Published online May 27, 2024. doi: 10.4254/wjh.v16.i5.800
Revised: April 10, 2024
Accepted: April 18, 2024
Published online: May 27, 2024
Processing time: 103 Days and 21.1 Hours
In recent years, approximately half of the newly diagnosed cases and mortalities attributed to hepatocellular carcinoma (HCC) have been reported in China. Despite the high incidence of HCC, there remains a paucity of data regarding the natural growth pattern and the determination of optimal surveillance intervals specific to the Chinese population.
To quantify the natural tumor growth pattern of HCC in regional China.
A retrospective analysis was performed on patients from a single institution in Southwest China who had undergone two or more serial dynamic computed tomography or magnetic resonance imaging scans between 2014 and 2020, without having received any anti-cancer therapy. Tumor growth was assessed using tumor volume doubling time (TVDT) and tumor growth rate (TGR), with volumes measured manually by experienced radiologists. Simple univariate linear regression and descriptive analysis were applied to explore associations between growth rates and clinical factors.
This study identifies the median TVDT for HCC as 163.4 d, interquartile range (IQR) 72.1 to 302.3 d, with a daily TGR of 0.42% (IQR 0.206%-0.97%). HCC growth patterns reveal that about one-third of tumors grow indolently with TVDT exceeding 270 d, another one-third of tumors exhibit rapid growth with TVDT under 90 d, and the remaining tumors show intermediate growth rates, with TVDT ranging between 3 to 9 months.
The identified TGRs support biannual surveillance and follow-up for HCC patients in certain regions of China. Given the observed heterogeneity in HCC growth, further investigation is warranted.
Core Tip: The present study offers real-world data, revealing a median tumor volume doubling time of 163 d for hepatocellular carcinoma (HCC) patients in Southwest China. These findings endorse the implementation of biannual surveillance and follow-up for this patient population. The observed heterogeneity in HCC growth, with approximately one-third of patients exhibiting indolent, intermediate, or rapid growth, highlights the necessity for individualized management and targeted treatment strategies based on specific tumor growth rate. Further research is essential to elucidate the mechanisms driving this growth heterogeneity and to inform clinical practice.
- Citation: Tu L, Xie H, Li Q, Lei PG, Zhao PL, Yang F, Gong C, Yao YL, Zhou S. Quantifying the natural growth rate of hepatocellular carcinoma: A real-world retrospective study in southwestern China. World J Hepatol 2024; 16(5): 800-808
- URL: https://www.wjgnet.com/1948-5182/full/v16/i5/800.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i5.800
Liver cancer, primarily hepatocellular carcinoma (HCC), stands as one of the most prevalent and lethal malignancies in numerous countries[1,2]. It ranks as the fourth leading cause of cancer-related death worldwide, with mortality rates on the rise in recent years, notably becoming the second most common cause of cancer mortality in some regions[3,4]. China, in particular, bears a significant burden of liver cancer, with nearly half of the world's diagnoses occurring within its borders. This burden is reflected in mortality rates, where approximately half of all liver cancer-related deaths worldwide are reported in China[5,6]. Since 2019, China has consistently recorded over 360,000 new cases of liver cancer annually[1,3]. Notably, between 1999 and 2019, HCC ascended from the seventh to the second most common cause of cancer mortality in China, trailing only behind lung cancer[1,3,7].
HCC, traditionally recognized as an aggressive malignancy, exhibits unique biological behaviors and underlying mechanisms that contribute to its poor prognosis for many patients. The rapid and uninterrupted growth of HCC is characteristic of its malignant nature, posing significant challenges to clinical treatment[8]. As tumor growth is a fundamental hallmark of cancer, understanding the natural tumor growth rate (TGR) assumes critical importance in managing HCC patients[9]. This understanding informs various aspects of patient care, including tumor surveillance, screening intervals, treatment planning, and prognostic communication. Rapid tumor growth often signifies the aggressive nature of the lesion, necessitating shorter surveillance intervals and intervention, whereas indolent growth provides patients with additional time to explore treatment options or await curative measures such as transplantation. A comprehensive understanding of tumor growth and its underlying mechanisms empowers clinicians to offer more precise recommendations for managing HCC patients in clinical practice[10,11].
Tailoring medical management strategies based on real-world experiences holds significant promise for optimizing patient care and conserving medical resources. However, the scarcity of real-world data on the natural growth pattern of HCC, particularly in mainland China-where the annual number of new cases and deaths accounts for nearly half of all global cases-poses a challenge to evidence-based practice in HCC management. This study aims to address this gap by providing evidence for follow-up intervals of HCC patients based on clinical cases, with the goal to facilitate precise and evidence-based management of HCC.
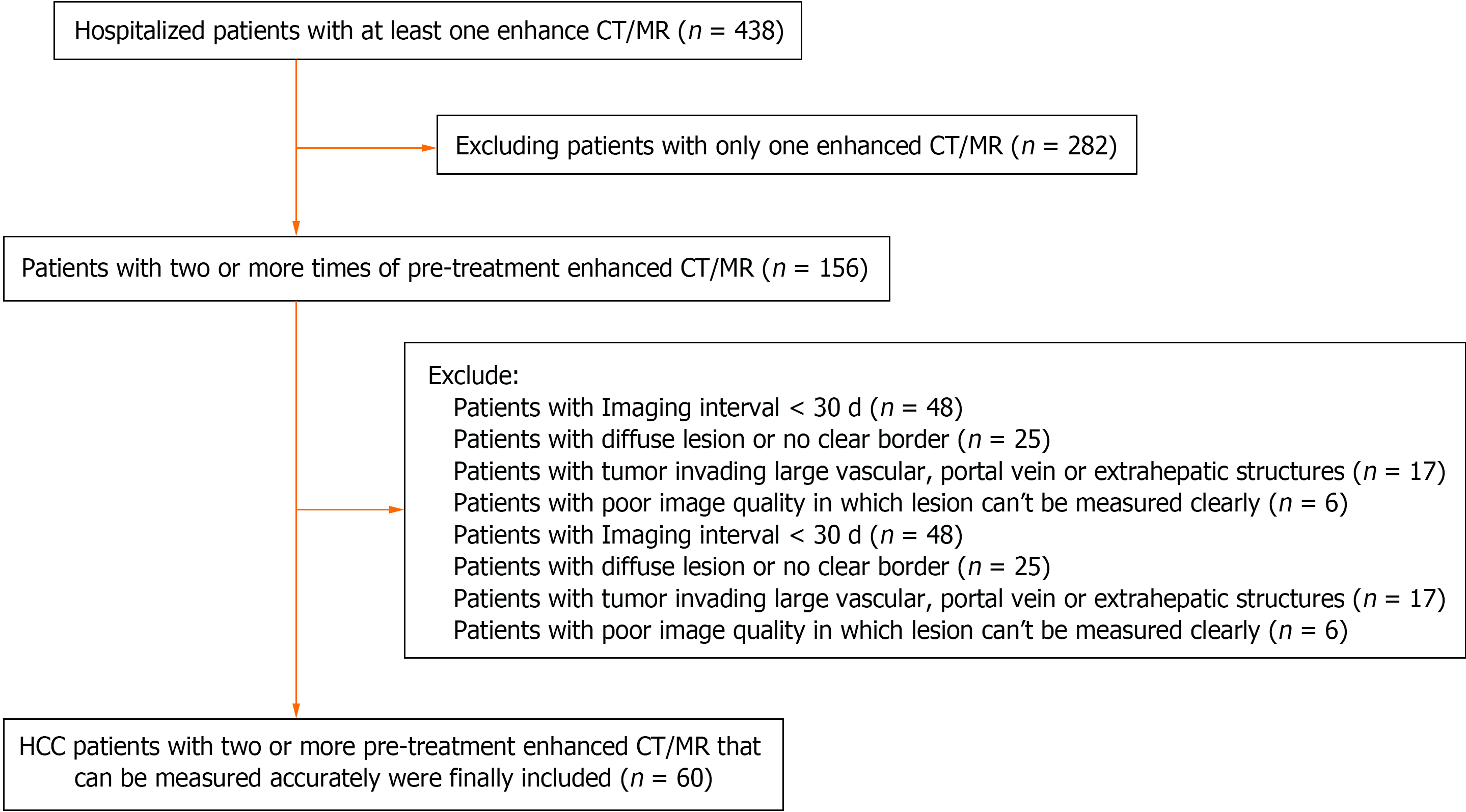
This is a retrospective study conducted at the Affiliated Hospital of Guizhou Medical University in southwest China, focusing on patients diagnosed with HCC between January 1, 2014, and December 30, 2020. A comprehensive search of the hospital's electronic database, comprising both clinical and radiological information systems, identified 438 patients with a primary diagnosis of HCC. To ensure accurate analysis and measurement, two experienced radiologists, Yao YL and Gong C, reviewed the medical records and imaging studies to ensure that there were at least two sets of contrast-enhanced computed tomography (CT) and/or magnetic resonance imaging (MRI) images available for analysis. Contrast-enhanced CT and MRI scans are indispensable for achieving accurate clinical and imaging diagnoses of liver cancer, as plain CT and MRI often proved insufficient to delineate the accurate extent of HCC. Consequently, 282 patients were excluded from the study as their medical records contained only a single enhanced CT and/or MRI image. Figure 1 outlines the process of patient selection.
Further exclusions were made from the initial cohort of 156 patients to maintain the integrity of the study. Forty-eight patients were excluded due to intervals of less than 30 d between imaging sessions, as shorter intervals could potentially affect the accuracy of measurements. Twenty-five patients with diffuse and indistinct tumor borders were omitted from the analysis, as precise measurement of tumor dimensions was deemed challenging. Additionally, 17 patients with tumors invading large vascular or extrahepatic structures, such as portal vein tumor thrombosis, were excluded to prevent inaccurate measurements. Six patients were excluded due to poor image quality, rendering the images unsuitable for lesion measurement. As a result of these exclusions, 60 patients were included in the final study group.
This study was conducted with the approval of the local institutional review boards of the Affiliated Hospital of Guizhou Medical University, No. 2022-734, ensuring adherence to ethical standards and patient confidentiality.
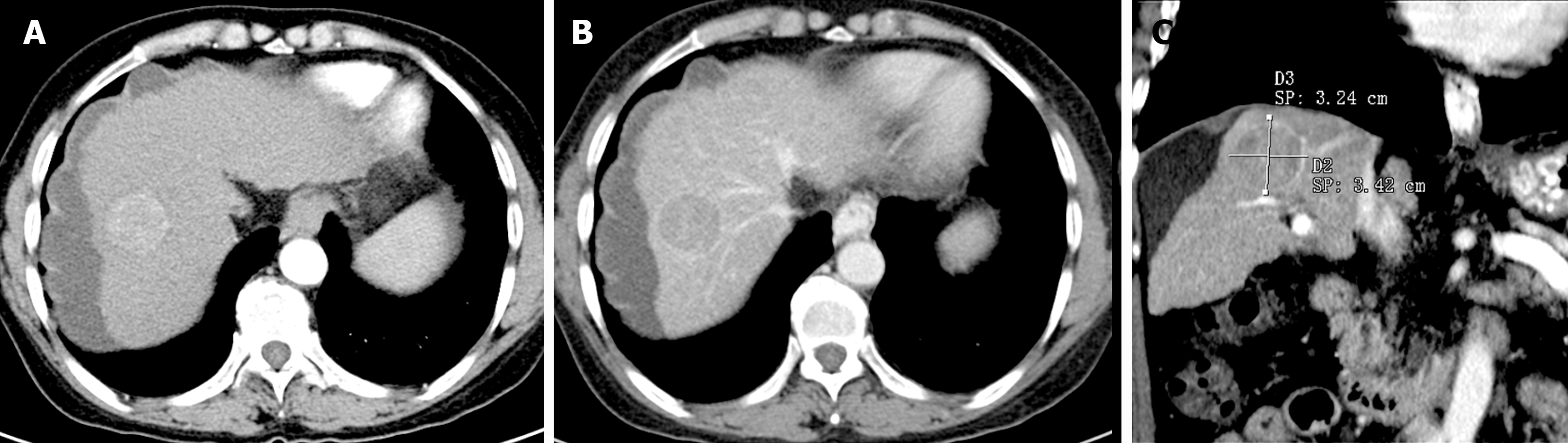
The diameters of HCC lesions were measured according to the Liver Cancer Diagnosis and Reporting System 2018 edition (LI-RADS-2018) criteria, utilizing phases or sequences that best outlined the lesion margins on contrast-enhanced images (CT or MRI)[12]. Figure 2 illustrates one typical case in this study on contrast-enhanced computed tomography. Two abdominal radiologists, Li Q with 10 years and Gong C with 3 years of diagnostic experience, independently performed the measurements of tumor diameters[12]. Prior to measurement, both radiologists were blinded to the patients’ pathological diagnoses. The measurement results were independently verified by Xie H, who has 10 years of diagnostic experience. In cases of significant discrepancies in measurement results, Xie H discussed with Li Q and Gong C to reach a consensus. In instances where patients presented with multiple focal HCCs, the diameters of the larger lesions were measured. For patients with more than two enhanced CT/MRI scans, earlier imaging and the last interval were utilized to calculate the imaging interval and TGR, aiming to provide a comprehensive reflection of macroscopic changes.
Tumor growth was assessed using two primary metrics: Tumor volume doubling time (TVDT) and TGR. TVDT, commonly reported in cancer studies, represents the time it takes for a tumor's volume to double. Conversely, TGR serves as a direct indicator of tumor growth and is often employed for statistical analysis. The tumor volume (V) and TGR, as well as TVDT, were calculated using the following equations. The maximum tumor diameters (a, b, c) were utilized, as most HCC lesions resemble an ellipsoid[13-15]. In the context amentioned, V1 and T1 respectively represent the volume of the tumor at the second examination and the date of the examination, while V0 and T0 represent the volume of the tumor at the initial examination and the date of the examination.
We used the Kruskal-Wallis test for continuous variables to compare groups, while Fisher’s exact test was used for categorical variables. Univariate logistic regression was used to determine the possible correlation between the TGR (indolent, indolent or rapid) and clinical factors, including age, sex, body mass index (BMI), liver disease etiology, Child-Pugh score, BCLC stage, and initial tumor diameter. All tests were performed at the 5% significance level. All the analyses were performed with the statistical software package Free software version 1.2 based on R-3 (http://www.R-project.org, The R Foundation).
The TGR was classified as rapid, intermediate, and indolent according to the TVDT of the lesion. A rapid growth rate was defined as a TVDT < 3 months, while an HCC was defined as exhibiting indolent growth if it had a TVDT longer than 9 months. A TVDT between 3 and 9 months was defined as intermediate growth. These cut-off points were selected because some guidelines recommend semiannual surveillance, while other regional surveillance programs suggest repeated imaging of indeterminate hepatic nodules at 3-month intervals. Data from all patients were collected and calculated using Microsoft Excel version 2010.
Among the 438 HCC patients reviewed during the study period, 60 patients were finally included in the analysis. The baseline characteristics of the cohort are summarized in Table 1. The study comprised 49 males and 11 females, with a mean age at diagnosis of HCC of 59.3 years. The majority of patients (86.7%) were infected with hepatitis B virus (HBV); however, in the non-HBV group, four patients exhibited heavy alcohol consumption and were diagnosed with alcoholic liver disease, while four patients did not have any known hepatic infection or risk factors for HCC. Among the cohort, 20 patients had Child-Pugh A cirrhosis, 31 patients had Child-Pugh B cirrhosis, and six had Child-Pugh C cirrhosis. Laboratory results for prothrombin time were missing for three patients, resulting in the absence of Child-Pugh and BCLC classification data for these individuals. Regarding BCLC staging, 26 patients were classified as stage A, 22 as stage B, six as stage C, and three as stage D. Twelve patients underwent surgery, including hepatic resection (n = 10) and transplantation (n = 2), while 20 patients received locoregional treatments such as transarterial chemoembolization (n = 19) or radiofrequency ablation (n = 1). The remaining 28 individuals did not receive treatment for HCC before loss of follow-up or death.
| Variable | Total (n = 60) |
| Age | 60.0 ± 11.1 |
| Sex | |
| Female | 11 (18.3) |
| Male | 49 (81.7) |
| Smoking | |
| No | 24 (40.7) |
| Yes | 35 (59.3) |
| Drinking | |
| No | 27 (45.8) |
| Yes | 32 (54.2) |
| BMI | 23.8 ± 3.1 |
| Hepatitis | |
| Non-HBV | 8 (13.3) |
| HBV | 52 (86.7) |
| AFP, median (IQR) | 14.3 (3.1, 120.4) |
| AVD, median (IQR) | 21.8 (15.2, 39.6) |
| Child-Pugh (%) | |
| A | 20 (33.3) |
| B | 31 (51.7) |
| C | 6 (10.0) |
| NA1 | 3 (5.0) |
| BCLC classification | |
| A | 26 (43.3) |
| B | 22 (36.7) |
| C | 6 (10.0) |
| D | 3 (5.0) |
| NA1 | 3 (5.0) |
The diameter of the tumors in the study ranged from 1.0 to 13.3 cm, with a median of 2.2 cm interquartile range (IQR) = 1.5, 4.0. The overall median TVDT was 163.4 d (IQR = 72.1, 302.3), and the median TGR in the overall study population was 0.42% (IQR = 0.206%, 0.97%) per day, as shown in Table 2.
| Variable | Total (n = 60) |
| TVDT, median (IQR) | 163.4 (72.1, 302.3) |
| TGR, median (IQR) | 42.4 (23.0, 96.1) |
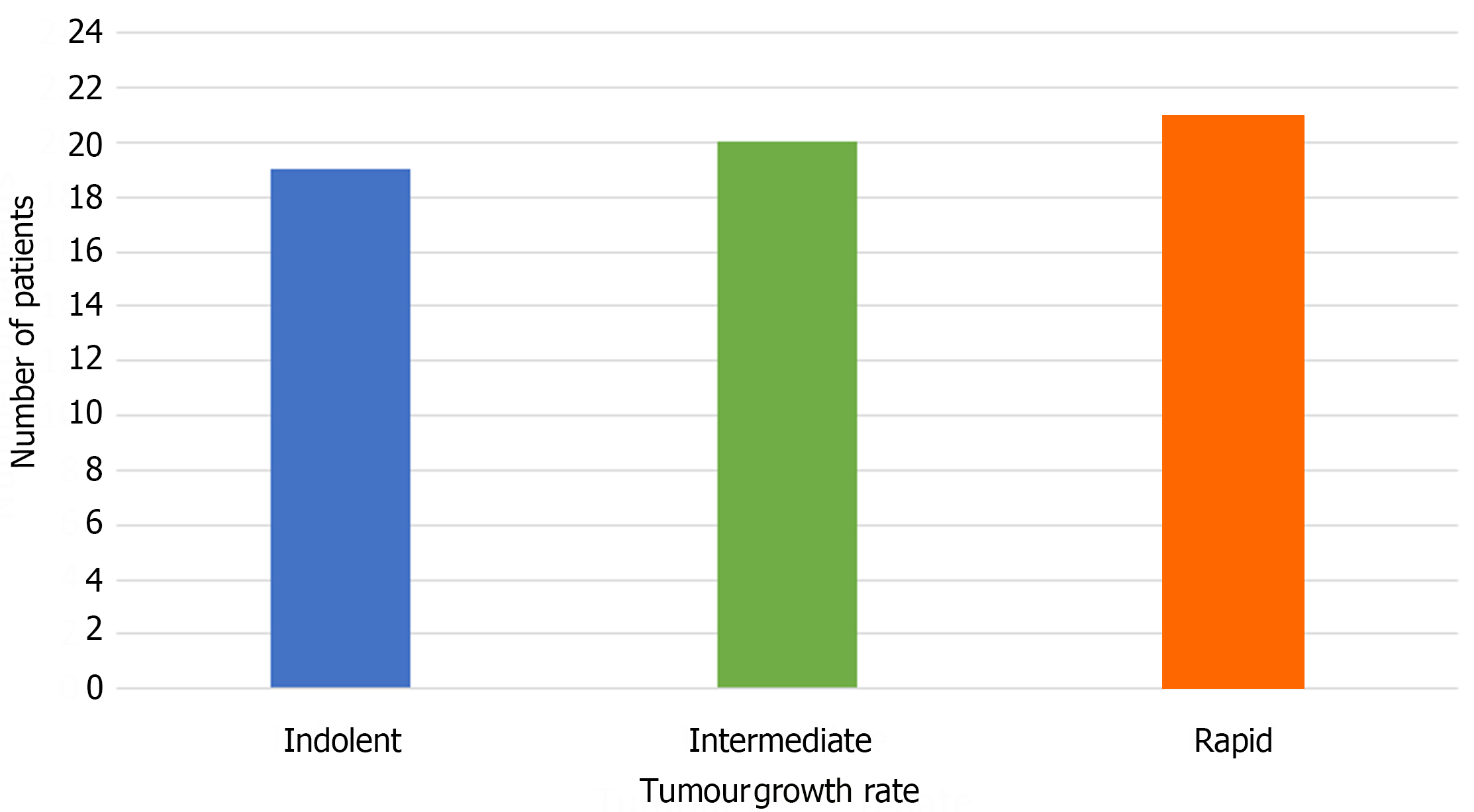
Using 3 months and 9 months as the cutoff points for rapid, intermediate, and indolent growth rates, HCCs in 19 patients exhibited indolent growth, HCCs in 21 patients exhibited rapid growth, and the remaining 20 HCCs exhibited an intermediate growth rate. The distribution of patients classified by TVDT in each group is illustrated in Figure 3, with each group contributing to nearly one-third of the study population.
In an attempt to explore potential associations between growth pattern and clinical variables using univariate linear regression analysis, tumors in the indolent growth group tended to have a larger diameter, while tumors in the rapid growth group had a smaller diameter. However, no statistically significant differences were identified between growth rate and BMI, alpha-fetoprotein (AFP) levels, or BCLC classifications.
The present study was conducted retrospectively to explore the growth pattern of HCC in a hospital in southwestern China, considering ethnic and practical factors. The median observed TVDT for HCC was 163.4 d, which is approximately two weeks shorter than 6 months. The median TGR was calculated at 0.42% per day. Using 3 months and 9 months as cut-off points, HCC lesions exhibited a heterogeneous growth pattern: nearly one-third of the lesions demonstrated a rapid growth rate with TVDT of less than 3 months (90 d), nearly one-third of the HCC lesions presented with an indolent growth rate, characterized by a TVDT longer than 9 months, and the remaining one-third of the HCC lesions demonstrated an intermediate growth rate, with TVDT between 3 months and 9 months.
The TVDT observed in our study was marginally longer compared to those reported in similar studies conducted in Eastern Asia, primarily originating from Japan and South Korea[16-18]. Previous research in these regions has reported shorter median TVDTs, typically ranging from 85 to 127 d, particularly among patients predominantly infected with HBV[11]. Interestingly, in our study, 52 out of the 60 patients were infected with HBV, a proportion similar to that found in studies conducted in South Korea[19,20], where TVDTs tended to be shorter. However, our findings contrast with those reported in Western nations, where TVDTs have been reported as longer[15,21,22]. For instance, a multicenter cohort study conducted by Rich et al[15] reported a median TVDT of 229 d in Western countries, where the predominant risk factors for HCC were hepatitis C virus, alcohol-related liver disease, and nonalcoholic fatty liver disease. Additionally, An et al[18] highlighted an association between growth rate and underlying etiology. Our study suggests that the median TVDT observed in the Chinese population falls somewhere between those reported in Eastern Asia and the Western world, indicating possible regional variations in HCC growth patterns[23]. To our knowledge, this study is the first from mainland China to report TGR and TVDT of HCC in the English literature. Given the substantial population of HCC patients in mainland China[24,25], these findings hold significant importance. They advocate for biannual surveillance and follow-up for individuals at high risk of developing or suffering from HCC[26].
In our study, we observed a special distribution of patients with different TGRs, with nearly one-third of the affected population distributed in each of the three groups. This finding aligns with previous research by Nathani et al[11] and Rich et al[15], suggesting that HCC may demonstrate a heterogeneous growth pattern. Understanding this phenomenon is crucial, as most studies on tumor growth patterns have suggested a constant, rapid growth rate in malignant tumors like HCC. However, a heterogeneous growth pattern carries important clinical implications, necessitating tailored management strategies. Consistent with findings by other authors[18], our study also observed that HCCs with larger diameters tend to grow more slowly, and we did not find a statistically significant association between TVDT and various clinical factors such as BMI, AFP levels, hepatitis etiology, liver function, or tumor stage[11,27,28], consistent with the findings of Sheu et al[29]. However, further studies are warranted to elucidate potential associations between clinical factors such as race, serological biochemical compounds, infection etiologies, and growth patterns[28,30]. Considering that the biological behavior of tumors is influenced by both host factors and underlying etiologies[31], additional research is needed to clarify the underlying mechanisms of tumor growth patterns in HCC[8].
This study has several limitations. Firstly, its retrospective design introduces inherent selection bias into the study population. Secondly, the relatively small sample size and the inclusion of patients from only one center in southwestern China limit the generalizability of the findings. Given China's significant contribution to global HCC cases and deaths, additional studies from mainland China are crucial to complement existing literatures[10,22] and enhance real-world knowledge of HCC management[32,33]. Thirdly, the reliance on imaging rather than pathological confirmation of lesions introduces potential misclassification bias, although the high positive predictive value of LI-RADS-5 in cirrhotic patients partially mitigates this concern[34]. Lastly, the study's reliance on certain hypotheses that HCC grows at a constant rate, and the volume of the lesions was calculated using the spherical shape, underscores the need for further research in related fields. Both of these hypotheses are still in development, and additional research is needed in related fields.
The natural growth rate observed in this study supports biannual surveillance and follow-up intervals for HCC patients in regional China. The study reveals a heterogeneous growth rate in HCC, underscoring the need for further research to explore the underlying mechanisms and clinical implications.
The author thanks Xiaocao Su for her assistance in the data cleaning process.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68667] [Article Influence: 13733.4] [Reference Citation Analysis (201)] |
| 2. | Rumgay H, Arnold M, Ferlay J, Lesi O, Cabasag CJ, Vignat J, Laversanne M, McGlynn KA, Soerjomataram I. Global burden of primary liver cancer in 2020 and predictions to 2040. J Hepatol. 2022;77:1598-1606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 1416] [Article Influence: 354.0] [Reference Citation Analysis (1)] |
| 3. | Xia C, Dong X, Li H, Cao M, Sun D, He S, Yang F, Yan X, Zhang S, Li N, Chen W. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1790] [Cited by in RCA: 2505] [Article Influence: 626.3] [Reference Citation Analysis (2)] |
| 4. | Singal AG, Kanwal F, Llovet JM. Global trends in hepatocellular carcinoma epidemiology: implications for screening, prevention and therapy. Nat Rev Clin Oncol. 2023;20:864-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 577] [Cited by in RCA: 533] [Article Influence: 177.7] [Reference Citation Analysis (2)] |
| 5. | Zheng R, Qu C, Zhang S, Zeng H, Sun K, Gu X, Xia C, Yang Z, Li H, Wei W, Chen W, He J. Liver cancer incidence and mortality in China: Temporal trends and projections to 2030. Chin J Cancer Res. 2018;30:571-579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 252] [Article Influence: 31.5] [Reference Citation Analysis (2)] |
| 6. | Qi J, Li M, Wang L, Hu Y, Liu W, Long Z, Zhou Z, Yin P, Zhou M. National and subnational trends in cancer burden in China, 2005-20: an analysis of national mortality surveillance data. Lancet Public Health. 2023;8:e943-e955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 327] [Article Influence: 109.0] [Reference Citation Analysis (2)] |
| 7. | Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl). 2021;134:783-791. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1624] [Cited by in RCA: 2006] [Article Influence: 401.2] [Reference Citation Analysis (3)] |
| 8. | Rebouissou S, Nault JC. Advances in molecular classification and precision oncology in hepatocellular carcinoma. J Hepatol. 2020;72:215-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 413] [Article Influence: 68.8] [Reference Citation Analysis (2)] |
| 9. | Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51728] [Cited by in RCA: 48796] [Article Influence: 3253.1] [Reference Citation Analysis (12)] |
| 10. | Friberg S, Mattson S. On the growth rates of human malignant tumors: implications for medical decision making. J Surg Oncol. 1997;65:284-297. [PubMed] [DOI] [Full Text] |
| 11. | Nathani P, Gopal P, Rich N, Yopp A, Yokoo T, John B, Marrero J, Parikh N, Singal AG. Hepatocellular carcinoma tumour volume doubling time: a systematic review and meta-analysis. Gut. 2021;70:401-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 12. | Chernyak V, Fowler KJ, Kamaya A, Kielar AZ, Elsayes KM, Bashir MR, Kono Y, Do RK, Mitchell DG, Singal AG, Tang A, Sirlin CB. Liver Imaging Reporting and Data System (LI-RADS) Version 2018: Imaging of Hepatocellular Carcinoma in At-Risk Patients. Radiology. 2018;289:816-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 869] [Article Influence: 108.6] [Reference Citation Analysis (1)] |
| 13. | SCHWARTZ M. A biomathematical approach to clinical tumor growth. Cancer. 1961;14:1272-1294. [PubMed] [DOI] [Full Text] |
| 14. | Yoshino M. Growth kinetics of hepatocellular carcinoma. Jpn J Clin Oncol. 1983;13:45-52. [PubMed] |
| 15. | Rich NE, John BV, Parikh ND, Rowe I, Mehta N, Khatri G, Thomas SM, Anis M, Mendiratta-Lala M, Hernandez C, Odewole M, Sundaram LT, Konjeti VR, Shetty S, Shah T, Zhu H, Yopp AC, Hoshida Y, Yao FY, Marrero JA, Singal AG. Hepatocellular Carcinoma Demonstrates Heterogeneous Growth Patterns in a Multicenter Cohort of Patients With Cirrhosis. Hepatology. 2020;72:1654-1665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 115] [Article Influence: 19.2] [Reference Citation Analysis (1)] |
| 16. | Kubota K, Ina H, Okada Y, Irie T. Growth rate of primary single hepatocellular carcinoma: determining optimal screening interval with contrast enhanced computed tomography. Dig Dis Sci. 2003;48:581-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (2)] |
| 17. | Okazaki N, Yoshino M, Yoshida T, Suzuki M, Moriyama N, Takayasu K, Makuuchi M, Yamazaki S, Hasegawa H, Noguchi M. Evaluation of the prognosis for small hepatocellular carcinoma based on tumor volume doubling time. A preliminary report. Cancer. 1989;63:2207-2210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (1)] |
| 18. | An C, Choi YA, Choi D, Paik YH, Ahn SH, Kim MJ, Paik SW, Han KH, Park MS. Growth rate of early-stage hepatocellular carcinoma in patients with chronic liver disease. Clin Mol Hepatol. 2015;21:279-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 19. | Negro F. Natural history of NASH and HCC. Liver Int. 2020;40 Suppl 1:72-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (2)] |
| 20. | But DY, Lai CL, Yuen MF. Natural history of hepatitis-related hepatocellular carcinoma. World J Gastroenterol. 2008;14:1652-1656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 145] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (2)] |
| 21. | Ebara M, Ohto M, Shinagawa T, Sugiura N, Kimura K, Matsutani S, Morita M, Saisho H, Tsuchiya Y, Okuda K. Natural history of minute hepatocellular carcinoma smaller than three centimeters complicating cirrhosis. A study in 22 patients. Gastroenterology. 1986;90:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 277] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 22. | Furlan A, Marin D, Agnello F, Di Martino M, Di Marco V, Lagalla R, Catalano C, Brancatelli G. Hepatocellular carcinoma presenting at contrast-enhanced multi-detector-row computed tomography or gadolinium-enhanced magnetic resonance imaging as a small (≤2 cm), indeterminate nodule: growth rate and optimal interval time for imaging follow-up. J Comput Assist Tomogr. 2012;36:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 23. | Kim JK, Kim HD, Jun MJ, Yun SC, Shim JH, Lee HC, Lee D, An J, Lim YS, Chung YH, Lee YS, Kim KM. Tumor Volume Doubling Time as a Dynamic Prognostic Marker for Patients with Hepatocellular Carcinoma. Dig Dis Sci. 2017;62:2923-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (1)] |
| 24. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6423] [Article Influence: 802.9] [Reference Citation Analysis (9)] |
| 25. | Valery PC, Laversanne M, Clark PJ, Petrick JL, McGlynn KA, Bray F. Projections of primary liver cancer to 2030 in 30 countries worldwide. Hepatology. 2018;67:600-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 253] [Article Influence: 31.6] [Reference Citation Analysis (1)] |
| 26. | Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, Bie P, Liu L, Wen T, Han G, Wang M, Liu R, Lu L, Ren Z, Chen M, Zeng Z, Liang P, Liang C, Yan F, Wang W, Ji Y, Yun J, Cai D, Chen Y, Cheng W, Cheng S, Dai C, Guo W, Hua B, Huang X, Jia W, Li Y, Liang J, Liu T, Lv G, Mao Y, Peng T, Ren W, Shi H, Shi G, Tao K, Wang X, Xiang B, Xing B, Xu J, Yang J, Yang Y, Ye S, Yin Z, Zhang B, Zhang L, Zhang S, Zhang T, Zhao Y, Zheng H, Zhu J, Zhu K, Shi Y, Xiao Y, Dai Z, Teng G, Cai J, Cai X, Li Q, Shen F, Qin S, Dong J, Fan J. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer. 2020;9:682-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 618] [Article Influence: 103.0] [Reference Citation Analysis (3)] |
| 27. | Wursthorn K, Manns MP, Wedemeyer H. Natural history: the importance of viral load, liver damage and HCC. Best Pract Res Clin Gastroenterol. 2008;22:1063-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 28. | Matsuhashi T, Yamada N, Shinzawa H, Takahashi T. Effect of alcohol on tumor growth of hepatocellular carcinoma with type C cirrhosis. Intern Med. 1996;35:443-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (1)] |
| 29. | Sheu JC, Sung JL, Chen DS, Yang PM, Lai MY, Lee CS, Hsu HC, Chuang CN, Yang PC, Wang TH. Growth rate of asymptomatic hepatocellular carcinoma and its clinical implications. Gastroenterology. 1985;89:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 314] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 30. | Shingaki N, Tamai H, Mori Y, Moribata K, Enomoto S, Deguchi H, Ueda K, Inoue I, Maekita T, Iguchi M, Kato J, Ichinose M. Serological and histological indices of hepatocellular carcinoma and tumor volume doubling time. Mol Clin Oncol. 2013;1:977-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 31. | Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 6045] [Article Influence: 1511.3] [Reference Citation Analysis (2)] |
| 32. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3370] [Article Influence: 481.4] [Reference Citation Analysis (45)] |
| 33. | Marks RM, Masch WR, Chernyak V. LI-RADS: Past, Present, and Future, From the AJR Special Series on Radiology Reporting and Data Systems. AJR Am J Roentgenol. 2021;216:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Kamath A, Roudenko A, Hecht E, Sirlin C, Chernyak V, Fowler K, Mitchell DG. CT/MR LI-RADS 2018: clinical implications and management recommendations. Abdom Radiol (NY). 2019;44:1306-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Tchilikidi KY, Russia S-Editor: Qu XL L-Editor: A P-Editor: Cai YX