Published online Oct 27, 2024. doi: 10.4254/wjh.v16.i10.1208
Revised: September 24, 2024
Accepted: October 11, 2024
Published online: October 27, 2024
Processing time: 102 Days and 21.5 Hours
In this letter, we comment on a recent publication by Mei et al, in the World Journal of Hepatology, investigating the hepatoprotective effects of the modified Xiaoyao San (MXS) formula in a male rat model of non-alcoholic steatohepatitis (NASH). The authors found that MXS treatment mitigated hepatic steatosis and inflammation in the NASH model, as evidenced by the reduction in lipid droplets (LDs), fibrosis markers and lipogenic factors. Interestingly, these hepatoprotective effects were associated with androgen upregulation (based on metabolomics analysis of male steroid hormone metabolites), adenosine 5’-monophosphate-activated protein kinase (AMPK) activation, and restoration of phosphatase and tensin homolog (PTEN) expression. However, the authors did not clearly discuss the relationships between MXS-induced hepatic steatosis reduction in the NASH model, and androgen upregulation, AMPK activation, and restoration of PTEN expression. This editorial emphasizes the reported mechanisms and explains how they act or interact with each other to reduce hepatic steatosis and inflammation in the NASH model. As a perspective, we propose additional mechanisms (such as autophagy/lipophagy activation in hepatocytes) for the clearance of LDs and suppression of hepatic steatosis by MXS in the NASH model. A proper understanding of the mechanisms of MXS-induced reduction of hepatic steatosis might help in the treatment of NASH and related diseases.
Core Tip: Modified Xiaoyao San (MXS) formula has hepato-protective effects in a male rat model of non-alcoholic steatohepatitis (NASH) via suppression of steatosis and inflammation. These protective effects are related to several mechanisms, including the regulation of sex hormone metabolism, androgen upregulation, adenosine 5’-monophosphate-activated protein kinase activation, and enhanced phosphatase and tensin homolog expression. A deeper understanding of the mechanisms behind MXS-induced reduction of hepatic steatosis could assist in the treatment of NASH and related diseases.
- Citation: Eid N, Bhatnagar P, Chan LL, Garcia-Macia M. Suppression of hepatic steatosis in non-alcoholic steatohepatitis model by modified Xiaoyao San formula: Evidence, mechanisms and perspective. World J Hepatol 2024; 16(10): 1208-1212
- URL: https://www.wjgnet.com/1948-5182/full/v16/i10/1208.htm
- DOI: https://dx.doi.org/10.4254/wjh.v16.i10.1208
Non-alcoholic fatty liver disease (NAFLD) is a chronic condition characterized by a marked accumulation of lipid droplets (LDs) in hepatocytes (steatosis), which can progress to non-alcoholic steatohepatitis (NASH). Morphologically, NASH is characterized by steatosis, hepatocyte injury and inflammatory hepatocellular ballooning, which can progress into fibrosis, cirrhosis and hepatocellular carcinoma. Currently, no licensed drugs have been approved for the treatment of NASH[1,2]. Xiaoyao San (XS) is a traditional Chinese medicine used for the treatment of liver stagnation and spleen deficiency syndrome, depression, various gynaecological diseases, cancer, steatosis and more. The XS formulation consists of eight Chinese herbal medicines: Bupleurum chinense DC., Angelica sinensis (Oliv.) Diels, Paeonia lactiflora Pall., Atractylodes macrocephala Koidz., Poria cocos (Schw.) Wolf, Glycyrrhiza uralensis Fisch., Zingiber officinale Roscoe and Mentha haplocalyx Briq. at a weight ratio of 3:3:3:3:3:1.5:1:1[3-9].
In an interesting paper, Mei et al[10] investigated the hepato-protective effects of a modified XS (MXS) formula on a male rat model of NASH using light microscopy, immunohistochemistry (IHC), Western blot, and metabolomics analysis of male steroid hormone metabolites. The authors found that six-week MXS treatment suppressed steatosis, fibrosis, and inflammation markers in the NASH model. This reduction of steatosis was associated with adenosine 5’-monophosphate-activated protein kinase (AMPK) activation, restoration of the phosphoinositide 3-kinase/phosphatase and tensin homolog (PTEN) expression and upregulation of androgens. These morphological and molecular findings were linked to a significant reduction in liver weight and liver weight/body weight ratio in the MXS-treated NASH group compared to the untreated NASH group. In the next paragraphs, we discuss these findings, explain the related mechanisms, and offer further perspectives.
Using oil-red staining for neutral lipids (a marker of LDs), Mei et al[10] reported a marked decrease in LDs in the MXS-treated NASH group compared to the untreated NASH group. LDs have specific proteins surrounding their fat core, known as perilipins. The upregulation of these proteins has been associated with enhanced hepatic steatosis in NAFLD and NASH[1,11]. Therefore, studying the expression of these proteins under MXS treatment in a NASH model using IHC and Western blot could provide interesting insights into the mechanisms involved. In agreement with Mei et al[10], two studies have reported the suppression of hepatic steatosis by XS through various mechanisms, including inhibition of the glucocorticoid receptor/perilipin-2 signaling pathway[5] and activation of the estrogen receptor α pathway in ovariectomized ApoE-/-mice[3]. Furthermore, XS decoction reduced hepatic fibrosis in rats via the transforming growth factor-β/Smad and protein kinase B/forkhead box O3 signaling pathways[4]. Moreover, Mei et al[10] reported the decreased expression of the fibrosis marker alpha smooth muscle actin (α-SMA), the inflammation marker cyclooxygenase-2 (COX-2), and lipogenesis factors, fatty acid synthase (FASN) and peroxisome proliferator-activated receptor gamma (PPARγ) in the livers of the MXS-treated NASH group compared to the untreated NASH group[10]. The findings were based on α-SMA and FASN IHC analysis and COX-2 and PPARγ Western blot analysis. However, in the study by Mei et al[10], IHC of hepatic α-SMA did not clearly show the morphology of the stellate cells, which are the main effectors for hepatic fibrosis in NASH[12]. Therefore, higher magnification imaging is needed to better visualize these cells. In addition, immunoelectron microscopy could be a valuable technique for detecting stellate cells using α-SMA immunogold labelling[11].
A key finding in the study by Mei et al[10] was the significant upregulation of androgens in the MXS-treated NASH group. Mechanistically, metabolomics analysis revealed that this upregulation occurred via regulation of the steroid sex hormone-related metabolic pathway. Androgen upregulation was associated with activation of AMPK and enhanced expression of PTEN in hepatocytes of the MXS-treated group. Despite these findings, several critical questions and unresolved issues warrant further investigation: (1) What is the relationship between androgen upregulation and the suppression of hepatic steatosis by MXS in the NASH model? (2) What is the mechanism of androgen upregulation with MXS treatment? (3) How do activation of AMPK and restoration of PTEN expression by MXS treatment reduce hepatic steatosis? And (4) Are there any possible additional mechanisms for the reduction of hepatic steatosis by MXS treatment?
A growing body of evidence indicates that androgen levels are reduced in men and animal models of NAFLD, NASH and models of obesity and metabolic syndrome[13,14]. Importantly, testosterone therapy was found to reduce hepatic steatosis in men with type 2 diabetes[15] and suppress the expression of FASN. Thus, this therapy appeared to protect against hepatic steatosis in cholesterol-fed androgen-deficient mice[16]. Moreover, endogenous testosterone has been reported to alleviate hepatic steatosis in protein-restricted male rats[17]. These findings align with the study by Mei et al[10], which reports that suppression of FASN in the MXS-treated NASH group was linked to reduced hepatic steatosis. Taken together, the androgens appear to reduce hepatic steatosis by suppressing lipogenic factors such as FASN, while hepatic steatosis itself can suppress androgen, hence answering the first and second questions raised. This bi-directional relationship underscores the need for further research to fully understand the mechanisms involved.
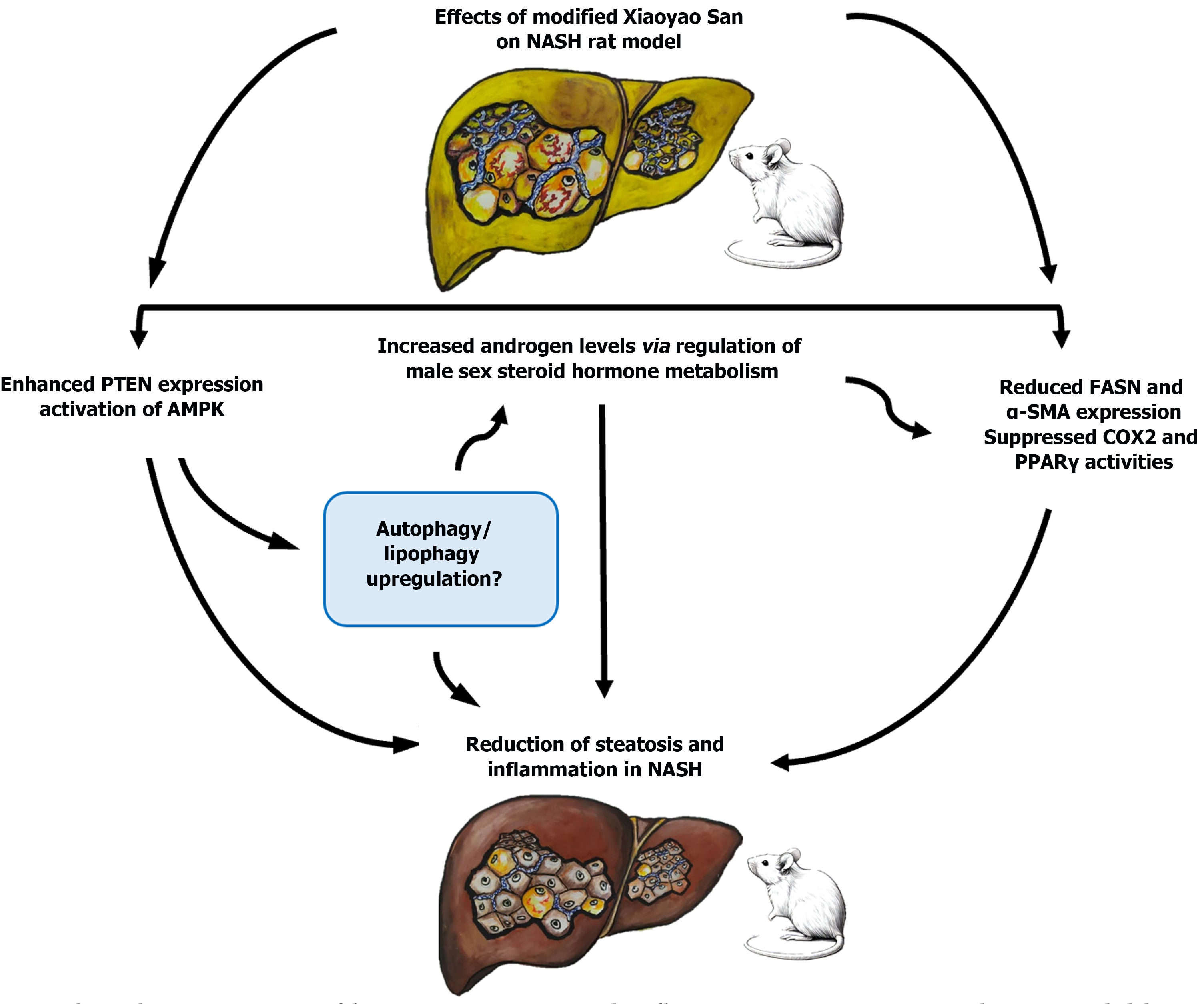
In NAFL and AFL diseases, activation of AMPK was reported to reduce hepatic steatosis by down-regulating lipogenic gene expression (FASN, sterol regulatory element-binding protein 1c, ACC and HMGCR). Activation of AMPK also enhances the expression of fatty acid oxidation proteins involved in lipolysis (carnitine palmitoyl transferase 1, peroxisome proliferator-activated receptor-γ coactivator 1, hormone-sensitive lipase, and adipose triglyceride lipase). Furthermore, AMPK activation improves mitochondrial function and integrity[18,19]. PTEN is another key regulator in cellular functions, including signaling, lipid and glucose metabolism, as well as cell survival, tumor suppression and apoptosis. The loss of hepatic PTEN results in increased de novo lipogenesis through robust induction of sterol regulatory element-binding protein and FASN expression[20,21]. These reports support the study by Mei et al[10], who observed AMPK activation and increased PTEN expression following MXS treatment in the NASH group. This observation was associated with suppression of the lipogenic factors FASN and PPARγ and answer the third question raised. Figure 1 summarizes the various mechanisms of MXS-related hepatoprotection in the NASH male rat model, based on the study Mei et al[10] and proposed mechanisms for future research.
Macroautophagy (hereafter referred to as autophagy) is a prosurvival bulk degradation pathway that degrades almost all cellular components via lysosomes and is specifically upregulated upon exposure to various stressors such as oxidative stress, mitochondrial damage and LD overload[11]. In addition, autophagy selectively clears damaged organelles such as mitochondria (via mitophagy), and LDs (via lipophagy)[11,22]. Impaired autophagy or lipophagy leads to various diseases, including NAFL/NASH and metabolic syndrome; conversely autophagy activation by natural products or drugs such as rapamycin reduces steatosis in AFL and NAFL[11,22,23]. Autophagy–related gene (ATG) products are the key components of autophagy; more than 40 ATGs are found in yeast such as ATG 8 and its mammalian homolog, microtubule-associated protein 1A/1B-light chain 3 (LC3). Autophagy is initiated by AMPK activation and mechanistic target of rapamycin inhibition, resulting in the formation of Beclin1-mediated autophagosomal membranes. These membranes mature into LC3-II- mediated autophagosomes, which engulf the cellular contents, then fuse with lysosomes to be later degraded by different lysosomal enzymes[11,22,23]. Importantly, XS has been reported to exert anti-depressant effects via activation of autophagy and formation of autophagosomes in mice hippocampal and hypothalamic neurons[7,8]. Therefore, we speculate that MXS could activate AMPK-mediated autophagy upregulation in hepatocytes of the NASH model, resulting in the suppression of steatosis and the reduction of liver weight and liver weight/body weight ratio in the NASH group after six weeks of MXS treatment[10]. This is because autophagy upregulation not only clears LDs but also abnormal proteins and damaged organelles from the liver of NASH models[11,22,23]. Additionally, autophagy activation by MXS could be a possible mechanism for androgen upregulation (Figure 1) based on recent studies, indicating that autophagy enhances testosterone production by Leydig cells[24-26]. This could be an additional answer to the second question. Further research is needed to explore the proautophagic effects of MXS on the liver and testis in the NASH model.
MXS-induced suppression of hepatic steatosis, fibrosis and inflammation in NASH male rat model was associated with androgen upregulation, AMPK activation and restoration of PTEN expression in hepatocytes. Further research is needed to investigate the effects of MXS in a female rat model of NASH. In addition, whether autophagy/lipophagy have roles in the suppression of hepatic steatosis by MXS warrants further research.
| 1. | Scorletti E, Carr RM. A new perspective on NAFLD: Focusing on lipid droplets. J Hepatol. 2022;76:934-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 2. | Harrison SA, Allen AM, Dubourg J, Noureddin M, Alkhouri N. Challenges and opportunities in NASH drug development. Nat Med. 2023;29:562-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 242] [Reference Citation Analysis (0)] |
| 3. | Hu T, Wei M, Hong G, Qi T, Xiang Y, Yang Y, Yi Y. Xiaoyao San attenuates hepatic steatosis through estrogen receptor α pathway in ovariectomized ApoE-/- mice. J Ethnopharmacol. 2022;282:114612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Zhou Y, Wu R, Cai FF, Zhou WJ, Lu YY, Zhang H, Chen QL, Su SB. Xiaoyaosan decoction alleviated rat liver fibrosis via the TGFβ/Smad and Akt/FoxO3 signaling pathways based on network pharmacology analysis. J Ethnopharmacol. 2021;264:113021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 5. | Gong L, Wang G, Ma Q, Hao W, Xian M, Wu Y, Kurihara H, He R, Chen J. Novel insights into the effect of Xiaoyao san on corticosterone-induced hepatic steatosis: inhibition of glucocorticoid receptor/perilipin-2 signaling pathway. Acupunct Herb Med. 2022;2:49-57. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Liu N, Yang J, Ma W, Li C, An L, Zhang X, Zou Q. Xiaoyao Powder in the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Ethnopharmacol. 2022;288:114999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Wang M, Bi Y, Zeng S, Liu Y, Shao M, Liu K, Deng Y, Wen G, Sun X, Zeng P, Jing L, Lv Z. Modified Xiaoyao San ameliorates depressive-like behaviors by triggering autophagosome formation to alleviate neuronal apoptosis. Biomed Pharmacother. 2019;111:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Yang FR, Zhu XX, Kong MW, Zou XJ, Ma QY, Li XJ, Chen JX. Xiaoyaosan Exerts Antidepressant-Like Effect by Regulating Autophagy Involves the Expression of GLUT4 in the Mice Hypothalamic Neurons. Front Pharmacol. 2022;13:873646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Tang KR, Mo XW, Zhou XY, Chen YY, Liu DD, He LL, Ma QY, Li XJ, Chen JX. Xiaoyao San, a Chinese herbal formula, ameliorates depression-like behavior in mice through the AdipoR1/AMPK/ACC pathway in hypothalamus. J Integr Med. 2022;20:442-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Mei XL, Wu SY, Wu SL, Luo XL, Huang SX, Liu R, Qiang Z. Hepatoprotective effects of Xiaoyao San formula on hepatic steatosis and inflammation via regulating the sex hormones metabolism. World J Hepatol. 2024;16:1051-1066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (6)] |
| 11. | Alim Al-Bari A, Ito Y, Thomes PG, Menon MB, García-Macia M, Fadel R, Stadlin A, Peake N, Faris ME, Eid N, Klionsky DJ. Emerging mechanistic insights of selective autophagy in hepatic diseases. Front Pharmacol. 2023;14:1149809. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 12. | Marcher AB, Bendixen SM, Terkelsen MK, Hohmann SS, Hansen MH, Larsen BD, Mandrup S, Dimke H, Detlefsen S, Ravnskjaer K. Transcriptional regulation of Hepatic Stellate Cell activation in NASH. Sci Rep. 2019;9:2324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 76] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 13. | Van de Velde F, Bekaert M, Hoorens A, Geerts A, T'Sjoen G, Fiers T, Kaufman JM, Van Nieuwenhove Y, Lapauw B. Histologically proven hepatic steatosis associates with lower testosterone levels in men with obesity. Asian J Androl. 2020;22:252-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Song MJ, Choi JY. Androgen dysfunction in non-alcoholic fatty liver disease: Role of sex hormone binding globulin. Front Endocrinol (Lausanne). 2022;13:1053709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Apostolov R, Gianatti E, Wong D, Kutaiba N, Gow P, Grossmann M, Sinclair M. Testosterone therapy reduces hepatic steatosis in men with type 2 diabetes and low serum testosterone concentrations. World J Hepatol. 2022;14:754-765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Kelly DM, Nettleship JE, Akhtar S, Muraleedharan V, Sellers DJ, Brooke JC, McLaren DS, Channer KS, Jones TH. Testosterone suppresses the expression of regulatory enzymes of fatty acid synthesis and protects against hepatic steatosis in cholesterol-fed androgen deficient mice. Life Sci. 2014;109:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Uchida K, Inoue K, Hasegawa Y, Hakuno F, Takahashi SI, Takenaka A. Endogenous testosterone reduces hepatic lipid accumulation in protein-restricted male rats. Nutrition. 2021;85:111130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am J Physiol Endocrinol Metab. 2016;311:E730-E740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 402] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 19. | Fang C, Pan J, Qu N, Lei Y, Han J, Zhang J, Han D. The AMPK pathway in fatty liver disease. Front Physiol. 2022;13:970292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 229] [Reference Citation Analysis (0)] |
| 20. | Peyrou M, Bourgoin L, Foti M. PTEN in liver diseases and cancer. World J Gastroenterol. 2010;16:4627-4633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Chen CY, Chen J, He L, Stiles BL. PTEN: Tumor Suppressor and Metabolic Regulator. Front Endocrinol (Lausanne). 2018;9:338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 437] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 22. | Eid N, Ito Y, Otsuki Y. The autophagic response to alcohol toxicity: the missing layer. J Hepatol. 2013;59:398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Zhang S, Peng X, Yang S, Li X, Huang M, Wei S, Liu J, He G, Zheng H, Yang L, Li H, Fan Q. The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders. Cell Death Dis. 2022;13:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 191] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 24. | Chen H, Chen K, Zhao F, Guo Y, Liang Y, Wang Z, Liu T, Chen S. Macroautophagy involved in testosterone synthesis in Leydig cells of male dairy goat (Capra hircus). Theriogenology. 2022;180:53-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 25. | Ma Y, Zhou Y, Zhu YC, Wang SQ, Ping P, Chen XF. Lipophagy Contributes to Testosterone Biosynthesis in Male Rat Leydig Cells. Endocrinology. 2018;159:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Gao F, Li G, Liu C, Gao H, Wang H, Liu W, Chen M, Shang Y, Wang L, Shi J, Xia W, Jiao J, Gao F, Li J, Chen L, Li W. Autophagy regulates testosterone synthesis by facilitating cholesterol uptake in Leydig cells. J Cell Biol. 2018;217:2103-2119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 169] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/