Published online May 27, 2024. doi: 10.4254/wjh.v16.i5.791
Peer-review started: October 10, 2023
First decision: November 27, 2023
Revised: March 7, 2024
Accepted: March 25, 2024
Article in press: March 25, 2024
Published online: May 27, 2024
Processing time: 224 Days and 23.7 Hours
Wilson disease (WD) is a progressive, potentially fatal degenerative disease affecting the liver and central nervous system. Given its low prevalence, collecting data on large cohorts of patients with WD is challenging. Comprehensive insur
To describe patients with WD in the United States, their treatment and clinical outcome, using a large insurance claims database.
This retrospective, longitudinal study was performed in the Clarivate Real-World Data Product database. All patients with ≥ 2 claims associated with an International Classification of Diseases 10 (ICD-10) diagnostic code for WD (E83.01) between 2016 and 2021 were included and followed until death or study end. Patients were divided into two groups by whether or not they were documented to have received a specific treatment for WD. Clinical manifestations, hospitalisations, liver transplantation and death were documented.
Overall, 5376 patients with an ICD-10 diagnostic code for WD were identified. The mean age at inclusion was 41.2 years and 52.0% were men. A specific WD treatment was documented for 885 patients (15.1%), although the number of patients taking zinc salts may be underestimated due to over the counter purchase. At inclusion, the mean age of patients with a documented treatment was 36.6 ± 17.8 years vs 42.2 ± 19.6 years in those without a documented treatment. During follow-up, 273 patients (5.1%) died. Compared with the American general population, the standardised mortality ratio was 2.19. The proportion of patients with a documented WD-specific treatment who died during follow-up was 4.0% and the mean age at death 52.7 years.
Patients treated for WD in the United States had an excess early mortality compared with the American population. These findings indicate that there is a significant unmet need for effective treatment for WD in the United States.
Core Tip: This insurance claims database study in the United States evaluated data on 5376 patients with a reimbursement claim for Wilson disease (WD), although only patients with a documented treatment of WD can be unequivocally assumed to have the disease. For patients receiving a specific WD treatment, the mean age at death was 57 years, and the standard mortality ratio with respect to the general United States population was 2.19. These findings indicate an important unmet medical need for more effective treatment.
- Citation: Daniel-Robin T, Kumar P, Benichou B, Combal JP. Characteristics of patients with Wilson disease in the United States: An insurance claims database study. World J Hepatol 2024; 16(5): 791-799
- URL: https://www.wjgnet.com/1948-5182/full/v16/i5/791.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i5.791
Wilson disease (WD) is a progressive, potentially fatal degenerative disease, principally affecting the liver and the central nervous system[1,2]. It is caused by loss-of-function mutations in the ATP7B gene, localised on chromosome 13, which encodes the copper-transporting ATPase ATP7B[3,4]. In the absence of a functional ATB7B protein, copper is no longer eliminated correctly in the bile and accumulates in tissues to toxic levels, where it may lead to irreversible hepatic injury and neurodegeneration[1,5]. Moreover, around 30%-40% of patients with WD present with psychiatric symptoms, such as depression or acute psychosis[6]. Based on clinical studies, the prevalence of WD has been estimated to be between 1:30,000 and 1:50,000 (2 to 3.3 cases per 100000) in most countries[7]. Genetic studies have suggested a higher prevalence[8], but this may be an over estimate due to inclusion of ATP7B gene mutations with low penetrance, or mutation that are not pathogenic. Accurate determination of prevalence is also complicated by frequent misdiagnosis[1]. Effective treatments exist, which can generally halt or slow down the progression of WD,[9,10] and the most recent treatment guidelines of the American Association for the Study of Liver Diseases recommend that these should be initiated as soon as possible after diagnosis and continued for life or until liver transplantation, which is be a cure for WD[11]. These specific treatments include copper chelators such as D-penicillamine and trientine, or zinc salts, although the long-term effectiveness of the latter may be less than that of copper chelators[12].
Although large prospective clinical studies in WD are challenging to perform due to the rarity of the disease, the existence of national reference centres in some countries may allow for retrospective analyses on relatively large patient cohorts. For example, a report from Poland describes a cohort of 929 patients attending the reference centre for WD in Warsaw for a period of over 60 years[13]. In addition, the availability of comprehensive insurance claims databases in recent years provides a powerful tool to collect retrospective data on large numbers of patients with rare diseases[14-16]. Insurance claims database studies of WD have now been reported from Taiwan[17], South Korea[18] and France[19,20].
As the structure of healthcare provision in general, and for rare diseases in particular, differs significantly between countries, it is of interest to extend these observations to other health systems. In this study, the objective was to explore the characteristics of patients with WD in the United States and to document treatment patterns.
This was a retrospective, longitudinal, health insurance claims database study of patients with WD in the United States. Data were extracted from the Clarivate Real-World Data Product (Clarivate RWD) for all patients with at least two submitted claims for WD between January 1, 2016 (the date when the full database was first available) and December 31, 2021 (the most recent full year of validated data available), identified by ICD-10 codes. The index date was defined as the date of the first claim associated with WD. Patients were followed from the index date until they died or until the end of the study period (December 31, 2021) if that occurred first (Figure 1).
The Clarivate RWD Product is a database of open healthcare reimbursement claims obtained from clearinghouses. The database links medical claims, pharmacy claims, and electronic health records from ≥ 98% of commercial and government health insurance plans using a direct-matching algorithm and an encrypted participant key. Data are updated from the source claims repositories daily. The nature of the database allows longitudinal tracking of individual patients over time if they change payers. The Clarivate RWD Product covers claims data from approximately 220 million unique lives and contains information on the patient, payer, and details of the consultation, medical procedure or prescription. Data can be extracted on patient demographics, geographic location, diagnoses and comorbidities, clinical tests, and therapeutic interventions. If a patient dies, then the date of death is documented, but not the cause of death.
Patients with WD were identified by the presence of two claims associated with the ICD-10 code E83.01 (WD) during the study period. The rationale for requiring two documented claims was that, in reimbursement databases in the United States, physicians requesting diagnostic tests (or other reimbursable healthcare resource use) can use the ICD-10 code for suspected but unconfirmed diagnoses. The requirement for two claims significantly increases the likelihood that the diagnosis will have been confirmed in these patients. Patients with no documented healthcare reimbursement during the 1-year pre-index period were excluded.
Information was extracted from the database on demographics at the index date. All reimbursed delivery of specific WD treatments (D-penicillamine, trientine or zinc acetate) was documented from national drug codes (NDCs) by date. All hospitalisations and any hospitalisations for liver transplantation identified by the appropriate procedure code throughout the post-index follow-up period, were documented by date. All claims associated with clinical manifestations of WD were determined using a list of hepatic, neurological and psychiatric signs and symptoms potentially attributable to WD, which were identified using the relevant ICD-10 codes or using NDCs for medications for these symptoms (Supplementary Table 1). For patients who died, the date was documented.
The study was purely descriptive and no statistical hypotheses were tested. For some analyses, two subgroups of patients were compared. These comprised patients with at least one documented delivery of a reimbursed WD-specific treatment during the study period (with documented treatment subgroup) or of patients with no such delivery (no documented treatment subgroup). Continuous variables were described by mean values with their standard deviation or median values with their interquartile range (IQR). Categorical variables were reported as frequency counts and percentage. Missing data were not replaced. Categorical variables were compared between the with documented treatment and the no documented treatment subgroup using the χ² test. Continuous variables were compared by Student’s t-test.
The study complied with all relevant international and national legislation on medical research and data privacy. The data were de-identified at the patient level and extracted using an encrypted participant key in compliance with the United States Health Insurance Portability and Accountability Act. As the data were anonymised prior to extraction, ethics committee approval and informed consent were not required.
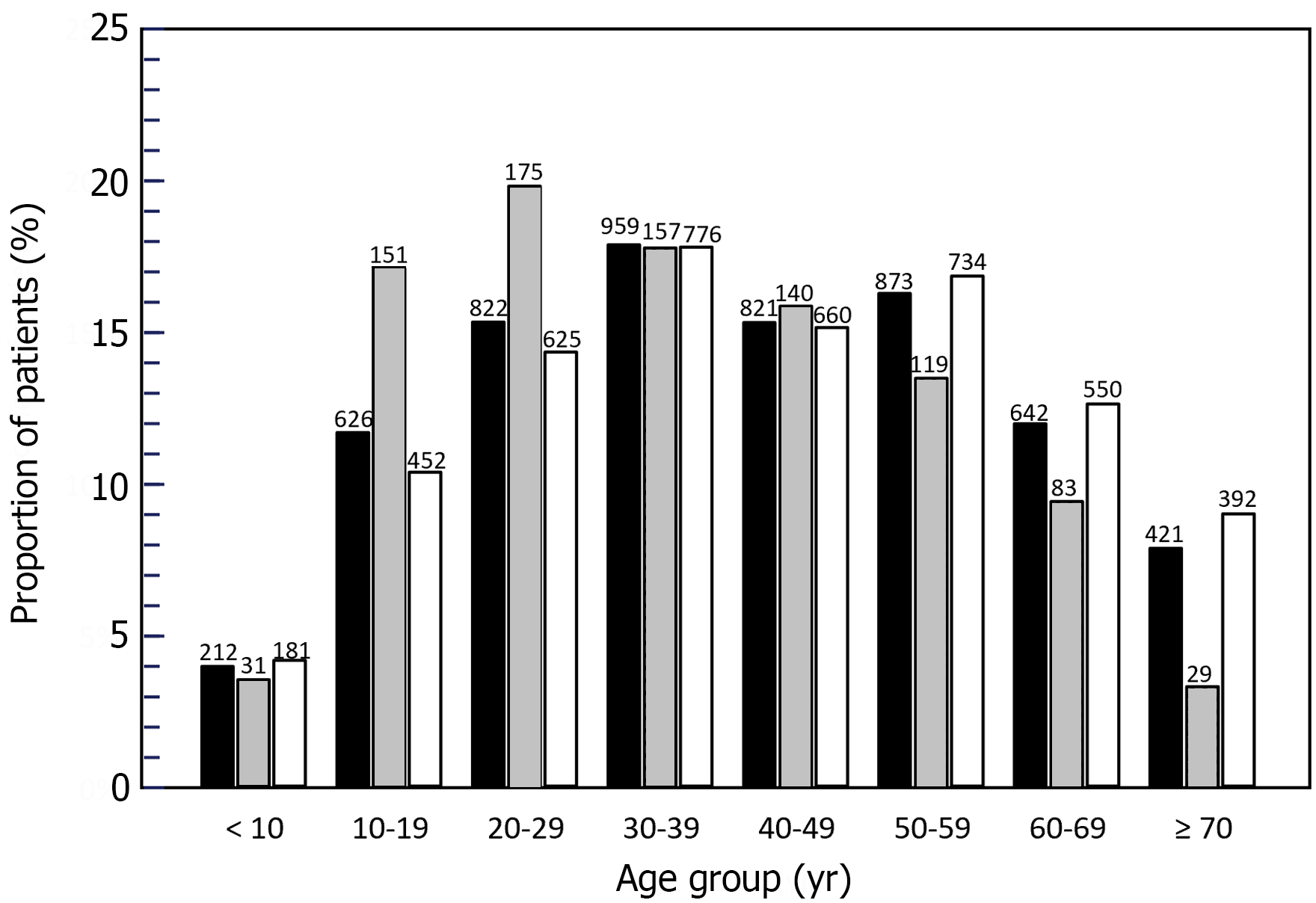
Over the study period, 5376 unique patients with at least two claims associated with an ICD-10 code for WD were identified and constituted the analysis population. Apart from the first year (2016), the number of unique patients present in the database each year was relatively stable, ranging from 2220 in 2020 to 2356 in 2021 (Table 1). Overall, 2926 patients (54.4%) were followed for at least 2 years and 658 (12.2%) for at least 5 years. The mean age of the analysis population at the index date was 41.2 ± 19.4 years (median 40 years; IQR: 26–56 years). Eight hundred and sixty-three patients (15.6%) were younger than 20 years of age and 1063 (19.8%) at least 60 years of age. The age distribution of the analysis population is presented in Figure 1. Overall, 2791 patients (52.0%) were men and 2582 (48.0%) were women.
In the analysis population, 885 patients (15.1%) were documented to receive a prescription for a WD-specific treatment at least once. This was most frequently trientine (n = 489; 8.4%), followed by zinc salts (n = 407; 6.9%) and D-penicillamine (n = 182; 2.9%). The remaining 4491 patients were never documented to have received a WD-specific treatment. At the index date, patients delivered a documented specific treatment for WD were younger than patients without (mean age: 36.6 ± 17.8 years vs 42.2 ± 19.6 respectively; P < 0.001; Student’s t-test). In the with documented treatment group, 182 (20.6%) were < 20 years of age and 112 (12.7%) were ≥ 60 years of age. In contrast, in the no documented treatment group, 633 (14.5%) were < 20 years of age and 942 (21.6%) were ≥ 60 years of age. The age distribution of these two patient groups is shown in Figure 1.
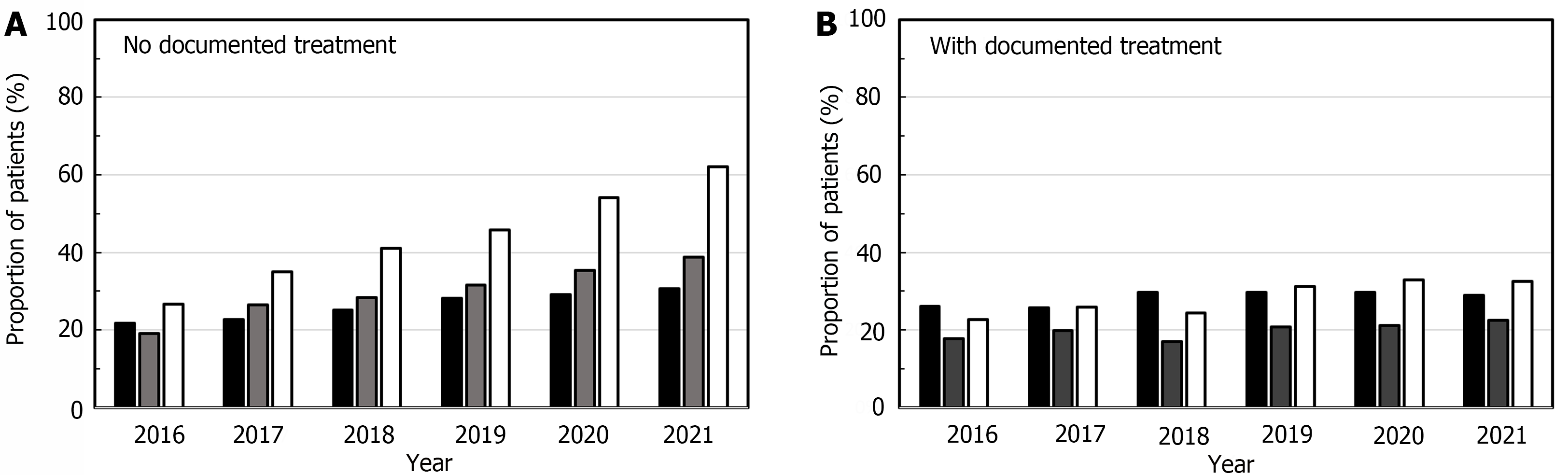
In the analysis population, 3829 patients presented at least one clinical manifestation suggestive of WD during the follow-up period, including the index date. This was most frequently a psychiatric manifestation (Table 2). Hepatic manifestations were more frequently documented in patients with documented treatment than in those without (P < 0.001; Table 2). No between-group difference was observed for the frequency of neurological and psychiatric manifestations (Table 2). Over the study period, the proportion of patients with documented clinical manifestations increased. The proportion of patients with any manifestation increased from 48.5% in 2016 to 72.4% in 2021. These increases were principally observed in the 4491 patients without documented treatment. The proportion remained relatively stable in patients with documented treatment. The increase was principally driven by patients with psychiatric (increased from 25.2% to 46.3%) and neurological manifestations (increased from 18.4% to 28.9%). The increase over time in the proportion of patients with documented hepatic manifestations was minimal (22.5% in 2016 and 22.9% in 2021) (Figure 2).
| Type of manifestation | All patients | Patients with documented treatment | Patients with no documented treatment | P value by χ² test |
| n = 5376 | n = 885 | n = 4491 | ||
| Hepatic manifestations | 1796 (33.4) | 389 (44.0) | 1288 (28.7) | < 0.001 |
| Neurological manifestations | 1935 (36.0) | 302 (34.1) | 1577 (35.1) | 0.572 |
| Psychiatric manifestations | 2433 (45.3) | 383 (43.3) | 1977 (44.0) | 0.683 |
| At least one of the above | 3829 (71.2) | 649 (73.3) | 3060 (68.1) | 0.002 |
During the follow-up period, 1564 patients (29.1%) were hospitalised overnight at least once. The median length of stay was 6 d (IQR: 2 d-16 d), and the duration did not differ significantly between patients with or without a documented treatment (Table 3). Overall, 121 patients (2.3%) were documented as having undergone a liver transplantation during the follow-up period (Table 3). The most frequently documented primary reasons for hospitalisation were WD (152 patients hospitalised), unspecified cirrhosis of the liver (112 patients), sepsis - unspecified organism (108 patients), and hepatic failure unspecified without coma (105 patients). Other individual reasons for hospitalisation were documented in no more than 100 patients.
| Parameter | All patients | Patients with documented treatment | Patients with no documented treatment | P value by χ² test | |
| n = 5376 | n = 885 | n = 4491 | |||
| Day hospitalisations, n (%) | 4991 (92.8) | 865 (97.7) | 4126 (91.9) | < 0.0001 | |
| Overnight hospitalisations, n (%) | 1564 (29.1) | 295 (33.3) | 1269 (28.3) | 0.0023 | |
| Length of stay1, median (IQR) | 6 (2–16) | 6 (2–17) | 6 (2–17) | NA | |
| Liver transplantation, n (%) | 121 (2.3) | 23 (2.6) | 98 (2.2) | NA | |
Over the follow-up period, a total of 273 patients in the analysis population (5.1%) died. This mortality rate is twice as high as that observed for all insurees present in the database (2.74%). The number of patients who died each year was around 60, except in the first (2016) and last (2021) years, with 2021 including incomplete death records (Table 4). For patients with a documented treatment, the proportion who died was 4.0% (n = 35) and in the subgroup without such a treatment, 5.3% (n = 232). This difference was not significant (P = 0.094; χ² test). The remaining six patients who died had received a liver transplant. The mean overall age at death was 57.9 ± 16.7 years, 52.7 years of age in patients with documented treatment and 58.8 years of age in those without documented treatment; P = 0.053). When compared with overall mortality in the American general population, the standardised mortality ratio in patients with WD was 2.19.
| Insurees with WD | All insurees | |||||
| Year | Total n | Deaths | Mortality rate, % | Total n | Deaths | Mortality rate, % |
| 20161 | 1887 | 27 | 1.43 | 203060882 | 1991215 | 0.98 |
| 2017 | 2239 | 51 | 2.28 | 223313801 | 2341297 | 1.05 |
| 2018 | 2253 | 58 | 2.57 | 223828962 | 2420297 | 1.08 |
| 2019 | 2356 | 61 | 2.59 | 219088011 | 2390049 | 1.09 |
| 2020 | 2220 | 63 | 2.84 | 215457629 | 2461627 | 1.14 |
| 20212 | 2277 | 13 | 0.57 | 239164264 | 205313 | 0.09 |
| Total | 5376 | 273 | 5.08 | 431039425 | 11809798 | 2.74 |
We identified 5376 unique patients with ≥ 2 claims associated with an ICD-10 code for WD in the Clarivate RWD database over a 6-year inclusion period, corresponding to around 2250 prevalent patients each year, with the exception of the first year (2016), when case identification was potentially not exhaustive. Assuming the diagnosis was correctly assigned in all cases and a prevalence of clinically-diagnosed WD between 1:30000 and 1:50000[7], these cases should account for between 50% and 80% of all patients with WD in the United States. With regard to treatment, we found that only 15% of patients had been documented to have received a WD-specific treatment at least once during the follow-up period. Overall, 273 patients died during the study period, corresponding to 5.1% of the WD cohort. Compared with the American general population, the standardised mortality ratio in patients with WD was 2.19. This suggests that WD was associated with excess mortality, as previously reported in other recent studies elsewhere[13,18,20]. In addition, the mean age at death was 57.9 years, compared with a life expectancy of 77 years in the American general population in 2020, representing a loss of around 20 life years. The observed mortality data are consistent with data from the Polish national WD registry, in which patients with WD have also shown around 20 life years lost compared with the general population[13]. A recent study of > 17,000 patients with WD identified between 2007 and 2017 in the United States National Inpatient Sample has also described an elevated mortality in patients with WD (unadjusted mortality rate: 2.51% in patients with WD vs 2.05% in patients without)[20].
At the index date, the median age of the patients was 40 years, similar to that reported in the French insurance claims database study (39 years)[21]. This age is older than the age of first diagnosis reported in WD registry studies, which is typically between 20 years and 30 years of age[13,22]. This is most simply explained by the fact that the Clarivate database only became available in 2016 and patients who were diagnosed prior to that date may appear in the database for the first time many years after they were originally diagnosed. In addition, there may be an issue with coding in certain insurance claims databases, where the ICD-10 code for WD is not consistently documented at the time of diagnosis. In the Taiwanese insurance claims database study, in which patients with WD were identified not by ICD codes associated with the claim, but by linking from the national WD patient registry, the incidence of first documentation in the database peaked in the 15-year-old to 19-year-old age group[17].
The observed proportion of treated patients (15%) is certainly an underestimate of the true treatment rate in this population. Zinc salts are available over the counter in the United States, and if purchased that way, are not reimbursed through health insurance and therefore not documented in the database. In addition, patients in the United States can purchase medications from online pharmacies in other countries such as Canada or India and may have an incentive to do so if that would be substantially cheaper. In the United States, copper chelators (D-penicillamine and trientine), even in generic formulations, are expensive, up to $91 a pill for trientine and up to $35 a pill for D-penicillamine, and there may thus have been an unknown amount of purchasing from online pharmacies. For these reasons, treatment of WD may have been undocumented in a potentially large number of patients. Documented treatment or prescription rates in insurance claims database studies from other countries range from 34.9% in South Korea[18] to 43.8% in France[21]. As well as underestimating the number of patients treated (the numerator), the number of patients with an ascertained diagnosis of WD (the denominator) may be overestimated, as it is not uncommon in the United States, for health insurance purposes, to assign an ICD code for WD before diagnostic confirmation, and the code be may not revised subsequently if the diagnosis was not confirmed. For this reason, the diagnosis remains uncertain in an unknown proportion of patients without an associated WD-specific treatment. In contrast, the diagnosis of WD should not be open to question in patients with a documented specific treatment. At the index date, no major differences between patients with a documented treatment and those without were observed, except that patients of the former group were younger and more frequently presented hepatic symptoms, consistent with previous reports for age in the French, South Korean and Polish setting[13,18,21]. However, over time the proportion of patients in the no documented treatment group who presented neurological or psychiatric manifestations tended to rise following the index date, whereas that was not the case in the documented treatment group. This may reflect the fact that WD is frequently diagnosed on the basis of hepatic symptoms, with neurological and psychiatric symptoms developing later[22]. It should be noted that persistence, and even aggravation of neurological symptoms is well-documented in patients with WD who are under treatment[10].
The most frequent clinical manifestations evocative of WD that were documented were psychiatric manifestations in 44.0% of patients. This finding was unanticipated, as in previous registry studies[13,22] and insurance claims database studies[17,18,21], hepatic manifestations tended to dominate, being observed in over half the patients. This is generally the case in patients with typical early-onset WD in adolescence or young adulthood[23]. Psychotropic medications such as anxiolytics and antidepressants, which contribute to the definition of psychiatric manifestations, may have been prescribed to manage the psychological distress arising from living with WD, rather than to treat psychiatric manifestations of the disease sensu stricto. This explanation may also account for the unexpectedly large rise in the proportion of patients with psychiatric manifestations over the years following the index date. In this study, only hepatic manifestations were observed in 33% of patients. It is possible that these hepatic manifestations require treatment generating a reimbursement claim less frequently than neurological or psychiatric manifestations, and given the relatively short follow-up duration, claims related to hepatic manifestations were under-represented. It should also be noted that the proportion of patients undergoing liver transplantation (2.3%) was relatively low compared to previous studies in Europe[19,22] and South Korea[18]. However, the number of liver transplantations is also probably underestimated as transplants occurring before the database became available in 2016 could not be identified.
The principal strength of the study resides in the large number of patients available for analysis (5376 unique patients). Limitations include the fact that WD was documented from reimbursement claims only and without independent clinical ascertainment: The proportion of patients whose clinical diagnosis of WD was not confirmed, without the disease code being corrected in the database is unknown. This may lead to an over-estimation of the number of patients with a confirmed and definite diagnosis of WD who do not receive a specific treatment. In addition, treatment rates were probably underestimated because, as mentioned above, over the counter and online drug purchases were not identified. This illustrates the need to reinforce database quality by ascertaining medical data and correcting coding when necessary so that data on rare diseases from reimbursement claims databases can be used with confidence. In addition, patients with a documented liver transplantation are not treated with copper-depleting drugs, and in this study they were assigned for this reason to the no documented treatment group. However, this only concerned 121 patients (2.3%). Another limitation is that the period over which individual patients could be followed in the database was also relatively short (around 2 years). In addition, the hepatic, neurological and psychiatric manifestations that were documented could not be ascribed unequivocally to WD. The data on deaths is probably incomplete, and in consequence, mortality might be underestimated, even though it was clearly elevated in patients with WD. During the first year of the database (2016), all deaths were probably not captured and this is also the case in the most recent year (2021). There is a lag time between the date of death in the community and the date when the information is transferred to health insurance databases. It should also be noted that the different health insurance plans in the United States do not provide identical coverage and what is actually reimbursed may differ from one plan to another. A future way forward may be to perform a bridging study between hospital patient registries and insurance claims databases to evaluate the completeness and accuracy of claims coding rare diseases such as WD.
This insurance claims database study describes a large cohort of 5376 patients with ICD-10 codes for WD in the United States, although the number of actual WD cases is expected to be lower due to unconfirmed diagnoses. Patients with a documented WD treatment together with a WD diagnosis code can be confidently assumed to have WD. These patients are frequently symptomatic and have an excess early mortality compared with the American general population, despite the fact that effective treatments can halt or slow the progression of disease. Despite the study limitations, our data suggest that a significant number of patients may not be receiving best standard of care in terms of treatment, compliance, and follow-up. These findings indicate that there is a significant unmet need for effective treatment for WD in the United States.
We wish to thank Adam Doble, Foxymed (Paris, France) for medical writing support and Claire Leboucher, Putnam PHMR (Lyon, France) for advice on data analysis.
| 1. | Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH, Schilsky ML. Wilson disease. Nat Rev Dis Primers. 2018;4:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 605] [Cited by in RCA: 626] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 2. | Bandmann O, Weiss KH, Kaler SG. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14:103-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 477] [Cited by in RCA: 679] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 3. | Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327-337. [PubMed] [DOI] [Full Text] |
| 4. | Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993;5:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 991] [Cited by in RCA: 918] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 5. | Stremmel W, Weiskirchen R. Therapeutic strategies in Wilson disease: pathophysiology and mode of action. Ann Transl Med. 2021;9:732. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 6. | Kipker N, Alessi K, Bojkovic M, Padda I, Parmar MS. Neurological-Type Wilson Disease: Epidemiology, Clinical Manifestations, Diagnosis, and Management. Cureus. 2023;15:e38170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 7. | Sandahl TD, Laursen TL, Munk DE, Vilstrup H, Weiss KH, Ott P. The Prevalence of Wilson's Disease: An Update. Hepatology. 2020;71:722-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 8. | Gao J, Brackley S, Mann JP. The global prevalence of Wilson disease from next-generation sequencing data. Genet Med. 2019;21:1155-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Mulligan C, Bronstein JM. Wilson Disease: An Overview and Approach to Management. Neurol Clin. 2020;38:417-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 10. | Litwin T, Dzieżyc K, Członkowska A. Wilson disease-treatment perspectives. Ann Transl Med. 2019;7:S68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Schilsky ML, Roberts EA, Bronstein JM, Dhawan A, Hamilton JP, Rivard AM, Washington MK, Weiss KH, Zimbrean PC. A multidisciplinary approach to the diagnosis and management of Wilson disease: 2022 Practice Guidance on Wilson disease from the American Association for the Study of Liver Diseases. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 54.0] [Reference Citation Analysis (1)] |
| 12. | Weiss KH, Gotthardt DN, Klemm D, Merle U, Ferenci-Foerster D, Schaefer M, Ferenci P, Stremmel W. Zinc monotherapy is not as effective as chelating agents in treatment of Wilson disease. Gastroenterology. 2011;140:1189-1198.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 13. | Członkowska A, Niewada M, Litwin T, Kraiński Ł, Skowrońska M, Piechal A, Antos A, Misztal M, Khanna I, Kurkowska-Jastrzębska I. Seven decades of clinical experience with Wilson's disease: Report from the national reference centre in Poland. Eur J Neurol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 14. | Bezin J, Duong M, Lassalle R, Droz C, Pariente A, Blin P, Moore N. The national healthcare system claims databases in France, SNIIRAM and EGB: Powerful tools for pharmacoepidemiology. Pharmacoepidemiol Drug Saf. 2017;26:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 444] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 15. | Lim SS, Lee W, Kim YK, Kim J, Park JH, Park BR, Yoon JH. The cumulative incidence and trends of rare diseases in South Korea: a nationwide study of the administrative data from the National Health Insurance Service database from 2011-2015. Orphanet J Rare Dis. 2019;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 16. | Ninomiya K, Okura M. Nationwide comprehensive epidemiological study of rare diseases in Japan using a health insurance claims database. Orphanet J Rare Dis. 2022;17:140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 17. | Lai CH, Tseng HF. Population-based epidemiologic study of Wilson's disease in Taiwan. Eur J Neurol. 2010;17:830-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Choe EJ, Choi JW, Kang M, Lee YK, Jeon HH, Park BK, Won SY, Cho YS, Seo JH, Lee CK, Chung JB. A population-based epidemiology of Wilson's disease in South Korea between 2010 and 2016. Sci Rep. 2020;10:14041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Poujois A, Woimant F, Samson S, Chaine P, Girardot-Tinant N, Tuppin P. Characteristics and prevalence of Wilson's disease: A 2013 observational population-based study in France. Clin Res Hepatol Gastroenterol. 2018;42:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 20. | Patel AH, Ghattu M, Mazzaferro N, Chen A, Catalano K, Minacapelli CD, Rustgi V. Demographics and Outcomes Related to Wilson's Disease Patients: A Nationwide Inpatient Cohort Study. Cureus. 2023;15:e44714. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Daniel-Robin T, Bénichou B, Leboucher C, Blein C, Combal JP. Epidemiology, treatment and burden of Wilson disease in France: A 10-year analysis of the national health insurance database. Clin Res Hepatol Gastroenterol. 2022;46:101992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 22. | Merle U, Schaefer M, Ferenci P, Stremmel W. Clinical presentation, diagnosis and long-term outcome of Wilson's disease: a cohort study. Gut. 2007;56:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 387] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 23. | Poujois A, Woimant F. Wilson's disease: A 2017 update. Clin Res Hepatol Gastroenterol. 2018;42:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wang YC, China S-Editor: Zhang L L-Editor: Filipodia P-Editor: Cai YX