heyward WL, Francis DP, Bender TR. The long-term serological course of asymptomatic hepatitis B virus carriers and the development of primary hepatocellular carcinoma. J Infect Dis. 1985;151:604-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 120] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Villeneuve JP, Desrochers M, Infante-Rivard C, Willems B, Raymond G, Bourcier M, Côté J, Richer G. A long-term follow-up study of asymptomatic hepatitis B surface antigen-positive carriers in Montreal. Gastroenterology. 1994;106:1000-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 128] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 27. | McMahon BJ, Holck P, Bulkow L, Snowball M. Serologic and clinical outcomes of 1536 Alaska Natives chronically infected with hepatitis B virus. Ann Intern Med. 2001;135:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 292] [Article Influence: 11.7] [Reference Citation Analysis (1)] |
| 28. | Manno M, Cammà C, Schepis F, Bassi F, Gelmini R, Giannini F, Miselli F, Grottola A, Ferretti I, Vecchi C, De Palma M, Villa E. Natural history of chronic HBV carriers in northern Italy: morbidity and mortality after 30 years. Gastroenterology. 2004;127:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 183] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Da Silva LC, Madruga CL, Carrilho FJ, Pinho JR, Saéz-Alquezar A, Santos C, Bassit L, Barreto C, Fonseca LE, Alves VA, Leitão R, Vianna R, Cardoso RA, França AV, Gayotto LC. Spontaneous hepatitis B surface antigen clearance in a long-term follow-up study of patients with chronic type B hepatitis. Lack of correlation with hepatitis C and D virus superinfection. J Gastroenterol. 1996;31:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Fattovich G, Giustina G, Sanchez-Tapias J, Quero C, Mas A, Olivotto PG, Solinas A, Almasio P, Hadziyannis S, Degos F, de Moura MC, Krogsgaard K, Pantalena M, Realdi G, Corrocher R, Schalm SW. Delayed clearance of serum HBsAg in compensated cirrhosis B: relation to interferon alpha therapy and disease prognosis. European Concerted Action on Viral Hepatitis (EUROHEP). Am J Gastroenterol. 1998;93:896-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 132] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Sánchez-Tapias JM, Costa J, Mas A, Bruguera M, Rodés J. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology. 2002;123:1848-1856. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 314] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 32. | Zhou K, Contag C, Whitaker E, Terrault N. Spontaneous loss of surface antigen among adults living with chronic hepatitis B virus infection: a systematic review and pooled meta-analyses. Lancet Gastroenterol Hepatol. 2019;4:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 87] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 33. | Yeo YH, Ho HJ, Yang HI, Tseng TC, Hosaka T, Trinh HN, Kwak MS, Park YM, Fung JYY, Buti M, Rodríguez M, Treeprasertsuk S, Preda CM, Ungtrakul T, Charatcharoenwitthaya P, Li X, Li J, Zhang J, Le MH, Wei B, Zou B, Le A, Jeong D, Chien N, Kam L, Lee CC, Riveiro-Barciela M, Istratescu D, Sriprayoon T, Chong Y, Tanwandee T, Kobayashi M, Suzuki F, Yuen MF, Lee HS, Kao JH, Lok AS, Wu CY, Nguyen MH. Factors Associated With Rates of HBsAg Seroclearance in Adults With Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology. 2019;156:635-646.e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 34. | Yeo YH, Tseng TC, Hosaka T, Cunningham C, Fung JYY, Ho HJ, Kwak MS, Trinh HN, Ungtrakul T, Yu ML, Kobayashi M, Le AK, Henry L, Li J, Zhang J, Sriprayoon T, Jeong D, Tanwandee T, Gane E, Cheung RC, Wu CY, Lok AS, Lee HS, Suzuki F, Yuen MF, Kao JH, Yang HI, Nguyen MH. Incidence, Factors, and Patient-Level Data for Spontaneous HBsAg Seroclearance: A Cohort Study of 11,264 Patients. Clin Transl Gastroenterol. 2020;11:e00196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Flink HJ, van Zonneveld M, Hansen BE, de Man RA, Schalm SW, Janssen HL; HBV 99-01 Study Group. Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. Am J Gastroenterol. 2006;101:297-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 36. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW, Chang WY, Berg T, Flisiak R, McCloud P, Pluck N; Peginterferon Alfa-2a HBeAg-Positive Chronic Hepatitis B Study Group. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1182] [Article Influence: 56.3] [Reference Citation Analysis (1)] |
| 37. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S, Lai MY, Button P, Pluck N; Peginterferon Alfa-2a HBeAg-Negative Chronic Hepatitis B Study Group. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 862] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 38. | Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J, Popescu M, Hadziyannis S; Peginterferon alfa-2a in HBeAg-negative Chronic Hepatitis B Study Group. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-2179.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 261] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 39. | Marcellin P, Bonino F, Yurdaydin C, Hadziyannis S, Moucari R, Kapprell HP, Rothe V, Popescu M, Brunetto MR. Hepatitis B surface antigen levels: association with 5-year response to peginterferon alfa-2a in hepatitis B e-antigen-negative patients. Hepatol Int. 2013;7:88-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 40. | Lee IC, Yang SS, Lee CJ, Su CW, Wang YJ, Lan KH, Lin HC, Hou MC, Peng CY, Huang YH. Incidence and Predictors of HBsAg Loss After Peginterferon Therapy in HBeAg-Negative Chronic Hepatitis B: A Multicenter, Long-term Follow-up Study. J Infect Dis. 2018;218:1075-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 41. | Wang Z, Sun L, Wu Y, Xia Q. Extended duration versus standard duration of peginterferon alfa-2a in treatment of chronic hepatitis B: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2016;40:195-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 42. | Ma Y, Wang J, Xiong F, Lu J. Extended duration therapy regimens based on Pegylated interferon for chronic hepatitis B patients focusing on hepatitis B surface antigen loss: A systematic review and meta-analysis. Infect Genet Evol. 2020;85:104492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Song A, Lin X, Lu J, Ren S, Cao Z, Zheng S, Hu Z, Li H, Shen C, Chen X. Pegylated Interferon Treatment for the Effective Clearance of Hepatitis B Surface Antigen in Inactive HBsAg Carriers: A Meta-Analysis. Front Immunol. 2021;12:779347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Jeng WJ, Lok AS. Should Treatment Indications for Chronic Hepatitis B Be Expanded? Clin Gastroenterol Hepatol. 2021;19:2006-2014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 45. | Yip TC, Wong GL, Chan HL, Tse YK, Lam KL, Lui GC, Wong VW. HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. J Hepatol. 2019;70:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 242] [Article Influence: 34.6] [Reference Citation Analysis (1)] |
| 46. | Wong RJ, Nguyen MT, Trinh HN, Chan C, Huynh A, Ly MT, Nguyen HA, Nguyen KK, Torres S, Yang J, Liu B, Garcia RT, Bhuket T, Baden R, Levitt B, da Silveira E, Gish RG. Hepatitis B surface antigen loss and sustained viral suppression in Asian chronic hepatitis B patients: A community-based real-world study. J Viral Hepat. 2017;24:1089-1097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Hsu YC, Yeh ML, Wong GL, Chen CH, Peng CY, Buti M, Enomoto M, Xie Q, Trinh H, Preda C, Liu L, Cheung KS, Yeo YH, Hoang J, Huang CF, Riveiro-Barciela M, Kozuka R, Istratescu D, Tsai PC, Accarino EV, Lee DH, Wu JL, Huang JF, Dai CY, Cheung R, Chuang WL, Yuen MF, Wong VW, Yu ML, Nguyen MH. Incidences and Determinants of Functional Cure During Entecavir or Tenofovir Disoproxil Fumarate for Chronic Hepatitis B. J Infect Dis. 2021;224:1890-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 48. | Chan HLY, Buti M, Lim YS, Agarwal K, Marcellin P, Brunetto M, Chuang WL, Janssen HLA, Fung S, Izumi N, Abdurakhmanov D, Jabłkowski M, Celen MK, Ma X, Caruntu F, Flaherty JF, Abramov F, Wang H, Camus G, Osinusi A, Pan CQ, Shalimar, Seto WK, Gane E; GS-US-320-0110 and GS-US-320-0108 investigators. Long-Term Treatment With Tenofovir Alafenamide for Chronic Hepatitis B Results in High Rates of Viral Suppression and Favorable Renal and Bone Safety. Am J Gastroenterol. 2024;119:486-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 39] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 49. | Gish RG, Chang TT, Lai CL, de Man R, Gadano A, Poordad F, Yang J, Brett-Smith H, Tamez R. Loss of HBsAg antigen during treatment with entecavir or lamivudine in nucleoside-naïve HBeAg-positive patients with chronic hepatitis B. J Viral Hepat. 2010;17:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 109] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 50. | Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K, Manns M, Kotzev I, Tchernev K, Buggisch P, Weilert F, Kurdas OO, Shiffman ML, Trinh H, Washington MK, Sorbel J, Anderson J, Snow-Lampart A, Mondou E, Quinn J, Rousseau F. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442-2455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 890] [Cited by in RCA: 918] [Article Influence: 51.0] [Reference Citation Analysis (1)] |
| 51. | Chang TT, Lai CL, Kew Yoon S, Lee SS, Coelho HS, Carrilho FJ, Poordad F, Halota W, Horsmans Y, Tsai N, Zhang H, Tenney DJ, Tamez R, Iloeje U. Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology. 2010;51:422-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 470] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 52. | Yang JM, Chen LP, Wang YJ, Lyu B, Zhao H, Shang ZY, Li J, Fan ZY, Wu SD, Ming X, Li X, Huang SP, Cheng JL. Entecavir add-on Peg-interferon therapy plays a positive role in reversing hepatic fibrosis in treatment-naïve chronic hepatitis B patients: a prospective and randomized controlled trial. Chin Med J (Engl). 2020;133:1639-1648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Bourlière M, Rabiega P, Ganne-Carrie N, Serfaty L, Marcellin P, Barthe Y, Thabut D, Guyader D, Hezode C, Picon M, Causse X, Leroy V, Bronowicki JP, Carrieri P, Riachi G, Rosa I, Attali P, Molina JM, Bacq Y, Tran A, Grangé JD, Zoulim F, Fontaine H, Alric L, Bertucci I, Bouvier-Alias M, Carrat F; ANRS HB06 PEGAN Study Group. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: a randomised, controlled, open-label trial. Lancet Gastroenterol Hepatol. 2017;2:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 91] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 54. | Marcellin P, Ahn SH, Ma X, Caruntu FA, Tak WY, Elkashab M, Chuang WL, Lim SG, Tabak F, Mehta R, Petersen J, Foster GR, Lou L, Martins EB, Dinh P, Lin L, Corsa A, Charuworn P, Subramanian GM, Reiser H, Reesink HW, Fung S, Strasser SI, Trinh H, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HL; Study 149 Investigators. Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology. 2016;150:134-144.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 286] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 55. | Ahn SH, Marcellin P, Ma X, Caruntu FA, Tak WY, Elkhashab M, Chuang WL, Tabak F, Mehta R, Petersen J, Guyer W, Jump B, Chan A, Subramanian M, Crans G, Fung S, Buti M, Gaeta GB, Hui AJ, Papatheodoridis G, Flisiak R, Chan HLY. Hepatitis B Surface Antigen Loss with Tenofovir Disoproxil Fumarate Plus Peginterferon Alfa-2a: Week 120 Analysis. Dig Dis Sci. 2018;63:3487-3497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 56. | Fonseca MA, Ling JZJ, Al-Siyabi O, Co-Tanko V, Chan E, Lim SG. The efficacy of hepatitis B treatments in achieving HBsAg seroclearance: A systematic review and meta-analysis. J Viral Hepat. 2020;27:650-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Yuen MF, Lim SG, Plesniak R, Tsuji K, Janssen HLA, Pojoga C, Gadano A, Popescu CP, Stepanova T, Asselah T, Diaconescu G, Yim HJ, Heo J, Janczewska E, Wong A, Idriz N, Imamura M, Rizzardini G, Takaguchi K, Andreone P, Arbune M, Hou J, Park SJ, Vata A, Cremer J, Elston R, Lukić T, Quinn G, Maynard L, Kendrick S, Plein H, Campbell F, Paff M, Theodore D; B-Clear Study Group. Efficacy and Safety of Bepirovirsen in Chronic Hepatitis B Infection. N Engl J Med. 2022;387:1957-1968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 185] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 58. | Hou J, Zhang W, Xie Q, Hua R, Tang H, Morano Amado LE, Yang SS, Peng CY, Su WW, Chuang WL, Kim DJ, Avihingsanon A, Kao JH, Leerapun A, Yuen MF, Asselah T, Liang X, Bo Q, Canducci F, Catanese MT, Chen E, Cheng C, Chughlay F, Das S, Glavini K, Guerreiro N, Huang Y, Kakrana P, Kazma R, Patil A, Pavlovic V, Surujbally B, Triyatni M, Upmanyu R, Wat C, Gane E; Piranga Study Group. Xalnesiran with or without an Immunomodulator in Chronic Hepatitis B. N Engl J Med. 2024;391:2098-2109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 44] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 59. | Jiang S, Guo S, Huang Y, Yin Y, Feng J, Zhou H, Guo Q, Wang W, Xin H, Xie Q. Predictors of HBsAg seroclearance in patients with chronic HBV infection treated with pegylated interferon-α: a systematic review and meta-analysis. Hepatol Int. 2024;18:892-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 60. | Wu JF, Tai CS, Chang KC, Chen YJ, Hsu CT, Chen HL, Ni YH, Chang MH. Predictors of Functional Cure of Chronic Hepatitis B Virus Infection: A Long-Term Follow-Up Study. Clin Gastroenterol Hepatol. 2025;23:583-590.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 61. | Liu Y, Jia M, Wu S, Jiang W, Feng Y. Predictors of relapse after cessation of nucleos(t)ide analog treatment in HBeAg-negative chronic hepatitis B patients: A meta-analysis. Int J Infect Dis. 2019;86:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 62. | Liu J, Li T, Zhang L, Xu A. The Role of Hepatitis B Surface Antigen in Nucleos(t)ide Analogues Cessation Among Asian Patients With Chronic Hepatitis B: A Systematic Review. Hepatology. 2019;70:1045-1055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 63. | Leung RH, Hui RW, Mak LY, Mao X, Liu KS, Wong DK, Fung J, Seto WK, Yuen MF. ALT to qHBsAg ratio predicts long-term HBsAg seroclearance after entecavir cessation in Chinese patients with chronic hepatitis B. J Hepatol. 2024;81:218-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 21] [Reference Citation Analysis (0)] |
| 64. | Ikeda Y, Murata A, Nago H, Yamaguchi M, Om R, Terai Y, Kita Y, Sato S, Sato S, Shimada Y, Genda T. Hepatitis B surface antigen (HBsAg) glycan isomer is predictive of HBsAg seroclearance in patients with chronic hepatitis B. Hepatol Res. 2024;54:888-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 65. | Cao J, Gong J, Tsia Hin Fong CJ, Xiao C, Lin G, Li X, Jie Y, Chong Y. Prediction Model of HBsAg Seroclearance in Patients with Chronic HBV Infection. Biomed Res Int. 2020;2020:6820179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 66. | Lee HL, Lee SK, Han JW, Yang H, Nam H, Sung PS, Kim HY, Lee SW, Song DS, Kwon JH, Kim CW, Bae SH, Choi JY, Yoon SK, Jang JW. Prediction of long-term HBsAg seroclearance in patients with HBeAg-negative chronic hepatitis B. JHEP Rep. 2025;7:101391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 67. | Terrault NA, Wahed AS, Feld JJ, Cooper SL, Ghany MG, Lisker-Melman M, Perrillo R, Sterling RK, Khalili M, Chung RT, Rosenthal P, Fontana RJ, Sarowar A, Lau DTY, Wang J, Lok AS, Janssen HLA. Incidence and prediction of HBsAg seroclearance in a prospective multi-ethnic HBeAg-negative chronic hepatitis B cohort. Hepatology. 2022;75:709-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 68. | Lei Z, Wang L, Gao H, Guo S, Kang X, Yuan J, Lv Z, Jiang Y, Yi J, Chen Z, Wang G. Mechanisms underlying the compromised clinical efficacy of interferon in clearing HBV. Virol J. 2024;21:314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 69. | Wang G, Guan J, Khan NU, Li G, Shao J, Zhou Q, Xu L, Huang C, Deng J, Zhu H, Chen Z. Potential capacity of interferon-α to eliminate covalently closed circular DNA (cccDNA) in hepatocytes infected with hepatitis B virus. Gut Pathog. 2021;13:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 70. | Nishio A, Bolte FJ, Takeda K, Park N, Yu ZX, Park H, Valdez K, Ghany MG, Rehermann B. Clearance of pegylated interferon by Kupffer cells limits NK cell activation and therapy response of patients with HBV infection. Sci Transl Med. 2021;13:eaba6322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 71. | Phillips S, Jagatia R, Chokshi S. Novel therapeutic strategies for chronic hepatitis B. Virulence. 2022;13:1111-1132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 29] [Reference Citation Analysis (0)] |
| 72. | Fanning GC, Zoulim F, Hou J, Bertoletti A. Therapeutic strategies for hepatitis B virus infection: towards a cure. Nat Rev Drug Discov. 2019;18:827-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 426] [Article Influence: 60.9] [Reference Citation Analysis (1)] |
| 73. | Seeger C, Sohn JA. Complete Spectrum of CRISPR/Cas9-induced Mutations on HBV cccDNA. Mol Ther. 2016;24:1258-1266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 74. | Sekiba K, Otsuka M, Ohno M, Yamagami M, Kishikawa T, Suzuki T, Ishibashi R, Seimiya T, Tanaka E, Koike K. Inhibition of HBV Transcription From cccDNA With Nitazoxanide by Targeting the HBx-DDB1 Interaction. Cell Mol Gastroenterol Hepatol. 2019;7:297-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 75. | Rossignol JF, Bréchot C. A Pilot Clinical Trial of Nitazoxanide in the Treatment of Chronic Hepatitis B. Hepatol Commun. 2019;3:744-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 76. | Sekiba K, Otsuka M, Ohno M, Yamagami M, Kishikawa T, Seimiya T, Suzuki T, Tanaka E, Ishibashi R, Funato K, Koike K. Pevonedistat, a Neuronal Precursor Cell-Expressed Developmentally Down-Regulated Protein 8-Activating Enzyme Inhibitor, Is a Potent Inhibitor of Hepatitis B Virus. Hepatology. 2019;69:1903-1915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 77. | Ma W, Zhao X, Wang K, Liu J, Huang G. Dichloroacetic acid (DCA) synergizes with the SIRT2 inhibitor Sirtinol and AGK2 to enhance anti-tumor efficacy in non-small cell lung cancer. Cancer Biol Ther. 2018;19:835-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 78. | Olson ME, Harris RS, Harki DA. APOBEC Enzymes as Targets for Virus and Cancer Therapy. Cell Chem Biol. 2018;25:36-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 79. | Taverniti V, Ligat G, Debing Y, Kum DB, Baumert TF, Verrier ER. Capsid Assembly Modulators as Antiviral Agents against HBV: Molecular Mechanisms and Clinical Perspectives. J Clin Med. 2022;11:1349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 80. | McFadden WM, Sarafianos SG. Biology of the hepatitis B virus (HBV) core and capsid assembly modulators (CAMs) for chronic hepatitis B (CHB) cure. Glob Health Med. 2023;5:199-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 81. | Yuen MF, Zhou X, Gane E, Schwabe C, Tanwandee T, Feng S, Jin Y, Triyatni M, Lemenuel-Diot A, Cosson V, Xue Z, Kazma R, Bo Q. Safety, pharmacokinetics, and antiviral activity of RO7049389, a core protein allosteric modulator, in patients with chronic hepatitis B virus infection: a multicentre, randomised, placebo-controlled, phase 1 trial. Lancet Gastroenterol Hepatol. 2021;6:723-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 82. | Klumpp K, Shimada T, Allweiss L, Volz T, Lütgehetmann M, Hartman G, Flores OA, Lam AM, Dandri M. Efficacy of NVR 3-778, Alone and In Combination With Pegylated Interferon, vs Entecavir In uPA/SCID Mice With Humanized Livers and HBV Infection. Gastroenterology. 2018;154:652-662.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 83. | Yuen MF, Locarnini S, Lim TH, Strasser SI, Sievert W, Cheng W, Thompson AJ, Given BD, Schluep T, Hamilton J, Biermer M, Kalmeijer R, Beumont M, Lenz O, De Ridder F, Cloherty G, Ka-Ho Wong D, Schwabe C, Jackson K, Lai CL, Gish RG, Gane E. Combination treatments including the small-interfering RNA JNJ-3989 induce rapid and sometimes prolonged viral responses in patients with CHB. J Hepatol. 2022;77:1287-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 84. | Gane E, Yuen MF, Kim DJ, Chan HL, Surujbally B, Pavlovic V, Das S, Triyatni M, Kazma R, Grippo JF, Buatois S, Lemenuel-Diot A, Krippendorff BF, Mueller H, Zhang Y, Kim HJ, Leerapun A, Lim TH, Lim YS, Tanwandee T, Kim W, Cheng W, Hu TH, Wat C. Clinical Study of Single-Stranded Oligonucleotide RO7062931 in Healthy Volunteers and Patients With Chronic Hepatitis B. Hepatology. 2021;74:1795-1808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 85. | Hui RW, Mak LY, Seto WK, Yuen MF. RNA interference as a novel treatment strategy for chronic hepatitis B infection. Clin Mol Hepatol. 2022;28:408-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 86. | Thi EP, Ye X, Snead NM, Lee ACH, Micolochick Steuer HM, Ardzinski A, Graves IE, Espiritu C, Cuconati A, Abbott C, Jarosz A, Teng X, Paratala B, McClintock K, Harasym T, Rijnbrand R, Lam AM, Sofia MJ. Control of Hepatitis B Virus with Imdusiran, a Small Interfering RNA Therapeutic. ACS Infect Dis. 2024;10:3640-3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 87. | Sneller L, Lin C, Price A, Kottilil S, Chua JV. RNA Interference Therapeutics for Chronic Hepatitis B: Progress, Challenges, and Future Prospects. Microorganisms. 2024;12:599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 88. | Yuen MF, Lim YS, Yoon KT, Lim TH, Heo J, Tangkijvanich P, Tak WY, Thanawala V, Cloutier D, Mao S, Arizpe A, Cathcart AL, Gupta SV, Hwang C, Gane E. VIR-2218 (elebsiran) plus pegylated interferon-alfa-2a in participants with chronic hepatitis B virus infection: a phase 2 study. Lancet Gastroenterol Hepatol. 2024;9:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 42] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 89. | Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One. 2016;11:e0156667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 90. | Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J, Schmid P, Le Gal F, Gordien E, Krawczyk A, Mijočević H, Karimzadeh H, Roggendorf M, Vaillant A. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 204] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 91. | Vaillant A. REP 2139: Antiviral Mechanisms and Applications in Achieving Functional Control of HBV and HDV Infection. ACS Infect Dis. 2019;5:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 92. | Bazinet M, Pântea V, Placinta G, Moscalu I, Cebotarescu V, Cojuhari L, Jimbei P, Iarovoi L, Smesnoi V, Musteata T, Jucov A, Dittmer U, Krawczyk A, Vaillant A. Safety and Efficacy of 48 Weeks REP 2139 or REP 2165, Tenofovir Disoproxil, and Pegylated Interferon Alfa-2a in Patients With Chronic HBV Infection Naïve to Nucleos(t)ide Therapy. Gastroenterology. 2020;158:2180-2194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 186] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 93. | Sausen DG, Shechter O, Bietsch W, Shi Z, Miller SM, Gallo ES, Dahari H, Borenstein R. Hepatitis B and Hepatitis D Viruses: A Comprehensive Update with an Immunological Focus. Int J Mol Sci. 2022;23:15973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 94. | Liu H, Zakrzewicz D, Nosol K, Irobalieva RN, Mukherjee S, Bang-Sørensen R, Goldmann N, Kunz S, Rossi L, Kossiakoff AA, Urban S, Glebe D, Geyer J, Locher KP. Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP. Nat Commun. 2024;15:2476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 95. | Wedemeyer H, Schöneweis K, Bogomolov PO, Voronkova N, Chulanov V, Stepanova T, Bremer B, Allweiss L, Dandri M, Burhenne J, Haefeli W, Ciesek S, Dittmer U, Alexandrov A, Urban S. GS-13-Final results of a multicenter, open-label phase 2 clinical trial (MYR203) to assess safety and efficacy of myrcludex B in cwith PEG-interferon Alpha 2a in patients with chronic HBV/HDV co-infection. J Hepatol. 2019;70:e81. [DOI] [Full Text] |
| 96. | Lucifora J, Esser K, Protzer U. Ezetimibe blocks hepatitis B virus infection after virus uptake into hepatocytes. Antiviral Res. 2013;97:195-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 97. | Watashi K, Sluder A, Daito T, Matsunaga S, Ryo A, Nagamori S, Iwamoto M, Nakajima S, Tsukuda S, Borroto-Esoda K, Sugiyama M, Tanaka Y, Kanai Y, Kusuhara H, Mizokami M, Wakita T. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology. 2014;59:1726-1737. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 98. | Luk A, Jiang Q, Glavini K, Triyatni M, Zhao N, Racek T, Zhu Y, Grippo JF. A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers. Clin Transl Sci. 2020;13:985-993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 99. | Lanford RE, Guerra B, Chavez D, Giavedoni L, Hodara VL, Brasky KM, Fosdick A, Frey CR, Zheng J, Wolfgang G, Halcomb RL, Tumas DB. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508-1517, 1517.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 323] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 100. | Mackman RL, Mish M, Chin G, Perry JK, Appleby T, Aktoudianakis V, Metobo S, Pyun P, Niu C, Daffis S, Yu H, Zheng J, Villasenor AG, Zablocki J, Chamberlain J, Jin H, Lee G, Suekawa-Pirrone K, Santos R, Delaney WE 4th, Fletcher SP. Discovery of GS-9688 (Selgantolimod) as a Potent and Selective Oral Toll-Like Receptor 8 Agonist for the Treatment of Chronic Hepatitis B. J Med Chem. 2020;63:10188-10203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 101. | Sato S, Li K, Kameyama T, Hayashi T, Ishida Y, Murakami S, Watanabe T, Iijima S, Sakurai Y, Watashi K, Tsutsumi S, Sato Y, Akita H, Wakita T, Rice CM, Harashima H, Kohara M, Tanaka Y, Takaoka A. The RNA sensor RIG-I dually functions as an innate sensor and direct antiviral factor for hepatitis B virus. Immunity. 2015;42:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 362] [Article Influence: 32.9] [Reference Citation Analysis (3)] |
| 102. | Phillips S, Mistry S, Riva A, Cooksley H, Hadzhiolova-Lebeau T, Plavova S, Katzarov K, Simonova M, Zeuzem S, Woffendin C, Chen PJ, Peng CY, Chang TT, Lueth S, De Knegt R, Choi MS, Wedemeyer H, Dao M, Kim CW, Chu HC, Wind-Rotolo M, Williams R, Cooney E, Chokshi S. Peg-Interferon Lambda Treatment Induces Robust Innate and Adaptive Immunity in Chronic Hepatitis B Patients. Front Immunol. 2017;8:621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 103. | Su TH, Liu CJ. Combination Therapy for Chronic Hepatitis B: Current Updates and Perspectives. Gut Liver. 2017;11:590-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 104. | Deng G, Ge J, Liu C, Pang J, Huang Z, Peng J, Sun J, Hou J, Zhang X. Impaired expression and function of TLR8 in chronic HBV infection and its association with treatment responses during peg-IFN-α-2a antiviral therapy. Clin Res Hepatol Gastroenterol. 2017;41:386-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 105. | Evans A, Riva A, Cooksley H, Phillips S, Puranik S, Nathwani A, Brett S, Chokshi S, Naoumov NV. Programmed death 1 expression during antiviral treatment of chronic hepatitis B: Impact of hepatitis B e-antigen seroconversion. Hepatology. 2008;48:759-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 135] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 106. | Gane E, Verdon DJ, Brooks AE, Gaggar A, Nguyen AH, Subramanian GM, Schwabe C, Dunbar PR. Anti-PD-1 blockade with nivolumab with and without therapeutic vaccination for virally suppressed chronic hepatitis B: A pilot study. J Hepatol. 2019;71:900-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 268] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 107. | Qian J, Xie Y, Mao Q, Xie Q, Gu Y, Chen X, Hu G, Yang Y, Lu J, Zou G, Zhang Q, Fu L, Chen Y, Guo X, Hou J, Yan Y, Wu JJ, Cui Y, Wang G. A randomized phase 2b study of subcutaneous PD-L1 antibody ASC22 in virally suppressed patients with chronic hepatitis B who are HBeAg-negative. Hepatology. 2025;81:1328-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 108. | Mon HC, Lee PC, Hung YP, Hung YW, Wu CJ, Lee CJ, Chi CT, Lee IC, Hou MC, Huang YH. Functional cure of hepatitis B in patients with cancer undergoing immune checkpoint inhibitor therapy. J Hepatol. 2025;82:51-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 23] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 109. | Novotny LA, Evans JG, Su L, Guo H, Meissner EG. Review of Lambda Interferons in Hepatitis B Virus Infection: Outcomes and Therapeutic Strategies. Viruses. 2021;13:1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 110. | Ye L, Schnepf D, Staeheli P. Interferon-λ orchestrates innate and adaptive mucosal immune responses. Nat Rev Immunol. 2019;19:614-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 208] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 111. | Chan HLY, Ahn SH, Chang TT, Peng CY, Wong D, Coffin CS, Lim SG, Chen PJ, Janssen HLA, Marcellin P, Serfaty L, Zeuzem S, Cohen D, Critelli L, Xu D, Wind-Rotolo M, Cooney E; LIRA-B Study Team. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: A randomized phase 2b study (LIRA-B). J Hepatol. 2016;64:1011-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 112. | Lok AS, Pan CQ, Han SH, Trinh HN, Fessel WJ, Rodell T, Massetto B, Lin L, Gaggar A, Subramanian GM, McHutchison JG, Ferrari C, Lee H, Gordon SC, Gane EJ. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol. 2016;65:509-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 113. | Boni C, Janssen HLA, Rossi M, Yoon SK, Vecchi A, Barili V, Yoshida EM, Trinh H, Rodell TC, Laccabue D, Alfieri A, Brillo F, Fisicaro P, Acerbi G, Pedrazzi G, Andreone P, Cursaro C, Margotti M, Santoro R, Piazzolla V, Brunetto MR, Coco B, Cavallone D, Zhao Y, Joshi A, Woo J, Lau AH, Gaggar A, Subramanian GM, Massetto B, Fung S, Ahn SH, Ma X, Mangia A, Ferrari C. Combined GS-4774 and Tenofovir Therapy Can Improve HBV-Specific T-Cell Responses in Patients With Chronic Hepatitis. Gastroenterology. 2019;157:227-241.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 114. | Ma H, Lim TH, Leerapun A, Weltman M, Jia J, Lim YS, Tangkijvanich P, Sukeepaisarnjaroen W, Ji Y, Le Bert N, Li D, Zhang Y, Hamatake R, Tan N, Li C, Strasser SI, Ding H, Yoon JH, Stace NH, Ahmed T, Anderson DE, Yan L, Bertoletti A, Zhu Q, Yuen MF. Therapeutic vaccine BRII-179 restores HBV-specific immune responses in patients with chronic HBV in a phase Ib/IIa study. JHEP Rep. 2021;3:100361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 115. | Tak WY, Chuang WL, Chen CY, Tseng KC, Lim YS, Lo GH, Heo J, Agarwal K, Bussey L, Teoh SL, Tria A, Brown A, Anderson K, Vardeu A, O'Brien S, Kopycinski J, Kolenovska R, Barnes E, Evans T. Phase Ib/IIa randomized study of heterologous ChAdOx1-HBV/MVA-HBV therapeutic vaccination (VTP-300) as monotherapy and combined with low-dose nivolumab in virally-suppressed patients with CHB. J Hepatol. 2024;81:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 116. | Lampertico P, Brunetto MR, Craxì A, Gaeta GB, Rizzetto M, Rozzi A, Colombo M; HERMES Study Group. Add-on peginterferon alfa-2a to nucleos(t)ide analogue therapy for Caucasian patients with hepatitis B 'e' antigen-negative chronic hepatitis B genotype D. J Viral Hepat. 2019;26:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 117. | Hu P, Shang J, Zhang W, Gong G, Li Y, Chen X, Jiang J, Xie Q, Dou X, Sun Y, Li Y, Liu Y, Liu G, Mao D, Chi X, Tang H, Li X, Xie Y, Chen X, Jiang J, Zhao P, Hou J, Gao Z, Fan H, Ding J, Zhang D, Ren H. HBsAg Loss with Peg-interferon Alfa-2a in Hepatitis B Patients with Partial Response to Nucleos(t)ide Analog: New Switch Study. J Clin Transl Hepatol. 2018;6:25-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 118. | Pockros PJ, Mulgaonkar A. Cessation of Nucleoside/Nucleotide Analogue Therapy in Chronic Hepatitis B HBeAg-Negative Patients. Gastroenterol Hepatol (N Y). 2022;18:320-325. [PubMed] |
| 119. | Broquetas T, Carrión JA. Current Perspectives on Nucleos(t)ide Analogue Therapy for the Long-Term Treatment of Hepatitis B Virus. Hepat Med. 2022;14:87-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 120. | Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, Chen DS, Chen HL, Chen PJ, Chien RN, Dokmeci AK, Gane E, Hou JL, Jafri W, Jia J, Kim JH, Lai CL, Lee HC, Lim SG, Liu CJ, Locarnini S, Al Mahtab M, Mohamed R, Omata M, Park J, Piratvisuth T, Sharma BC, Sollano J, Wang FS, Wei L, Yuen MF, Zheng SS, Kao JH. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10:1-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1985] [Cited by in RCA: 2052] [Article Influence: 205.2] [Reference Citation Analysis (12)] |
| 121. | Chi H, Hansen BE, Yim C, Arends P, Abu-Amara M, van der Eijk AA, Feld JJ, de Knegt RJ, Wong DK, Janssen HL. Reduced risk of relapse after long-term nucleos(t)ide analogue consolidation therapy for chronic hepatitis B. Aliment Pharmacol Ther. 2015;41:867-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 122. | Hadziyannis SJ, Sevastianos V, Rapti I, Vassilopoulos D, Hadziyannis E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology. 2012;143:629-636.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 289] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 123. | Berg T, Simon KG, Mauss S, Schott E, Heyne R, Klass DM, Eisenbach C, Welzel TM, Zachoval R, Felten G, Schulze-Zur-Wiesch J, Cornberg M, Op den Brouw ML, Jump B, Reiser H, Gallo L, Warger T, Petersen J; FINITE CHB study investigators [First investigation in stopping TDF treatment after long-term virological suppression in HBeAg-negative chronic hepatitis B]. Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. J Hepatol. 2017;67:918-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 250] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 124. | European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67:370-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3745] [Cited by in RCA: 4009] [Article Influence: 445.4] [Reference Citation Analysis (1)] |
| 125. | García-López M, Lens S, Pallett LJ, Testoni B, Rodríguez-Tajes S, Mariño Z, Bartres C, García-Pras E, Leonel T, Perpiñán E, Lozano JJ, Rodríguez-Frías F, Koutsoudakis G, Zoulim F, Maini MK, Forns X, Pérez-Del-Pulgar S. Viral and immune factors associated with successful treatment withdrawal in HBeAg-negative chronic hepatitis B patients. J Hepatol. 2021;74:1064-1074. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 126. | Jeng WJ, Chen YC, Chien RN, Sheen IS, Liaw YF. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2018;68:425-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 265] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 127. | Hirode G, Choi HSJ, Chen CH, Su TH, Seto WK, Van Hees S, Papatheodoridi M, Lens S, Wong G, Brakenhoff SM, Chien RN, Feld J, Sonneveld MJ, Chan HLY, Forns X, Papatheodoridis GV, Vanwolleghem T, Yuen MF, Hsu YC, Kao JH, Cornberg M, Hansen BE, Jeng WJ, Janssen HLA; RETRACT-B Study Group. Off-Therapy Response After Nucleos(t)ide Analogue Withdrawal in Patients With Chronic Hepatitis B: An International, Multicenter, Multiethnic Cohort (RETRACT-B Study). Gastroenterology. 2022;162:757-771.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 128. | Sonneveld MJ, Park JY, Kaewdech A, Seto WK, Tanaka Y, Carey I, Papatheodoridi M, van Bömmel F, Berg T, Zoulim F, Ahn SH, Dalekos GN, Erler NS, Höner Zu Siederdissen C, Wedemeyer H, Cornberg M, Yuen MF, Agarwal K, Boonstra A, Buti M, Piratvisuth T, Papatheodoridis G, Maasoumy B; CREATE Study Group. Prediction of Sustained Response After Nucleo(s)tide Analogue Cessation Using HBsAg and HBcrAg Levels: A Multicenter Study (CREATE). Clin Gastroenterol Hepatol. 2022;20:e784-e793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 129. | Jeng WJ, Chen YC, Sheen IS, Lin CL, Hu TH, Chien RN, Liaw YF. Clinical Relapse After Cessation of Tenofovir Therapy in Hepatitis B e Antigen-Negative Patients. Clin Gastroenterol Hepatol. 2016;14:1813-1820.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 130. | Chong CH, Lim SG. When can we stop nucleoside analogues in patients with chronic hepatitis B? Liver Int. 2017;37 Suppl 1:52-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/